Upregulated Wnt-11 and miR-21 Expression Trigger Epithelial Mesenchymal Transition in Aggressive Prostate Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Situ Hybridization, Cell Blocks, and Immunostaining
2.3. RNA Extraction and qRT-PCR
2.4. Assays for Colony Formation and Soft Agar Colony Formation Spheroids
2.5. Assays for Cell Invasion, Migration, and Apoptosis
2.6. Hanging Drop Assay
2.7. Boyden Chamber Assay
2.8. Western Blotting
2.9. Data Analysis
3. Results
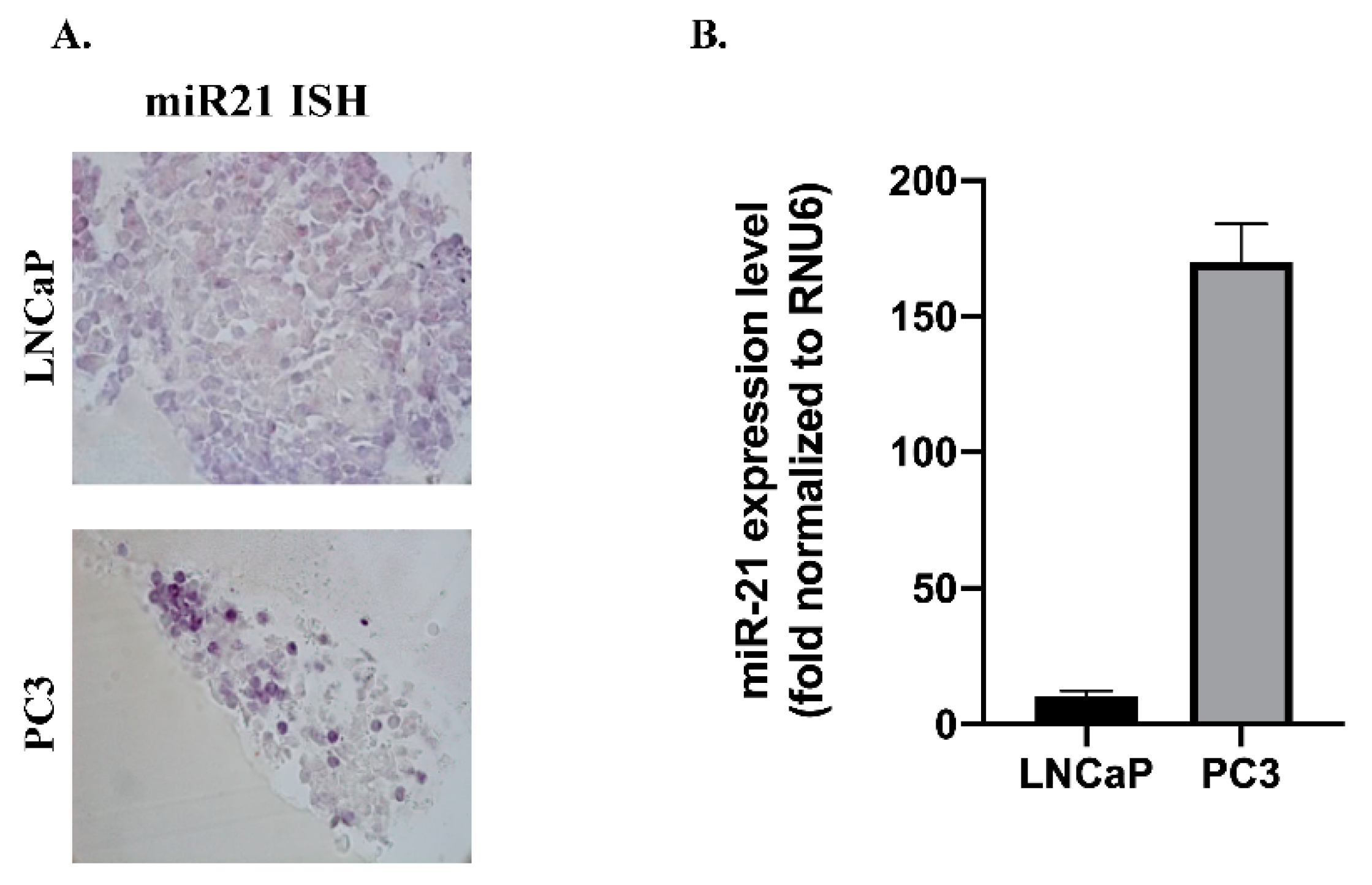
3.1. miR-21 and Wnt-11 Expression Profiling
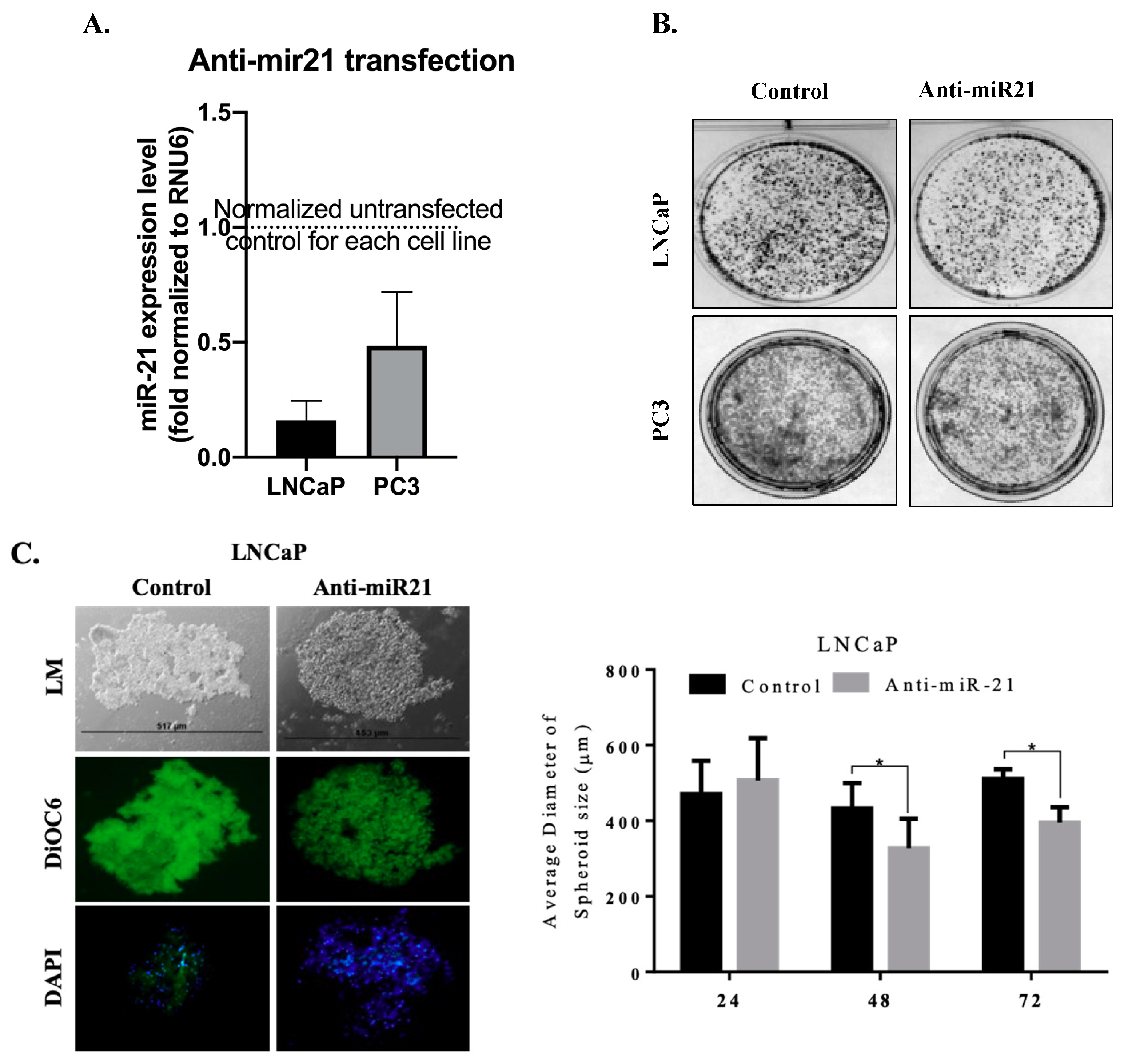
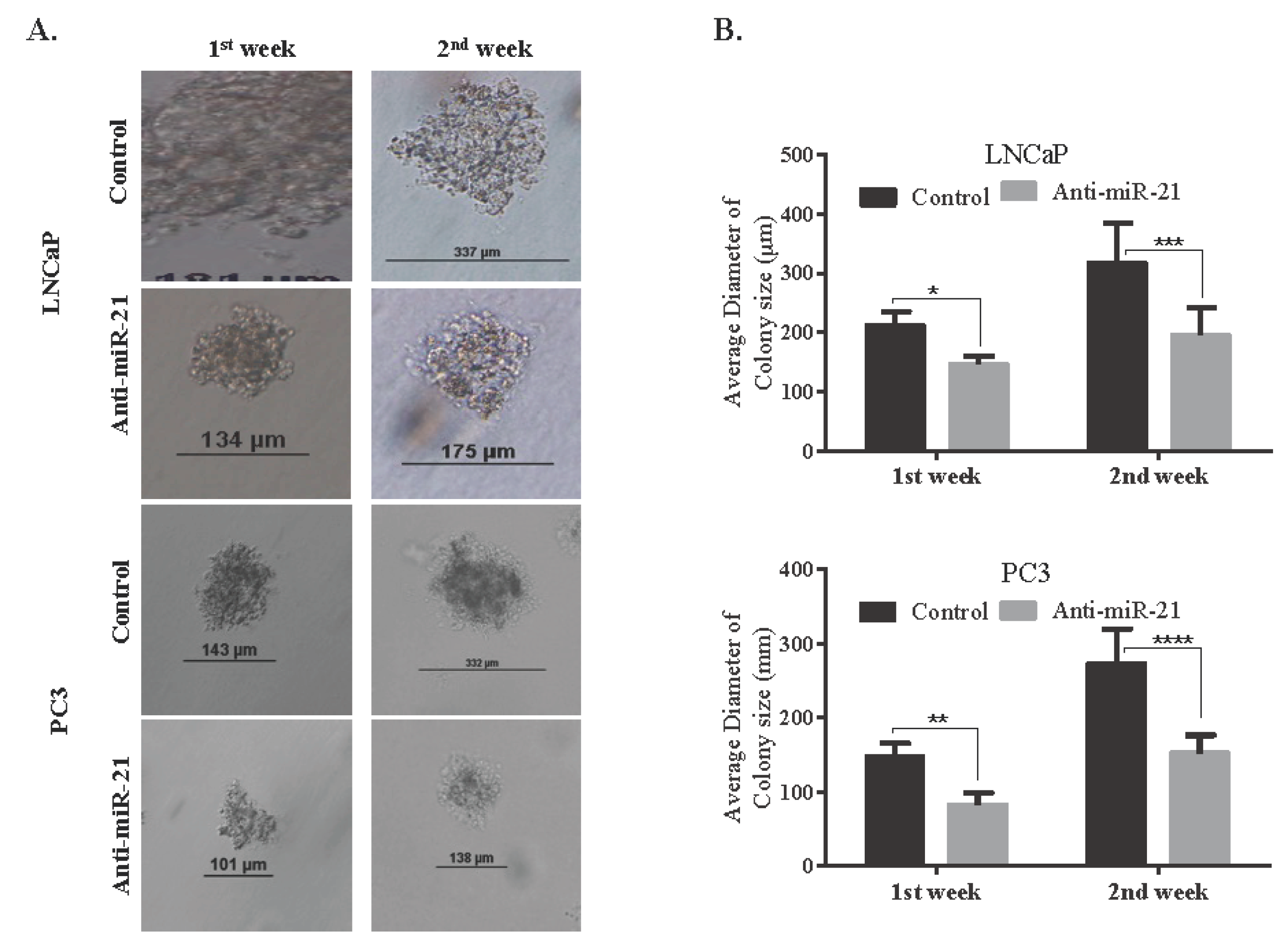
3.2. miR-21 Involves in Cell Survival and Colony Formation
3.3. miR-21 is a Strong Regulator on EMT Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbieri, C.; Shoag, J. Clinical variability and molecular heterogeneity in prostate cancer. Asian J. Androl. 2016, 18, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Srigley, J.R.; Delahunt, B.; Samaratunga, H.; Billis, A.; Cheng, L.; Clouston, D.; Evans, A.; Furusato, B.; Kench, J.; Leite, K.; et al. Controversial issues in Gleason and International Society of Urological Pathology (ISUP) prostate cancer grading: Proposed recommendations for international implementation. Pathology 2019, 51, 463–473. [Google Scholar] [CrossRef]
- Humphrey, P.A. Gleason grading and prognostic factors in carcinoma of the prostate. Mod. Pathol. 2004, 17, 292–306. [Google Scholar] [CrossRef]
- Bavelloni, A.; Ramazzotti, G.; Poli, A.; Piazzi, M.; Focaccia, E.; Blalock, W.L.; Faenza, I. MiRNA-210: A Current Overview. Anticancer Res. 2017, 37, 6511–6521. [Google Scholar]
