Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms
Abstract
1. Introduction
2. Pathology
3. Cardinal Motor Symptoms in PD Identification
3.1. Bradykinesia
3.2. Tremor
3.3. Rigidity
3.4. Postural Instability
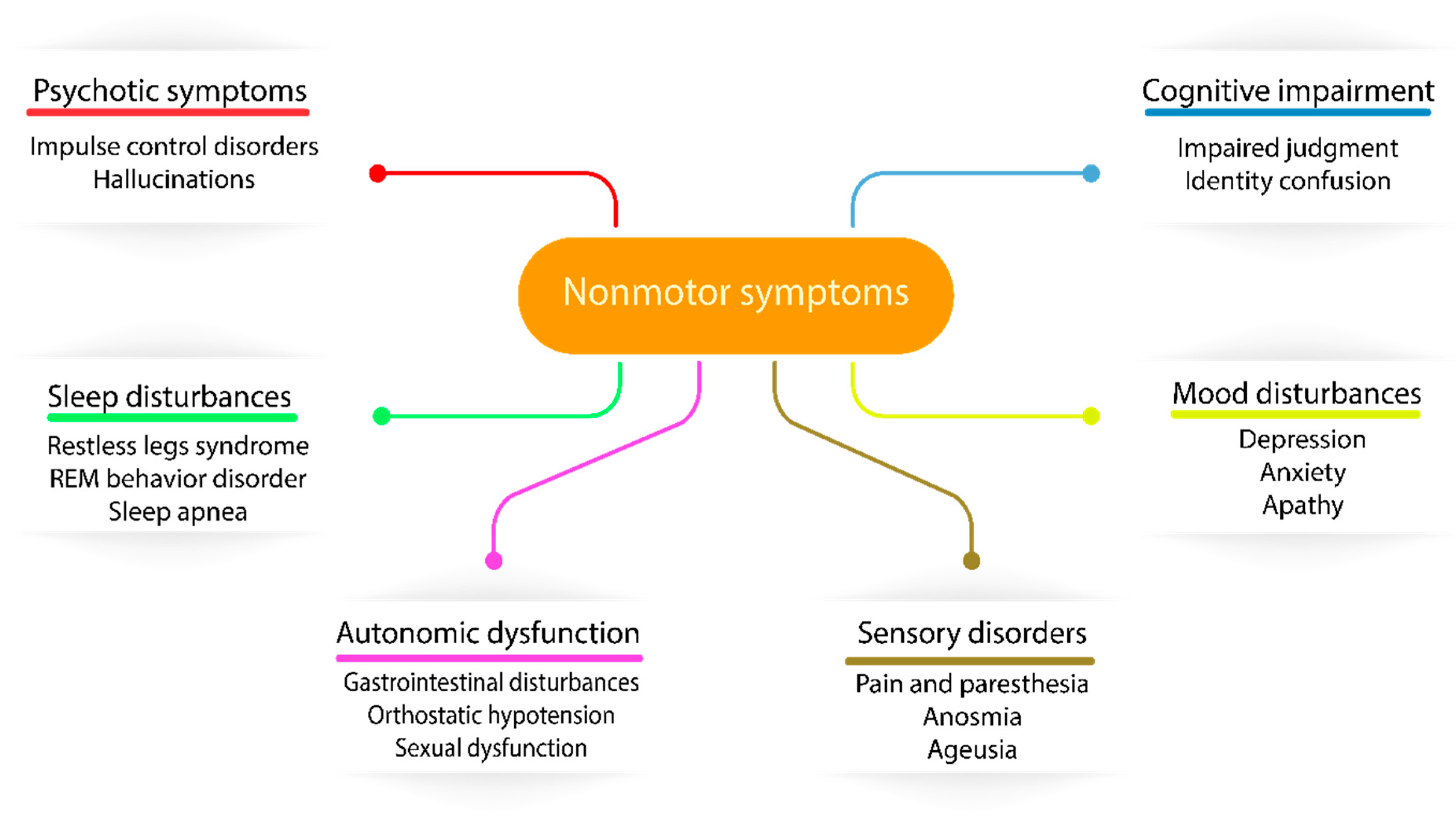
4. Recognition of Nonmotor Features in PD
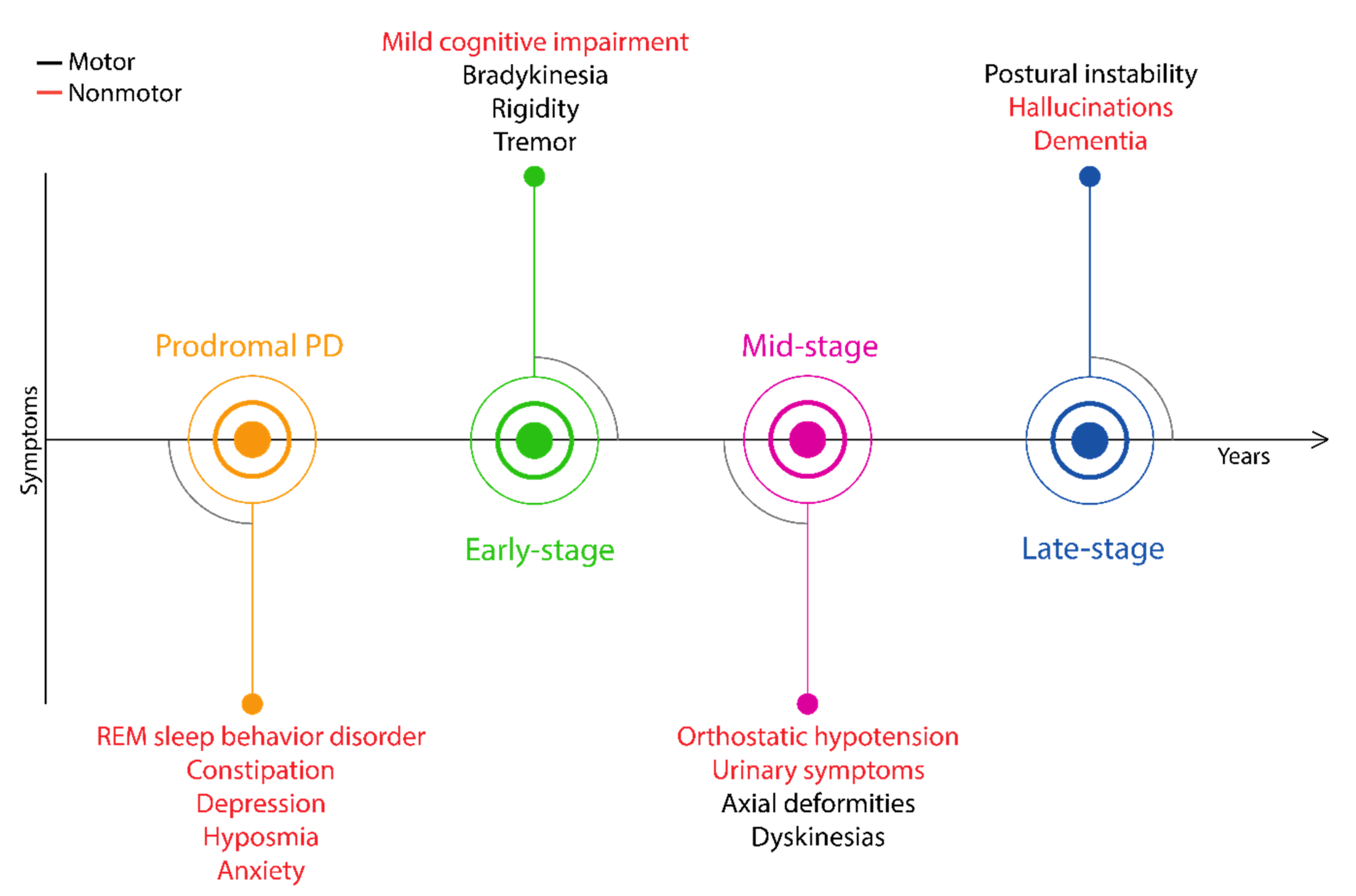
5. The Importance of Prodromal Symptoms
5.1. Rapid Eye Movement Sleep Behavior Disorder
5.2. Constipation
5.3. Olfactory Dysfunction
5.4. Depression
6. The Evolution of Critical Symptoms Involved in Diagnostic Procedures of PD
- Diagnostic criteria: presence of bradykinesia and at least one of the following symptoms: muscular rigidity, 4–6 Hz rest tremor and postural instability (not caused by other disorders)
- Exclusion criteria: history of repeated strokes or head injury, encephalitis, early severe autonomic involvement or dementia, Babinski sign, negative response to levodopa treatment and MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) exposure
- Supportive criteria (at least three required): unilateral onset, rest tremor, progressive course, persistent asymmetry, excellent response to dopaminergic therapy, levodopa-induced dyskinesia, positive levodopa response five years or more and clinical course of ten years or more
7. Potential Future Perspectives
7.1. Molecular Markers for PD Identification
7.2. Smart Devices
8. Conclusions
Funding
Conflicts of Interest
References
- Parkinson, J. An essay on the Shaking Palsy. Arch. Neurol. 1969, 20, 441–445. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s disease. Subcell Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.A.; Logroscino, G.; Gaziano, J.M.; Kurth, T. Incidence and remaining lifetime risk of Parkinson disease in advanced age. Neurology 2009, 72, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Cha, J.; Chung, S.J.; Yoo, H.S.; Sohn, Y.H.; Ye, B.S.; Lee, P.H. Beneficial effect of estrogen on nigrostriatal dopaminergic neurons in drug-naïve postmenopausal Parkinson’s disease. Sci. Rep. 2019, 9, 10531. [Google Scholar] [CrossRef]
- Wirdefeldt, K.; Gatz, M.; Pawitan, Y.; Pedersen, N.L. Risk and protective factors for Parkinson’s disease: A study in Swedish twins. Ann. Neurol. 2005, 57, 27–33. [Google Scholar] [CrossRef]
- Prediger, R.D. Effects of caffeine in Parkinson’s disease: From neuroprotection to the management of motor and non-motor symptoms. J. Alzheimer’s Dis. JAD 2010, 20, S205–S220. [Google Scholar] [CrossRef]
- Huse, D.M.; Schulman, K.; Orsini, L.; Castelli-Haley, J.; Kennedy, S.; Lenhart, G. Burden of illness in Parkinson’s disease. Mov. Disord.: Off. J. Mov. Disord. Soc. 2005, 20, 1449–1454. [Google Scholar] [CrossRef]
