MYB Transcription Factors as Regulators of Secondary Metabolism in Plants
Abstract
1. Introduction
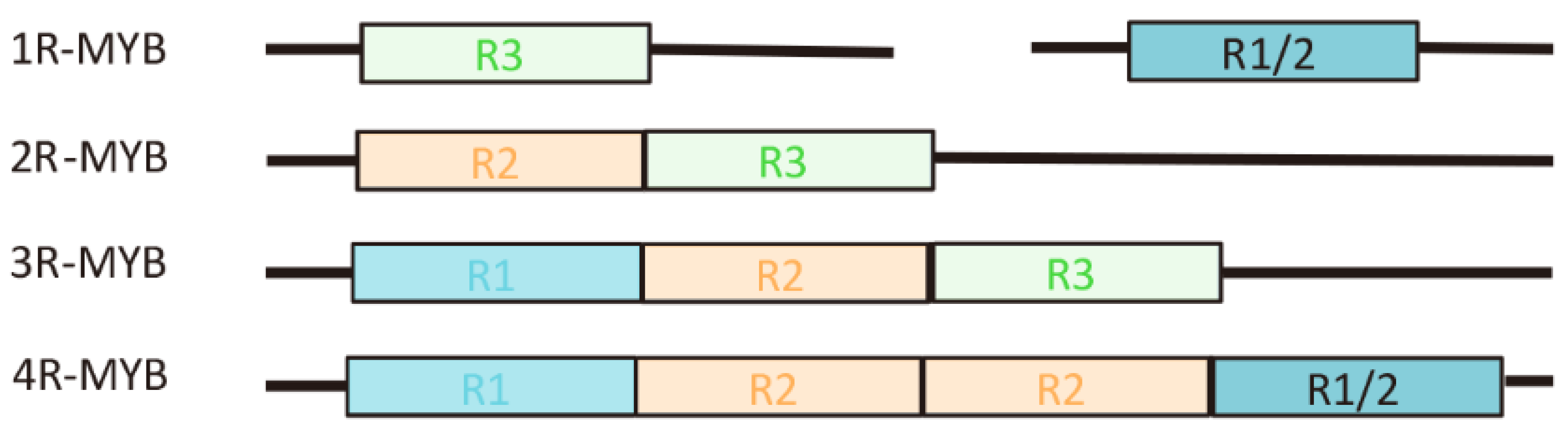
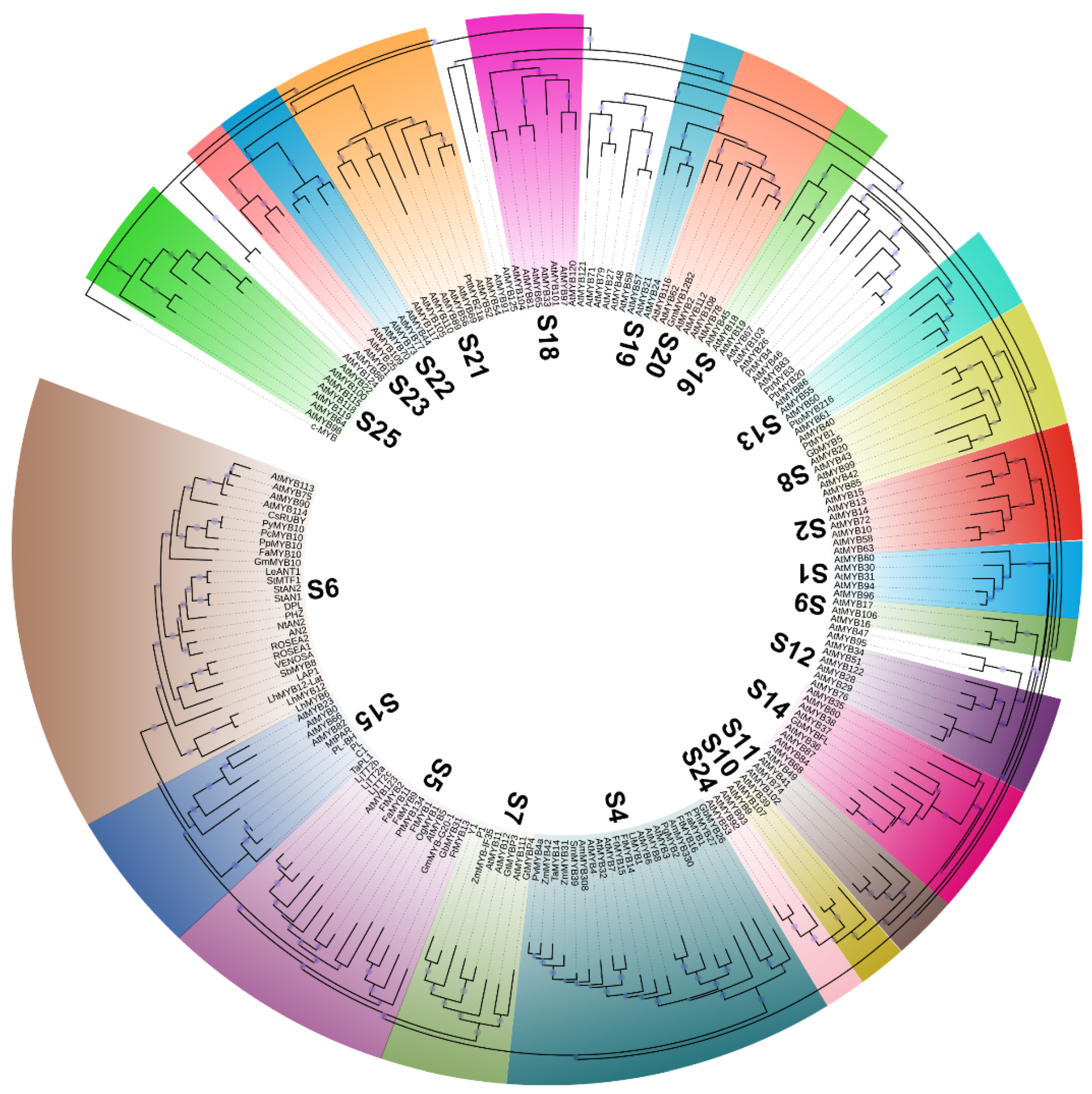
2. Diversity and Structure of the MYB TFs
3. Biological Function of MYB TFs
4. MYB TF Regulation of Secondary Metabolic Pathways
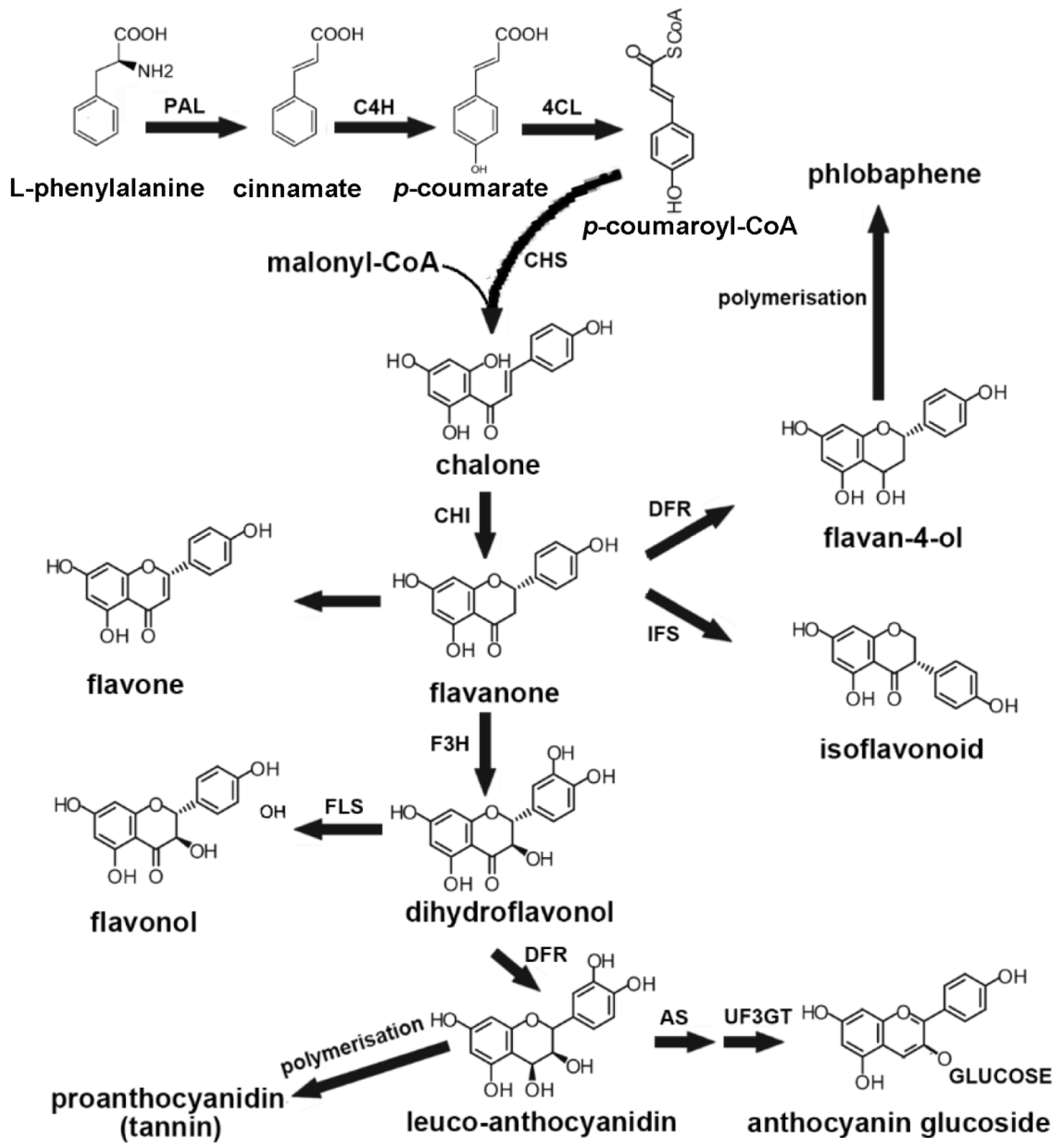
4.1. The Role of MYB TFs in the Biosynthesis of Flavonoids Secondary Metabolism
4.2. The role of MYB TFs in the Biosynthesis of Secondary Metabolism of Organic Acids
4.3. The Role of MYB TFs in the Biosynthesis of Lignins Secondary Metabolism
5. The Regulation Mechanism of MYB TFs
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Martin, C.; Paz-Ares, J.J. Myb transcription factors in plants. Trends Genet. 1997, 13, 67–73. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L.J. Myb transcription factors in arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Weston, K.; Bishop, J.M.J. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell 1989, 58, 85–93. [Google Scholar] [CrossRef]
- Stracke, R.; Werber, M.; Weisshaar, B.J. The r2r3-myb gene family in arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef]
- Rosinski, J.A.; Atchley, W.R.J. Molecular evolution of the myb family of transcription factors: Evidence for polyphyletic origin. J. Mol. Evol. 1998, 46, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Han, Y.; Li, D.; Lin, Y.; Cai, Y.J. Myb transcription factors in chinese pear (pyrus bretschneideri rehd.): Genome-wide identification, classification, and expression profiling during fruit development. Front. Plant Sci. 2016, 7, 577. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.-Z.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.; Samaha, R.J. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; He, K.; Liu, M.; Li, J.; Gao, Z.; Lin, Z.; Zhang, Y.; Wang, X.; Qiu, X.; et al. The myb transcription factor superfamily of arabidopsis: Expression analysis and phylogenetic comparison with the rice myb family. Plant Mol. Biol. 2006, 60, 107–124. [Google Scholar] [CrossRef]
- Paz-Ares, J.; Ghosal, D.; Wienand, U.; Peterson, P.; Saedler, H.J. The regulatory c1 locus of zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987, 6, 3553–3558. [Google Scholar] [CrossRef]
- Urao, T.; Yamaguchi-Shinozaki, K.; Urao, S.; Shinozaki, K. An arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved myb recognition sequence. Plant Cell 1993, 5, 1529–1539. [Google Scholar]
- Jin, H.; Martin, C.J. Multifunctionality and diversity within the plant myb-gene family. Plant Mol. Biol. 1999, 41, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Osbourn, A.; Ma, P.J. Myb transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Ramya, M.; Kwon, O.K.; An, H.R.; Park, P.M.; Baek, Y.S.; Park, P.H.J. Floral scent: Regulation and role of myb transcription factors. Phytochem. Lett. 2017, 19, 114–120. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L.J. Transcriptional control of flavonoid biosynthesis by myb–bhlh–wdr complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Meng, D.; Han, Y.; Chen, T.; Jiao, C.; Chen, Y.; Jin, Q.; Cai, Y.J. Comparative analysis of b-box genes and their expression pattern analysis under various treatments in dendrobium officinale. BMC Plant Biol. 2019, 19, 245. [Google Scholar] [CrossRef]
- Lee, P.-L.; Chen, J.-T.J. Plant regeneration via callus culture and subsequent in vitro flowering of dendrobium huoshanense. Acta Physiol. Plant. 2014, 36, 2619–2625. [Google Scholar] [CrossRef]
- Cao, Y.; Li, X.; Jiang, L.J. Integrative analysis of the core fruit lignification toolbox in pear reveals targets for fruit quality bioengineering. Biomolecules 2019, 9, 504. [Google Scholar] [CrossRef]
- Tamagnone, L.; Merida, A.; Parr, A.; Mackay, S.; Culianez-Macia, F.A.; Roberts, K.; Martin, C.J. The ammyb308 and ammyb330 transcription factors from antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. Plant Cell 1998, 10, 135–154. [Google Scholar] [CrossRef]