- Frixa, T.; Donzelli, S.; Blandino, G. Oncogenic MicroRNAs: Key Players in Malignant Transformation. Cancers 2015, 7, 2466–2485. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Tsao, C.-J. Emerging role of microRNA-21 in cancer. Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Zaragoza, O.; Deas, J.; Acosta, A.M.; De La O-Gómez, F.; Fernández-Tilapa, G.; Gómez-Cerón, C.; Benítez-Boijseauneau, O.; Burguete-Garcia, A.I.; Torres-Poveda, K.; Bermúdez-Morales, V.H.; et al. Relevance of miR-21 in regulation of tumor suppressor gene PTEN in human cervical cancer cells. BMC Cancer 2016, 16, 215. [Google Scholar]
- Wu, Y.; Song, Y.; Xiong, Y.; Wang, X.; Xu, K.; Han, B.; Bai, Y.; Li, L.; Zhang, Y.; Zhou, L. MicroRNA-21 (Mir-21) Promotes Cell Growth and Invasion by Repressing Tumor Suppressor PTEN in Colorectal Cancer. Cell. Physiol. Biochem. 2017, 43, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, N.; Baker, S.J. PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 127–150. [Google Scholar]
- Shi, G.-H.; Ye, D.-W.; Yao, X.-D.; Zhang, S.-L.; Dai, B.; Zhang, H.-L.; Shen, Y.-J.; Zhu, Y.; Zhu, Y.-P.; Xiao, W.-J.; et al. Involvement of microRNA-21 in mediating chemo-resistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol. Sin. 2010, 31, 867–873. [Google Scholar] [CrossRef]
- Abba, M.; Patil, N.; Allgayer, H. MicroRNAs in the Regulation of MMPs and Metastasis. Cancers 2014, 6, 625–645. [Google Scholar] [PubMed]
- Dart, D.A.; Arisan, D.E.; Owen, S.; Hao, C.; Jiang, W.G.; Uysal-Onganer, P. Wnt-11 Expression Promotes Invasiveness and Correlates with Survival in Human Pancreatic Ductal Adeno Carcinoma. Genes 2019, 10, 921. [Google Scholar]