- Findley, L.J. The economic impact of Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13, S8–S12. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Rizek, P.; Kumar, N.; Jog, M.S. An update on the diagnosis and treatment of Parkinson disease. CMAJ: Can. Med. Assoc. J. J. l’Assoc. Med. Can. 2016, 188, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, D.F.; Sheehan, L.J.; Phillips, P.A.; Moore-Smith, B. The levodopa test in Parkinson’s disease. Age Ageing 1995, 24, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Massano, J.; Bhatia, K.P. Clinical approach to Parkinson’s disease: Features, diagnosis, and principles of management. Cold Spring Harb. Perspect. Med. 2012, 2, a008870. [Google Scholar] [CrossRef] [PubMed]
- Meara, J.; Bhowmick, B.K.; Hobson, P. Accuracy of diagnosis in patients with presumed Parkinson’s disease. Age Ageing 1999, 28, 99–102. [Google Scholar] [CrossRef]
- Nandipati, S.; Litvan, I. Environmental Exposures and Parkinson’s Disease. Int. J. Environ. Res. Public Health 2016, 13, 881. [Google Scholar] [CrossRef]
- Lock, E.A.; Zhang, J.; Checkoway, H. Solvents and Parkinson disease: A systematic review of toxicological and epidemiological evidence. Toxicol. Appl. Pharmacol. 2013, 266, 345–355. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Chou, M.-C.; Lin, C.-L.; Kao, C.-H. Increased risk of Parkinson disease in patients with carbon monoxide intoxication: A population-based cohort study. Medicine 2015, 94, e869. [Google Scholar] [CrossRef]
- Kenborg, L.; Rugbjerg, K.; Lee, P.-C.; Ravnskjær, L.; Christensen, J.; Ritz, B.; Lassen, C.F. Head injury and risk for Parkinson disease: Results from a Danish case-control study. Neurology 2015, 84, 1098–1103. [Google Scholar] [CrossRef]
- Selvaraj, S.; Piramanayagam, S. Impact of gene mutation in the development of Parkinson’s disease. Genes Dis. 2019, 6, 120–128. [Google Scholar] [CrossRef]
- Hauser, D.N.; Hastings, T.G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol. Dis. 2013, 51, 35–42. [Google Scholar] [CrossRef] [PubMed]
- McCann, H.; Stevens, C.H.; Cartwright, H.; Halliday, G.M. alpha-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 2014, 20, S62–S67. [Google Scholar] [CrossRef]
- Burré, J. The Synaptic Function of α-Synuclein. J. Parkinson’s Dis. 2015, 5, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Guardia-Laguarta, C.; Area-Gomez, E.; Schon, E.A.; Przedborski, S. Novel subcellular localization for α-synuclein: Possible functional consequences. Front. Neuroanat. 2015, 9, 17. [Google Scholar] [CrossRef]
- Siddiqui, I.J.; Pervaiz, N.; Abbasi, A.A. The Parkinson Disease gene SNCA: Evolutionary and structural insights with pathological implication. Sci. Rep. 2016, 6, 24475. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Tanji, K.; Mori, F.; Takahashi, H. The Lewy body in Parkinson’s disease: Molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2007, 27, 494–506. [Google Scholar] [CrossRef]