- Bai, Y.C.; Li, C.L.; Zhang, J.W.; Li, S.J.; Luo, X.P.; Yao, H.P.; Chen, H.; Zhao, H.X.; Park, S.U.; Wu, Q.J. Characterization of two tartary buckwheat r2r3-myb transcription factors and their regulation of proanthocyanidin biosynthesis. Physiol. Plantarum 2014, 152, 431–440. [Google Scholar] [CrossRef]
- He, C.; da Silva, J.A.T.; Wang, H.; Si, C.; Zhang, M.; Zhang, X.; Li, M.; Tan, J.; Duan, J.J. Mining myb transcription factors from the genomes of orchids (phalaenopsis and dendrobium) and characterization of an orchid r2r3-myb gene involved in water-soluble polysaccharide biosynthesis. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Lipsick, J.S.J. One billion years of myb. Oncogene 1996, 13, 223–235. [Google Scholar] [PubMed]
- Zhou, L.; He, Y.; Li, J.; Liu, Y.; Chen, H. Cbfs function in anthocyanin biosynthesis by interacting with myb113 in eggplant (Solanum melongena L). Plant Cell Physiol. 2020, 61, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Baranowskij, N.; Frohberg, C.; Prat, S.; Willmitzer, L.J. A novel DNA binding protein with homology to myb oncoproteins containing only one repeat can function as a transcriptional activator. EMBO J. 1994, 13, 5383–5392. [Google Scholar] [CrossRef] [PubMed]
- Feller, A.; Machemer, K.; Braun, E.L.; Grotewold, E.J. Evolutionary and comparative analysis of myb and bhlh plant transcription factors. Plant J. 2011, 66, 94–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Gu, J.; Chopra, S.; Gu, X.; Peterson, T.J. Ordered origin of the typical two-and three-repeat myb genes. Gene 2004, 326, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Shelton, D.; Stranne, M.; Mikkelsen, L.; Pakseresht, N.; Welham, T.; Hiraka, H.; Tabata, S.; Sato, S.; Paquette, S.; Wang, T.L.J. Transcription factors of lotus: Regulation of isoflavonoid biosynthesis requires coordinated changes in transcription factor activity. Plant Physiol. 2012, 159, 531–547. [Google Scholar] [CrossRef]
- Peng, X.; Liu, H.; Wang, D.; Shen, S.J. Genome-wide identification of the jatropha curcas myb family and functional analysis of the abiotic stress responsive gene jcmyb2. BMC Genom. 2016, 17, 251. [Google Scholar] [CrossRef]
- Borevitz, J.O.; Xia, Y.; Blount, J.; Dixon, R.A.; Lamb, C.J. Activation tagging identifies a conserved myb regulator of phenylpropanoid biosynthesis. Plant Cell 2000, 12, 2383–2393. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.-M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M.J. Genetics and biochemistry of seed flavonoids. Ann. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M.J. Regulation of the anthocyanin biosynthetic pathway by the ttg1/bhlh/myb transcriptional complex in arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Heppel, S.C.; Jaffé, F.W.; Takos, A.M.; Schellmann, S.; Rausch, T.; Walker, A.R.; Bogs, J.J. Identification of key amino acids for the evolution of promoter target specificity of anthocyanin and proanthocyanidin regulating myb factors. Plant Mol. Biol. 2013, 82, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Fornalé, S.; Lopez, E.; Salazar-Henao, J.E.; Fernández-Nohales, P.; Rigau, J.; Caparros-Ruiz, D.J. Atmyb7, a new player in the regulation of uv-sunscreens in arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q.J. Iq-tree: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, W.; Zhao, Q.; Long, H.; Li, Z.; Liu, M.; Zhou, X.; Zhang, L.J. Integrative analysis reveals evolutionary patterns and potential functions of sweet transporters in euphorbiaceae. Int. J. Biol. Macromol. 2019, 139, 1–11. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P.J. Interactive tree of life (itol) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef]
- Katiyar, A.; Smita, S.; Lenka, S.K.; Rajwanshi, R.; Chinnusamy, V.; Bansal, K.C.J. Genome-wide classification and expression analysis of myb transcription factor families in rice and arabidopsis. BMC Genom. 2012, 13, 544. [Google Scholar] [CrossRef]