- Gorroño-Etxebarria, I.; Larracoechea, U.A.; Sanchez, S.; González, N.; Escobar, A.; Zabalza, I.; Quintana, M.; Vivanco, M.D.; Waxman, J.; Kypta, R. Wnt-11 as a Potential Prognostic Biomarker and Therapeutic Target in Colorectal Cancer. Cancers 2019, 11, 908. [Google Scholar]
- Uysal-Onganer, P.; Kawano, Y.; Caro, M.; Walker, M.M.; Diez, S.; Darrington, R.S.; Waxman, J.; Kypta, R. Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol. Cancer 2010, 9, 55. [Google Scholar] [PubMed]
- Peng, Y.; Zhang, X.; Feng, X.; Fan, X.; Jin, Z. The crosstalk between microRNAs and the Wnt/β-catenin signaling pathway in cancer. Oncotarget 2016, 8, 14089–14106. [Google Scholar]
- Zhou, H.; Zhu, X. MicroRNA-21 and microRNA-30c as diagnostic biomarkers for prostate cancer: A meta-analysis. Cancer Manag. Res. 2019, 11, 2039–2050. [Google Scholar]
- Sekhon, K.; Bucay, N.; Majid, S.; Dahiya, R.; Saini, S. MicroRNAs and epithelial-mesenchymal transition in prostate cancer. Oncotarget 2016, 7, 67597–67611. [Google Scholar]
- Petrylak, D.P. Current state of castration-resistant prostate cancer. Am. J. Manag. Care 2013, 19, 358–365. [Google Scholar]
- Zhang, H.-L.; Yang, L.-F.; Zhu, Y.; Yao, X.-D.; Zhang, S.-L.; Dai, B.; Zhu, Y.; Shen, Y.; Shi, G.-H.; Ye, D.-W. Serum miRNA-21: Elevated levels in patients with metastatic hormone-refractory prostate cancer and potential predictive factor for the efficacy of docetaxel-based chemotherapy. Prostate 2010, 71, 326–331. [Google Scholar]