- Gibson, G.E.; Park, L.C.; Sheu, K.F.; Blass, J.P.; Calingasan, N.Y. The alpha-ketoglutarate dehydrogenase complex in neurodegeneration. Neurochem. Int. 2000, 36, 97–112. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Cooper, J.M.; Dexter, D.; Clark, J.B.; Jenner, P.; Marsden, C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. J. Neurochem. 1990, 54, 823–827. [Google Scholar] [CrossRef]
- McNaught, K.; Olanow, C. Proteolytic stress: A unifying concept for the etiopathogenesis of Parkinson’s disease. Ann. Neurol. 2003, 53, S73–S84. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Wahlster, L.; McLean, P.J. Molecular chaperones in Parkinson’s disease--present and future. J. Parkinson’s Dis. 2011, 1, 299–320. [Google Scholar] [CrossRef]
- Murphy, K.E.; Gysbers, A.M.; Abbott, S.K.; Spiro, A.S.; Furuta, A.; Cooper, A.; Garner, B.; Kabuta, T.; Halliday, G.M. Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson’s disease. Mov. Disord. 2015, 30, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Riboldi, G.M.; Di Fonzo, A.B. GBA, Gaucher Disease, and Parkinson’s Disease: From Genetic to Clinic to New Therapeutic Approaches. Cells 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Berardelli, A.; Rothwell, J.C.; Thompson, P.D.; Hallett, M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain 2001, 124, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoets, F.J.; Schulzer, M.; Calne, D.B.; Snow, B.J. Which clinical sign of Parkinson’s disease best reflects the nigrostriatal lesion? Ann. Neurol. 1997, 41, 58–64. [Google Scholar] [CrossRef]
- Jankovic, J.; Schwartz, K.S.; Ondo, W. Re-emergent tremor of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1999, 67, 646–650. [Google Scholar] [CrossRef]
- Shahed, J.; Jankovic, J. Exploring the relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13, 67–76. [Google Scholar] [CrossRef]
- Riley, D.; Lang, A.E.; Blair, R.D.; Birnbaum, A.; Reid, B. Frozen shoulder and other shoulder disturbances in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1989, 52, 63–66. [Google Scholar] [CrossRef]
- Williams, D.R.; Watt, H.C.; Lees, A.J. Predictors of falls and fractures in bradykinetic rigid syndromes: A retrospective study. J. Neurol. Neurosurg. Psychiatry 2006, 77, 468–473. [Google Scholar] [CrossRef]
- Shivitz, N.; Koop, M.M.; Fahimi, J.; Heit, G.; Bronte-Stewart, H.M. Bilateral subthalamic nucleus deep brain stimulation improves certain aspects of postural control in Parkinson’s disease, whereas medication does not. Mov. Disord. Off. J. Mov. Disord. 2006, 21, 1088–1097. [Google Scholar] [CrossRef]