- Ng, D.W.; Abeysinghe, J.K.; Kamali, M.J. Regulating the regulators: The control of transcription factors in plant defense signaling. Int. J. Mol. Sci. 2018, 19, 3737. [Google Scholar] [CrossRef]
- Ma, D.; Constabel, C.P.J. Myb repressors as regulators of phenylpropanoid metabolism in plants. Trends Plant Sci. 2019, 24, 275–289. [Google Scholar] [CrossRef]
- Ge, L.; Dou, Y.; Li, M.; Qu, P.; He, Z.; Liu, Y.; Xu, Z.; Chen, J.; Chen, M.; Ma, Y.J.I. Simyb3 in foxtail millet (setaria italica) confers tolerance to low-nitrogen stress by regulating root growth in transgenic plants. Int. J. Mol. Sci. 2019, 20, 5741. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, X.; Wang, H.; Tang, X.; Liu, C.; Yin, H.; Ye, S.; Jiang, Y.; Duan, Y.; Luo, K.J. The poplar r2r3 myb transcription factor ptrmyb94 coordinates with abscisic acid signaling to improve drought tolerance in plants. Tree Physiol. 2019, 40, 46–59. [Google Scholar] [CrossRef]
- Lai, B.; Cheng, Y.; Liu, H.; Wang, Q.; Wang, Q.; Wang, C.; Su, R.; Chen, F.; Wang, H.; Du, L.J. Differential anthocyanin accumulation in radish taproot: Importance of rsmyb1 gene structure. Plant Cell Rep. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Sun, Z.; Ding, M.; Logacheva, M.D.; Kreft, I.; Wang, D.; Yan, M.; Shao, J.; Tang, Y.; Wu, Y.J. Ftsad2 and ftjaz1 regulate activity of the ftmyb11 transcription repressor of the phenylpropanoid pathway in fagopyrum tataricum. New Phytol. 2017, 216, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Choi, M.-G.; Kang, C.-S.; Park, C.-S.; Choi, S.-B.; Park, Y.-I.J. A wheat r2r3-myb protein purple plant1 (tapl1) functions as a positive regulator of anthocyanin biosynthesis. Biochem. Biophys. Res. Commun. 2016, 469, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Wang, C.; Guo, H.-Y.; Wang, Y.-C.J. Bplmyb46 from betula platyphylla can form homodimers and heterodimers and is involved in salt and osmotic stresses. Int. J. Mol. Sci. 2019, 20, 1171. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Li, Q.; Fan, X.; Yang, W.; Xue, Y.J. The r2r3 myb transcription factor ghmyb109 is required for cotton fiber development. Genetics 2008, 180, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.; Liang, X.; Pu, L.; Zhang, Y.; Xue, Y.J. Identification of ghmyb109 encoding a r2r3 myb transcription factor that expressed specifically in fiber initials and elongating fibers of cotton (Gossypium hirsutum L.). Biochim. et Biophys. Acta (BBA)-Gene Struct. Expr. 2003, 1630, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Khaldun, A.; Chen, J.; Zhang, C.; Lv, H.; Yuan, L.; Wang, Y.J. A r2r3-myb transcription factor regulates the flavonol biosynthetic pathway in a traditional chinese medicinal plant, epimedium sagittatum. Front. Plant Sci. 2016, 7, 1089. [Google Scholar] [CrossRef]
- Liu, T.; Luo, T.; Guo, X.; Zou, X.; Zhou, D.; Afrin, S.; Li, G.; Zhang, Y.; Zhang, R.; Luo, Z.J. Pgmyb2, a meja-responsive transcription factor, positively regulates the dammarenediol synthase gene expression in panax ginseng. Int. J. Mol. Sci. 2019, 20, 2219. [Google Scholar] [CrossRef]
- Cao, Z.-H.; Zhang, S.-Z.; Wang, R.-K.; Zhang, R.-F.; Hao, Y.-J.J. Genome wide analysis of the apple myb transcription factor family allows the identification of mdomyb121 gene confering abiotic stress tolerance in plants. PLoS ONE 2013, 8, e69955. [Google Scholar] [CrossRef]
- Du, H.; Yang, S.-S.; Liang, Z.; Feng, B.-R.; Liu, L.; Huang, Y.-B.; Tang, Y.-X.J. Genome-wide analysis of the myb transcription factor superfamily in soybean. BMC Plant Biol. 2012, 12, 106. [Google Scholar] [CrossRef]
- Sonbol, F.-M.; Fornalé, S.; Capellades, M.; Encina, A.; Tourino, S.; Torres, J.-L.; Rovira, P.; Ruel, K.; Puigdomenech, P.; Rigau, J.J. The maize zmmyb42 represses the phenylpropanoid pathway and affects the cell wall structure, composition and degradability in arabidopsis thaliana. Plant Mol. Biol. 2009, 70, 283. [Google Scholar] [CrossRef] [PubMed]