- Ribas, J.; Ni, X.; Haffner, M.; Wentzel, E.A.; Salmasi, A.H.; Chowdhury, W.; Kudrolli, T.A.; Yegnasubramanian, S.; Luo, J.; Rodriguez, R.; et al. miR-21: An androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res. 2009, 69, 7165–7169. [Google Scholar]
- Kosgodage, U.S.; Uysal-Onganer, P.; MacLatchy, A.; Mould, R.; Nunn, A.V.; Guy, G.W.; Kraev, I.; Chatterton, N.P.; Thomas, E.L.; Inal, J.; et al. Cannabidiol Affects Extracellular Vesicle Release, miR21 and miR126, and Reduces Prohibitin Protein in Glioblastoma Multiforme Cells. Transl. Oncol. 2018, 12, 513–522. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Allache, R.; Lachance, S.; Guyot, M.C.; De Marco, P.; Merello, E.; Justice, M.J.; Capra, V.; Kibar, Z. Novel mutations in Lrp6 orthologs in mouse and human neural tube defects affect a highly dosage-sensitive Wnt non-canonical planar cell polarity pathway. Hum. Mol. Genet. 2013, 23, 1687–1699. [Google Scholar] [PubMed]
- Katoh, M. WNT Signaling Pathway and Stem Cell Signaling Network. Clin. Cancer Res. 2007, 13, 4042–4045. [Google Scholar] [PubMed]
- Yokoyama, N.N.; Shao, S.; Hoang, B.H.; Mercola, D.; Zi, X. Wnt signaling in castration-resistant prostate cancer: Implications for therapy. Am. J. Clin. Exp. Urol. 2014, 2, 27–44. [Google Scholar] [PubMed]
- Vatansever, H.S.; Gumus, B.; Aydogdu, O.; Sivrikoz, O.N.; Turkoz-Uluer, E.; Kivanc, M.; Atesci, Y.Z.; Bugdayci, H. The role of stem/progenitor cells and Wnt/beta-catenin signaling pathway in the patients with prostate cancer. Minerva Urol. Nefrol. 2014, 66, 249–255. [Google Scholar]
- Van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar]