- Carlsson, A.; Lindqvist, M.; Magnusson, T.O.R. 3,4-Dihydroxyphenylalanine and 5-Hydroxytryptophan as Reserpine Antagonists. Nature 1957, 180, 1200. [Google Scholar] [CrossRef]
- Goldenberg, M.M. Medical management of Parkinson’s disease. Pharm. Ther. 2008, 33, 590–606. [Google Scholar]
- Papavasiliou, P.S.; Cotzias, G.C.; Duby, S.E.; Steck, A.J.; Fehling, C.; Bell, M.A. Levodopa in Parkinsonism: Potentiation of central effects with a peripheral inhibitor. N. Engl. J. Med. 1972, 286, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H.; Lang, A.E. Levodopa-related motor complications—Phenomenology. Mov. Disord. 2008, 23, S509–S514. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.; Olanow, C.W. Principles Of Treatment In Parkinson’s Disease; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Jankovic, J.; Aguilar, L.G. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.; Uchihara, T.; Kanazawa, T.; Itoh, Y.; Wakabayashi, K.; Kakita, A.; Takahashi, H. Unmyelinated axons are more vulnerable to degeneration than myelinated axons of the cardiac nerve in Parkinson’s disease. Neuropathol. Appl. Neurobiol. 2011, 37, 791–802. [Google Scholar] [CrossRef] [PubMed]
- The Dopaminergic and Non-Dopaminergic Features of Parkinson’s Disease. Parkinsons’s Dis. 2011, 1–6. [CrossRef]
- Frucht, S.J. Parkinson disease: An update. Neurologist 2004, 10, 185–194. [Google Scholar] [CrossRef]
- Braak, H.; Tredici, K.D.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Potashkin, J.A.; Blume, S.R.; Runkle, N.K. Limitations of animal models of Parkinson’s disease. Parkinsons’s Dis. 2010, 2011, 658083. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. A timeline for Parkinson’s disease. Parkinsonism Relat. Disord. 2010, 16, 79–84. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, P.; Seppi, K.; Poewe, W. The Concept of Prodromal Parkinson’s Disease. J. Parkinsons’s Dis. 2015, 5, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.H.; Depboylu, C.; Krenzer, M.; Vadasz, D.; Ries, V.; Sixel-Doring, F.; Mayer, G. REM sleep behavior disorder as a prodromal stage of alpha-synucleinopathies: Symptoms, epidemiology, pathophysiology, diagnosis and therapy. Nervenarzt 2014, 85, 19–25. [Google Scholar] [CrossRef]
- Yu, Q.-J.; Yu, S.-Y.; Zuo, L.-J.; Lian, T.-H.; Hu, Y.; Wang, R.-D.; Piao, Y.-S.; Guo, P.; Liu, L.; Jin, Z.; et al. Parkinson disease with constipation: Clinical features and relevant factors. Sci. Rep. 2018, 8, 567. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Benarroch, E.E. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol. Dis. 2012, 46, 559–564. [Google Scholar] [CrossRef]