- Fornalé, S.; Shi, X.; Chai, C.; Encina, A.; Irar, S.; Capellades, M.; Fuguet, E.; Torres, J.L.; Rovira, P.; Puigdomènech, P.J. Zmmyb31 directly represses maize lignin genes and redirects the phenylpropanoid metabolic flux. Plant J. 2010, 64, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Legay, S.; Lacombe, E.; Goicoechea, M.; Briere, C.; Séguin, A.; Mackay, J.; Grima-Pettenati, J.J. Molecular characterization of egmyb1, a putative transcriptional repressor of the lignin biosynthetic pathway. Plant Sci. 2007, 173, 542–549. [Google Scholar] [CrossRef]
- Shen, H.; He, X.; Poovaiah, C.R.; Wuddineh, W.A.; Ma, J.; Mann, D.G.; Wang, H.; Jackson, L.; Tang, Y.; Neal Stewart, C.J., Jr. Functional characterization of the switchgrass (panicum virgatum) r2r3-myb transcription factor pvmyb4 for improvement of lignocellulosic feedstocks. New Phytol. 2012, 193, 121–136. [Google Scholar] [CrossRef]
- Omer, S.; Kumar, S.; Khan, B.M.J. Over-expression of a subgroup 4 r2r3 type myb transcription factor gene from leucaena leucocephala reduces lignin content in transgenic tobacco. Plant Cell Rep. 2013, 32, 161–171. [Google Scholar] [CrossRef]
- Zhu, L.; Shan, H.; Chen, S.; Jiang, J.; Gu, C.; Zhou, G.; Chen, Y.; Song, A.; Chen, F.J. The heterologous expression of the chrysanthemum r2r3-myb transcription factor cmmyb1 alters lignin composition and represses flavonoid synthesis in arabidopsis thaliana. PLoS ONE 2013, 8, e65680. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffé, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R.J. Light-induced expression of a myb gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef]
- Ban, Y.; Honda, C.; Hatsuyama, Y.; Igarashi, M.; Bessho, H.; Moriguchi, T.J. Isolation and functional analysis of a myb transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol. 2007, 48, 958–970. [Google Scholar] [CrossRef]
- Vimolmangkang, S.; Han, Y.; Wei, G.; Korban, S.S.J. An apple myb transcription factor, mdmyb3, is involved in regulation of anthocyanin biosynthesis and flower development. BMC Plant Biol. 2013, 13, 176. [Google Scholar] [CrossRef]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C.J. Red colouration in apple fruit is due to the activity of the myb transcription factor, mdmyb10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef]
- Chagné, D.; Lin-Wang, K.; Espley, R.V.; Volz, R.K.; How, N.M.; Rouse, S.; Brendolise, C.; Carlisle, C.M.; Kumar, S.; De Silva, N.J. An ancient duplication of apple myb transcription factors is responsible for novel red fruit-flesh phenotypes. Plant Physiol. 2013, 161, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchio, F.; Wing, J.F.; Va, K.; Mol, J.N.; Koes, R.J. Analysis of bhlh and myb domain proteins: Species-specific regulatory differences are caused by divergent evolution of target anthocyanin genes. Plant J. 1998, 13, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchio, F.; Wing, J.; van der Woude, K.; Souer, E.; de Vetten, N.; Mol, J.; Koes, R.J. Molecular analysis of the anthocyanin2 gene of petunia and its role in the evolution of flower color. Plant Cell 1999, 11, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Schwinn, K.E.; Jameson, P.E.; Davies, K.M.J. Members of an r2r3-myb transcription factor family in petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J. 2011, 65, 771–784. [Google Scholar] [CrossRef]
- Yamagishi, M.; Shimoyamada, Y.; Nakatsuka, T.; Masuda, K.J. Two r2r3-myb genes, homologs of petunia an2, regulate anthocyanin biosyntheses in flower tepals, tepal spots and leaves of asiatic hybrid lily. Plant Cell Physiol. 2010, 51, 463–474. [Google Scholar] [CrossRef]
- Yamagishi, M.; Toda, S.; Tasaki, K.J. The novel allele of the lh myb 12 gene is involved in splatter-type spot formation on the flower tepals of asiatic hybrid lilies (lilium spp.). New Phytol. 2014, 201, 1009–1020. [Google Scholar] [CrossRef]
- Pandey, A.; Misra, P.; Chandrashekar, K.; Trivedi, P.K.J. Development of atmyb12-expressing transgenic tobacco callus culture for production of rutin with biopesticidal potential. Plant Cell Rep. 2012, 31, 1867–1876. [Google Scholar] [CrossRef]