- Uysal-Onganer, P.; Kypta, R. Wnt11 in 2011—the regulation and function of a non-canonical Wnt. Acta Physiol. 2011, 204, 52–64. [Google Scholar]
- Peng, X.; Ye, L.; Zhang, X.; Wang, M.; Lin, C.; Huang, S.; Guo, W.; Lai, Y.; Du, H.; Li, J.; et al. FZD8, a target of p53, promotes bone metastasis in prostate cancer by activating canonical Wnt/β-catenin signaling. Cancer Lett. 2017, 402, 166–176. [Google Scholar]
- Murillo-Garzón, V.; Gorroño-Etxebarria, I.; Åkerfelt, M.; Puustinen, M.C.; Sistonen, L.; Nees, M.; Carton, J.; Waxman, J.; Kypta, R. Frizzled-8 integrates Wnt-11 and transforming growth factor-β signaling in prostate cancer. Nat. Commun. 2018, 9, 1747. [Google Scholar]
- Charron, F.; Tessier-Lavigne, M. The Hedgehog, TGF-β/BMP and Wnt Families of Morphogens in Axon Guidance. Adv. Exp. Med. Biol. 2008, 621, 116–133. [Google Scholar]
- Fang, H.; Xie, J.; Zhang, M.; Zhao, Z.; Wan, Y.; Yao, Y. miRNA-21 promotes proliferation and invasion of triple-negative breast cancer cells through targeting PTEN. Am. J. Transl. Res. 2017, 9, 953–961. [Google Scholar] [PubMed]
- Yang, Y.; Guo, J.-X.; Shao, Z.-Q. miR-21 targets and inhibits tumor suppressor gene PTEN to promote prostate cancer cell proliferation and invasion: An experimental study. Asian Pac. J. Trop. Med. 2017, 10, 87–91. [Google Scholar] [PubMed]
- Mandhani, A.; Mitash, N.; Agnihotri, S.; Tiwari, S.; Agrawal, V. MicroRNA-21 could be a molecular marker to predict the recurrence of nonmuscle invasive bladder cancer. Indian J. Urol. 2017, 33, 283–290. [Google Scholar]
- Folini, M.; Gandellini, P.; Longoni, N.; Profumo, V.; Callari, M.; Pennati, M.; Colecchia, M.; Supino, R.; Veneroni, S.; Salvioni, R.; et al. miR-21: An oncomir on strike in prostate cancer. Mol. Cancer 2010, 9, 12. [Google Scholar]