- Ansari, K.A.; Johnson, A. Olfactory function in patients with Parkinson’s disease. J. Chronic Dis. 1975, 28, 493–497. [Google Scholar] [CrossRef]
- Fullard, M.E.; Morley, J.F.; Duda, J.E. Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 2017, 33, 515–525. [Google Scholar] [CrossRef]
- Ross, G.W.; Petrovitch, H.; Abbott, R.D.; Tanner, C.M.; Popper, J.; Masaki, K.; Launer, L.; White, L.R. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 2008, 63, 167–173. [Google Scholar] [CrossRef]
- Goldman, J.G.; Postuma, R. Premotor and nonmotor features of Parkinson’s disease. Curr. Opin. Neurol. 2014, 27, 434–441. [Google Scholar] [CrossRef]
- Doty, R.L.; Shaman, P.; Dann, M. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function. Physiol. Behav. 1984, 32, 489–502. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’ sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- D’Ath, P.; Katona, P.; Mullan, E.; Evans, S.; Katona, C. Screening, detection and management of depression in elderly primary care attenders. I: The acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Fam. Pract. 1994, 11, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.J.; Himmerich, H.; Kienzle, B.; Szegedi, A. Differentiating moderate and severe depression using the Montgomery-Asberg depression rating scale (MADRS). J. Affect. Disord. 2003, 77, 255–260. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D. Advances in markers of prodromal Parkinson disease. Nat. Rev. Neurol. 2016, 12, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Schwab, R.S. Projection technique for evaluating surgery in Parkinson’s disease. In Proceedings of the Third Symposium on Parkinson’s Disease, Edinburgh, Scotland, 20–22 May 1968; pp. 152–157. [Google Scholar]
- Gibb, W.; Lees, A. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef]
- Calne, D.B.; Snow, B.J.; Lee, C. Criteria for diagnosing Parkinson’s disease. Ann. Neurol. 1992, 32, S125–S127. [Google Scholar] [CrossRef]
- Larsen, J.P.; Dupont, E.; Tandberg, E. Clinical diagnosis of Parkinson’s disease. Proposal of diagnostic subgroups classified at different levels of confidence. Acta Neurol. Scand. 1994, 89, 242–251. [Google Scholar] [CrossRef]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Jiang, F.; Wu, Q.; Sun, S.; Bi, G.; Guo, L. Identification of potential diagnostic biomarkers for Parkinson’s disease. FEBS Openbio 2019, 9, 1460–1468. [Google Scholar] [CrossRef]
- Sakharkar, M.K.; Kashmir Singh, S.K.; Rajamanickam, K.; Mohamed Essa, M.; Yang, J.; Chidambaram, S.B. A systems biology approach towards the identification of candidate therapeutic genes and potential biomarkers for Parkinson’s disease. PLoS ONE 2019, 14, e0220995. [Google Scholar] [CrossRef]