- Pandey, A.; Misra, P.; Khan, M.P.; Swarnkar, G.; Tewari, M.C.; Bhambhani, S.; Trivedi, R.; Chattopadhyay, N.; Trivedi, P.K.J. Co-expression of arabidopsis transcription factor, at myb 12, and soybean isoflavone synthase, gm ifs 1, genes in tobacco leads to enhanced biosynthesis of isoflavones and flavonols resulting in osteoprotective activity. Plant Biotechnol. J. 2014, 12, 69–80. [Google Scholar] [CrossRef]
- Misra, P.; Pandey, A.; Tiwari, M.; Chandrashekar, K.; Sidhu, O.P.; Asif, M.H.; Chakrabarty, D.; Singh, P.K.; Trivedi, P.K.; Nath, P.J. Modulation of transcriptome and metabolome of tobacco by arabidopsis transcription factor, atmyb12, leads to insect resistance. Plant Physiol. 2010, 152, 2258–2268. [Google Scholar] [CrossRef]
- Schaart, J.G.; Dubos, C.; Romero De La Fuente, I.; Van Houwelingen, A.M.; de Vos, R.C.; Jonker, H.H.; Xu, W.; Routaboul, J.M.; Lepiniec, L.; Bovy, A.G.J. Identification and characterization of myb-b hlh-wd 40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef]
- Zhang, K.; Logacheva, M.D.; Meng, Y.; Hu, J.; Wan, D.; Li, L.; Janovská, D.; Wang, Z.; Georgiev, M.I.; Yu, Z.J. Jasmonate-responsive myb factors spatially repress rutin biosynthesis in fagopyrum tataricum. J. Exp. Bot. 2018, 69, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.-L.; Tang, Y.; Zhang, K.-X.; Li, F.-L.; Yang, P.-Y.; Tang, Y.-X.; Wu, Y.-M.; Shao, J.-R.J. Identification of tt2 gene from floral transcriptome in fagopyrum tataricum. Food Res. Int. 2013, 54, 1331–1333. [Google Scholar] [CrossRef]
- Matsui, K.; Umemura, Y.; Ohme-Takagi, M.J. Atmybl2, a protein with a single myb domain, acts as a negative regulator of anthocyanin biosynthesis in arabidopsis. Plant J. 2008, 55, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Z.J. Rnai-mediated suppression of the phenylalanine ammonia-lyase gene in salvia miltiorrhiza causes abnormal phenotypes and a reduction in rosmarinic acid biosynthesis. J. Plant Res. 2011, 124, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Celenza, J.L.; Quiel, J.A.; Smolen, G.A.; Merrikh, H.; Silvestro, A.R.; Normanly, J.; Bender, J.J. The arabidopsis atr1 myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol. 2005, 137, 253–262. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, P.; Yang, D.; Li, W.; Liang, Z.; Liu, Y.; Liu, F.J. Cloning and characterization of a putative r2r3 myb transcriptional repressor of the rosmarinic acid biosynthetic pathway from salvia miltiorrhiza. PLoS ONE 2013, 8, e73259. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.-P.; Wang, Z.-Z.J. The arabidopsis pap1 transcription factor plays an important role in the enrichment of phenolic acids in salvia miltiorrhiza. J. Agric. Food Chem. 2010, 58, 12168–12175. [Google Scholar] [CrossRef]
- Schwinn, K.; Venail, J.; Shang, Y.; Mackay, S.; Alm, V.; Butelli, E.; Oyama, R.; Bailey, P.; Davies, K.; Martin, C.J. A small family of myb-regulatory genes controls floral pigmentation intensity and patterning in the genus antirrhinum. Plant Cell 2006, 18, 831–851. [Google Scholar] [CrossRef]
- Liu, X.; Yu, W.; Zhang, X.; Wang, G.; Cao, F.; Cheng, H.J. Identification and expression analysis under abiotic stress of the r2r3-myb genes in ginkgo biloba l. Physiol. Mol. Biol. Plants 2017, 23, 503–516. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, F.; Cheng, S.; Liao, Y.J. Characterization and functional analysis of a myb gene (gbmybfl) related to flavonoid accumulation in ginkgo biloba. Genes Genom. 2018, 40, 49–61. [Google Scholar] [CrossRef]
- Huang, W.; Sun, W.; Lv, H.; Luo, M.; Zeng, S.; Pattanaik, S.; Yuan, L.; Wang, Y.J. A r2r3-myb transcription factor from epimedium sagittatum regulates the flavonoid biosynthetic pathway. PLoS ONE 2013, 8, e70778. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Khaldun, A.; Lv, H.; Du, L.; Zhang, C.; Wang, Y.J. Isolation and functional characterization of a r2r3-myb regulator of the anthocyanin biosynthetic pathway from epimedium sagittatum. Plant Cell Rep. 2016, 35, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Qi, L.; Yang, J.; Wu, C.; Liu, Y.; Huang, L.J. A scutellaria baicalensis r2r3-myb gene, sbmyb8, regulates flavonoid biosynthesis and improves drought stress tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 120, 961–972. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Saito, M.; Yamada, E.; Fujita, K.; Kakizaki, Y.; Nishihara, M.J. Isolation and characterization of gtmybp3 and gtmybp4, orthologues of r2r3-myb transcription factors that regulate early flavonoid biosynthesis, in gentian flowers. J. Exp. Bot. 2012, 63, 6505–6517. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Yamazaki, M.; Saito, K.J.M. A light-inducible myb-like gene that is specifically expressed in red perilla frutescens and presumably acts as a determining factor of the anthocyanin forma. Mol. Gen. Genet. 1999, 262, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Verdier, J.; Zhao, J.; Torres-Jerez, I.; Ge, S.; Liu, C.; He, X.; Mysore, K.S.; Dixon, R.A.; Udvardi, M.K.J. Mtpar myb transcription factor acts as an on switch for proanthocyanidin biosynthesis in medicago truncatula. Proc. Natl. Acad. Sci. USA 2012, 109, 1766–1771. [Google Scholar] [CrossRef]
- Farooqui, A.A. Beneficial effects of flavonoids on neurological disorders. In Phytochemicals, Signal Transduction, and Neurological Disorders; Springer: Berlin/Heidelberg, Germany, 2013; pp. 83–115. [Google Scholar]
- Katsumoto, Y.; Fukuchi-Mizutani, M.; Fukui, Y.; Brugliera, F.; Holton, T.A.; Karan, M.; Nakamura, N.; Yonekura-Sakakibara, K.; Togami, J.; Pigeaire, A.J.P.; et al. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R.J. The flavonoid biosynthetic pathway in arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V.J. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef]
- Grotewold, E.; Athma, P.; Peterson, T.J. Alternatively spliced products of the maize p gene encode proteins with homology to the DNA-binding domain of myb-like transcription factors. Proc. Natl. Acad. Sci. USA 1991, 88, 4587–4591. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Guo, L.; Gong, N.; Pang, Y.; Jiang, W.; Liu, Y.; Jiang, X.; Zhao, L.; Wang, Y.J. Functional characterization of tea (camellia sinensis) myb4a transcription factor using an integrative approach. Front. Plant Sci. 2017, 8, 943. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Wang, Y.-X.; Li, H.; Liu, Z.-W.; Cui, X.; Zhuang, J. Two myb transcription factors (csmyb2 and csmyb26) are involved in flavonoid biosynthesis in tea plant [camellia sinensis (l.) o. Kuntze]. BMC Plant Biol. 2018, 18, 288. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Bains, S.; Pathania, S.; Sharma, S.; Kaur, R.; Singh, K. Comparative transcriptomics reveals candidate transcription factors involved in costunolide biosynthesis in medicinal plant-saussurea lappa. Int. J. Biol. Macromol. 2020, 150, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Sablowski, R.; Moyano, E.; Culianez-Macia, F.A.; Schuch, W.; Martin, C.; Bevan, M. A flower-specific myb protein activates transcription of phenylpropanoid biosynthetic genes. EMBO J. 1994, 13, 128–137. [Google Scholar] [CrossRef]
- Shang, Y.; Venail, J.; Mackay, S.; Bailey, P.C.; Schwinn, K.E.; Jameson, P.E.; Martin, C.R.; Davies, K.M.J. The molecular basis for venation patterning of pigmentation and its effect on pollinator attraction in flowers of antirrhinum. New Phytol. 2011, 189, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.M.; Davies, K.M.; Deroles, S.C.; Bloor, S.J.; Lewis, D.H.J. The maize lc regulatory gene up-regulates the flavonoid biosynthetic pathway of petunia. Plant J. 1998, 13, 381–392. [Google Scholar] [CrossRef]
- Jiang, H.; Wood, K.V.; Morgan, J. Metabolic engineering of the phenylpropanoid pathway in saccharomyces cerevisiae. Appl. Environ. Microbiol 2005, 71, 2962–2969. [Google Scholar] [CrossRef]