- Wen, M.-C.; Xu, Z.; Lu, Z.; Chan, L.L.; Tan, E.K.; Tan, L.C.S. Microstructural network alterations of olfactory dysfunction in newly diagnosed Parkinson’s disease. Sci. Rep. 2017, 7, 12559. [Google Scholar] [CrossRef]
- Li, C.; Cui, L.; Yang, Y.; Miao, J.; Zhao, X.; Zhang, J.; Cui, G.; Zhang, Y. Gut Microbiota Differs Between Parkinson’s Disease Patients and Healthy Controls in Northeast China. Front. Mol. Neurosci. 2019, 12. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, C.; Zhu, D.; Wang, X.; Fang, L.; Zhong, J.; Mao, Q.; Sun, L.; Gong, X.; Xia, J.; et al. Serotonin-1A receptor alterations in depression: A meta-analysis of molecular imaging studies. BMC Psychiatry 2016, 16, 319. [Google Scholar] [CrossRef]
- Politis, M.; Loane, C. Serotonergic dysfunction in Parkinson’s disease and its relevance to disability. Sci. World J. 2011, 11, 1726–1734. [Google Scholar] [CrossRef]
- Zhang, F.; Niu, L.; Liu, X.; Liu, Y.; Li, S.; Yu, H.; Le, W. Rapid Eye Movement Sleep Behavior Disorder and Neurodegenerative Diseases: An Update. Aging Dis. 2020, 11, 315–326. [Google Scholar] [CrossRef]
- Tal, A.; Shinar, Z.; Shaki, D.; Codish, S.; Goldbart, A. Validation of Contact-Free Sleep Monitoring Device with Comparison to Polysomnography. J. Clin. Sleep Med. 2017, 13, 517–522. [Google Scholar] [CrossRef]
- Park, S.-M.; Won, D.D.; Lee, B.J.; Escobedo, D.; Esteva, A.; Aalipour, A.; Ge, T.J.; Kim, J.H.; Suh, S.; Choi, E.H.; et al. A mountable toilet system for personalized health monitoring via the analysis of excreta. Nat. Biomed. Eng. 2020. [Google Scholar] [CrossRef]
- Hilton, D.; Stephens, M.; Kirk, L.; Edwards, P.; Potter, R.; Zajicek, J.; Broughton, E.; Hagan, H.; Carroll, C. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014, 127, 235–241. [Google Scholar] [CrossRef]
- McGinnis, R.S.; McGinnis, E.W.; Hruschak, J.; Lopez-Duran, N.L.; Fitzgerald, K.; Rosenblum, K.L.; Muzik, M. Rapid Anxiety and Depression Diagnosis in Young Children Enabled by Wearable Sensors and Machine Learning. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 3983–3986. [Google Scholar]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Váradi, C. Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms. Biology 2020, 9, 103. https://doi.org/10.3390/biology9050103
Váradi C. Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms. Biology. 2020; 9(5):103. https://doi.org/10.3390/biology9050103
Chicago/Turabian StyleVáradi, Csaba. 2020. "Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms" Biology 9, no. 5: 103. https://doi.org/10.3390/biology9050103
APA StyleVáradi, C. (2020). Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms. Biology, 9(5), 103. https://doi.org/10.3390/biology9050103