- Payyavula, R.S.; Singh, R.K.; Navarre, D.A.J. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. J. Exp. Bot. 2013, 64, 5115–5131. [Google Scholar] [CrossRef]
- Zhong, R.; Richardson, E.A.; Ye, Z.-H. The myb46 transcription factor is a direct target of snd1 and regulates secondary wall biosynthesis in arabidopsis. Plant Cell 2007, 19, 2776–2792. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, R.L.; Ye, Z.-H. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in arabidopsis. Plant Cell 2008, 20, 2763–2782. [Google Scholar] [CrossRef]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z.-H. Myb58 and myb63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Zhang, S.; Liu, J.; Zhao, C.; Wu, J.; Cao, Y.; Fu, C.; Han, X.; He, H.; Zhao, Q. Myb20, myb42, myb43, and myb85 regulate phenylalanine and lignin biosynthesis during secondary cell wall formation. Plant Physiol. 2020, 182, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wang, X.; Li, C.; Lu, W.; Yang, L.; Jiang, Y.; Luo, K. Functional characterization of the poplar r2r3-myb transcription factor ptomyb216 involved in the regulation of lignin biosynthesis during wood formation. PLoS ONE 2013, 8, e76369. [Google Scholar] [CrossRef]
- McCarthy, R.L.; Zhong, R.; Fowler, S.; Lyskowski, D.; Piyasena, H.; Carleton, K.; Spicer, C.; Ye, Z.-H. The poplar myb transcription factors, ptrmyb3 and ptrmyb20, are involved in the regulation of secondary wall biosynthesis. Plant Cell Physiol. 2010, 51, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Dalton, S.; Roberts, L.A.; Moron-Garcia, O.M.; Iacono, R.; Kosik, O.; Gallagher, J.A.; Bosch, M. Modified expression of zmmyb167 in brachypodium distachyon and zea mays leads to increased cell wall lignin and phenolic content. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Patzlaff, A.; McInnis, S.; Courtenay, A.; Surman, C.; Newman, L.J.; Smith, C.; Bevan, M.W.; Mansfield, S.; Whetten, R.W.; Sederoff, R.R. Characterisation of a pine myb that regulates lignification. Plant J. 2003, 36, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Patzlaff, A.; Newman, L.J.; Dubos, C.; Whetten, R.W.; Smith, C.; McInnis, S.; Bevan, M.W.; Sederoff, R.R.; Campbell, M.M. Characterisation of ptmyb1, an r2r3-myb from pine xylem. Plant Mol. Biol. 2003, 53, 597–608. [Google Scholar] [CrossRef]
- Newman, L.J.; Perazza, D.E.; Juda, L.; Campbell, M.M. Involvement of the r2r3-myb, atmyb61, in the ectopic lignification and dark-photomorphogenic components of the det3 mutant phenotype. Plant J. 2004, 37, 239–250. [Google Scholar] [CrossRef]
- Karpinska, B.; Karlsson, M.; Srivastava, M.; Stenberg, A.; Schrader, J.; Sterky, F.; Bhalerao, R.; Wingsle, G. Myb transcription factors are differentially expressed and regulated during secondary vascular tissue development in hybrid aspen. Plant Mol. Biol. 2004, 56, 255–270. [Google Scholar] [CrossRef]
- Zhu, L.; Guan, Y.; Zhang, Z.; Song, A.; Chen, S.; Jiang, J.; Chen, F. Cmmyb8 encodes an r2r3 myb transcription factor which represses lignin and flavonoid synthesis in chrysanthemum. Plant Physiol. Biochem. 2020, 149, 217–224. [Google Scholar] [CrossRef]
- Legay, S.; Sivadon, P.; Blervacq, A.S.; Pavy, N.; Baghdady, A.; Tremblay, L.; Levasseur, C.; Ladouce, N.; Lapierre, C.; Séguin, A. Egmyb1, an r2r3 myb transcription factor from eucalyptus negatively regulates secondary cell wall formation in arabidopsis and poplar. New Phytol. 2010, 188, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Tak, H.; Negi, S.; Ganapathi, T. Overexpression of musamyb31, a r2r3 type myb transcription factor gene indicate its role as a negative regulator of lignin biosynthesis in banana. PLoS ONE 2017, 12, e0172695. [Google Scholar] [CrossRef]
- Ramsay, N.A.; Glover, B.J.J. Myb–bhlh–wd40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005, 10, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Puranik, S.; Prasad, M.J. Structure and regulatory networks of wd40 protein in plants. J. Plant Biochem. Biotechnol. 2012, 21, 32–39. [Google Scholar] [CrossRef]
- Thakare, D.; Tang, W.; Hill, K.; Perry, S.E.J. The mads-domain transcriptional regulator agamous-like15 promotes somatic embryo development in arabidopsis and soybean. Plant Physiol. 2008, 146, 1663–1672. [Google Scholar] [CrossRef]
- Morse, A.M.; Whetten, R.W.; Dubos, C.; Campbell, M.M.J. Post-translational modification of an r2r3-myb transcription factor by a map kinase during xylem development. New Phytol. 2009, 183, 1001–1013. [Google Scholar] [CrossRef]
- Reyes, J.L.; Chua, N.H.J. Aba induction of mir159 controls transcript levels of two myb factors during arabidopsis seed germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef]
- Cui, F.; Brosché, M.; Sipari, N.; Tang, S.; Overmyer, K.J. Regulation of aba dependent wound induced spreading cell death by myb 108. New Phytol. 2013, 200, 634–640. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K.J. Aba-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef]
| Plant Name | Transcription Factor | Biological Functions | References |
|---|---|---|---|
| Fagopyrum tataricum | FtMYB1, FtMYB2, | Overexpression enhances the synthesis and accumulation of anthocyanins | [19] |
| FtMYB13, FtMYB14, FtMYB15, FtMYB16 | Inhibition of biosynthesis of rutin | [71] | |
| FtMYB123L | Regulation of flavonoid biosynthesis | [72] | |
| FtMYB11 | Repress phenylpropanoid biosynthesis | [42] | |
| Lilium brownii var | LhsorMYB12 | Participate in the synthesis of anthocyanidins in leaves | [66] |
| Arabidopsis thaliana | AtMYBL2 | Inhibition of anthocyanin synthesis | [73] |
| AtMYB21, AtMYB24 | Promote the accumulation of anthocyanins | [74] | |
| AtMYB3, AtMYB4, AtMYB32 | Inhibit the accumulation of anthocyanins | [29] | |
| AtMYB34 | Regulation of the synthesis of glucosinolates | [75] | |
| Triticum aestivum | TaPL1, | Enhance the synthesis and accumulation of anthocyanins | [43] |
| Salvia miltiorrhiza | SmMYB39 | Inhibit the accumulation of phenolic acids | [76] |
| SmMYB4 | Affect the biosynthesis of rosmarinic acid | [77] | |
| Antirrhinum majus | AmMYB308, AmMYB330 | Reduce phenolic acid | [18] |
| Rosea1, Rosea2 and Venosa | Regulation of anthocyanin production | [78] | |
| Ginkgo biloba | GbMYB5, GbMYB26, GbMYB31 | Can participate in the biosynthesis of flavonoids under adverse conditions | [79] |
| GbMYBFL | Enhance the accumulation of flavonoids and anthocyanin | [80] | |
| Dendranthema morifolium | DmMYB1 | Negative regulation of the synthesis of flavonoids | [56] |
| Panax Ginseng | PgMYB2 | Play a key regulatory role in ginsenoside synthesis | [48] |
| Epimedium sagittatum | EsMYBF1 | Increased flavonol content and the decreased anthocyanin content in flowers | [47] |
| EsMYBA1 | Regulate anthocyanin biosynthesis | [81] | |
| EsAN2 | Regulate anthocyanin biosynthesis | [82] | |
| Scutellaria baicalensis | SbMYB8 | Regulate flavonoid biosynthesis | [83] |
| Gentiana triflora | GtMYBP3, GtMYBP4 | Activate the expression of flavonol synthesis genes | [84] |
| Perilla frutescens | MYB-P1 | Determined factor of the anthocyanin forma | [85] |
| Medicago truncatula | MtPAR | Regulate proanthocyanidin (PA) biosynthesis | [86] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Li, K.; Li, Y.; Zhao, X.; Wang, L. MYB Transcription Factors as Regulators of Secondary Metabolism in Plants. Biology 2020, 9, 61. https://doi.org/10.3390/biology9030061
Cao Y, Li K, Li Y, Zhao X, Wang L. MYB Transcription Factors as Regulators of Secondary Metabolism in Plants. Biology. 2020; 9(3):61. https://doi.org/10.3390/biology9030061
Chicago/Turabian StyleCao, Yunpeng, Kui Li, Yanli Li, Xiaopei Zhao, and Lihu Wang. 2020. "MYB Transcription Factors as Regulators of Secondary Metabolism in Plants" Biology 9, no. 3: 61. https://doi.org/10.3390/biology9030061
APA StyleCao, Y., Li, K., Li, Y., Zhao, X., & Wang, L. (2020). MYB Transcription Factors as Regulators of Secondary Metabolism in Plants. Biology, 9(3), 61. https://doi.org/10.3390/biology9030061