- Blower, P.E.; Chung, J.-H.; Verducci, J.S.; Lin, S.; Park, J.K.; Dai, Z.; Liu, C.-G.; Schmittgen, T.D.; Reinhold, W.; Croce, C.M.; et al. MicroRNAs modulate the chemosensitivity of tumor cells. Mol. Cancer Ther. 2008, 7, 1–9. [Google Scholar]
- Ravenna, L.; Principessa, L.; Verdina, A.; Salvatori, L.; Russo, M.A.; Petrangeli, E. Distinct Phenotypes of Human Prostate Cancer Cells Associate with Different Adaptation to Hypoxia and Pro-Inflammatory Gene Expression. PLoS ONE 2014, 9, e96250. [Google Scholar]
- Qin, W.; Zhao, B.; Shi, Y.; Yao, C.; Jin, L.; Jin, Y. BMPRII is a direct target of miR-21. Acta Biochim. Biophys. Sin. 2009, 41, 618–623. [Google Scholar]
- Haigl, B.; Vanas, V.; Setinek, U.; Hegedűs, B.; Gsur, A.; Sutterlüty-Fall, H. Expression of microRNA-21 in non-small cell lung cancer tissue increases with disease progression and is likely caused by growth conditional changes during malignant transformation. Int. J. Oncol. 2014, 44, 1325–1334. [Google Scholar]
- Luo, G.; Luo, W.; Sun, X.; Lin, J.; Wang, M.; Zhang, Y.; Luo, W.; Zhang, Y. MicroRNA-21 promotes migration and invasion of glioma cells via activation of Sox2 and β-catenin signaling. Mol. Med. Rep. 2016, 15, 187–193. [Google Scholar]
- Appert-Collin, A.; Bennasroune, A.; Jeannesson, P.; Terryn, C.; Fuhrmann, G.; Morjani, H.; Dedieu, S. Role of LRP-1 in cancer cell migration in 3-dimensional collagen matrix. Cell Adhes. Migr. 2016, 11, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Díaz-González, S.D.M.; Rodríguez-Aguilar, E.D.; Acosta, A.M.; Valadez-Graham, V.; Deas, J.; Gómez-Cerón, C.; Tavira-Montalván, C.A.; Arizmendi-Heras, A.; Ramirez, O.B.; Zurita, M.; et al. Transregulation of microRNA miR-21 promoter by AP-1 transcription factor in cervical cancer cells. Cancer Cell Int. 2019, 19, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Echevarría-Vargas, I.M.; Valiyeva, F.; Vivas-Mejia, P.E. Upregulation of miR-21 in Cisplatin Resistant Ovarian Cancer via JNK-1/c-Jun Pathway. PLoS ONE 2014, 9, e97094. [Google Scholar] [CrossRef] [PubMed]
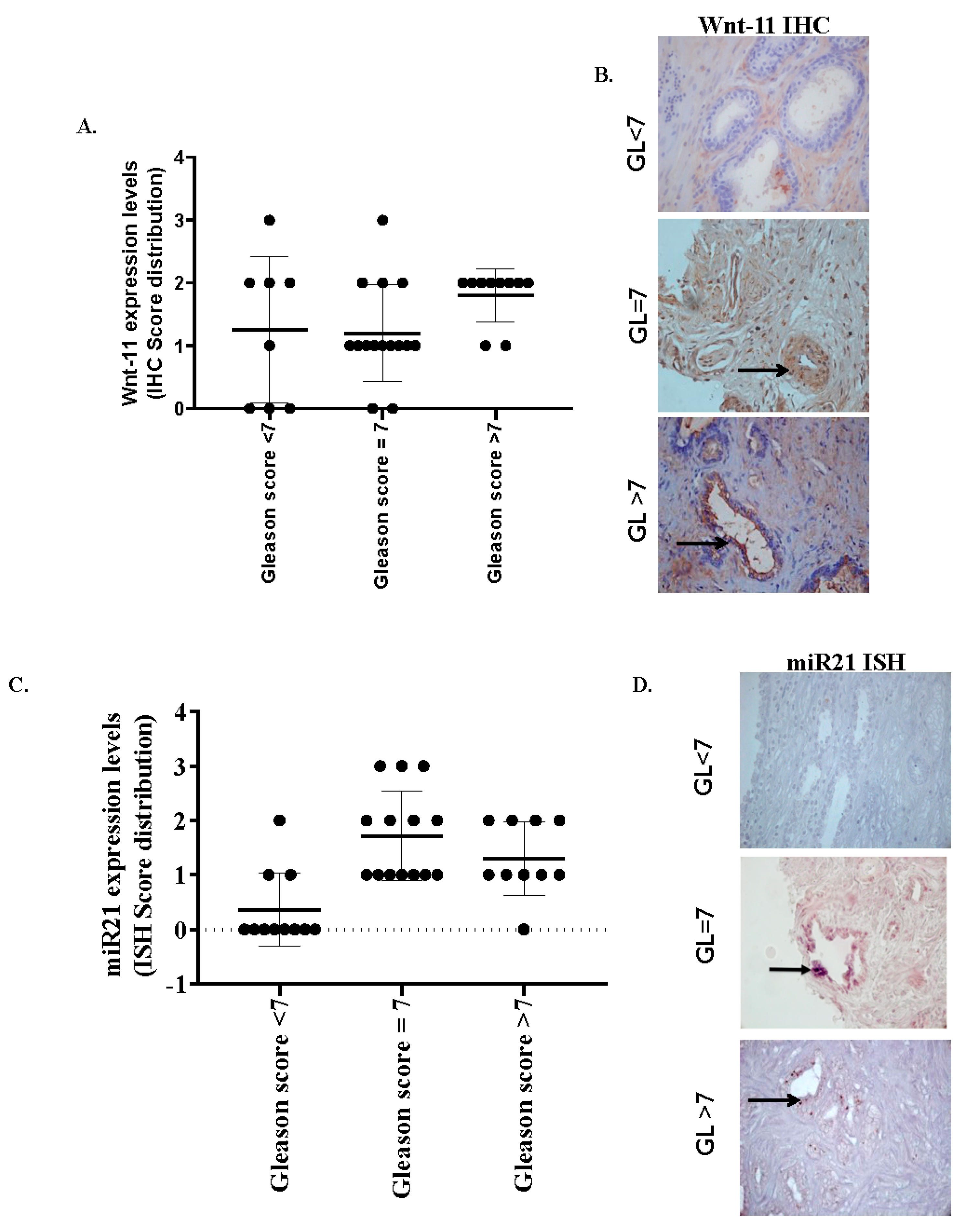


| Histological Grade | Wnt-11-Staining 25/36 (69.4%) | miR-21-Staining 25/36 (69%) |
|---|---|---|
| Gleason < 7 (n = 10) | 1/10 (10%) strong/moderate 1/10 (10%) weak 8/10 (80%) cases nonspecific | 1/9 (10%) strong/moderate 2/10 (20%) weak 7/10 (70%) negative/nonspecific |
| Gleason 7 (n = 20) | 8/17 (48%) strong/moderate | 7/17 (42%) |
| 7/17 (42%) weak | 7/17 (42%) weak | |
| 2/17 (10%) nonspecific | 3/17 (16%) nonspecific | |
| Gleason > 7 (n = 10) | 3/9 (33%) strong/moderate | 4/9 (44%) strong/moderate |
| 5/9 (56%) weak | 4/9 (44%) weak | |
| 1/9 (11%) nonspecific | 1/9 (11%) nonspecific |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arisan, E.D.; Rencuzogullari, O.; Freitas, I.L.; Radzali, S.; Keskin, B.; Kothari, A.; Warford, A.; Uysal-Onganer, P. Upregulated Wnt-11 and miR-21 Expression Trigger Epithelial Mesenchymal Transition in Aggressive Prostate Cancer Cells. Biology 2020, 9, 52. https://doi.org/10.3390/biology9030052
Arisan ED, Rencuzogullari O, Freitas IL, Radzali S, Keskin B, Kothari A, Warford A, Uysal-Onganer P. Upregulated Wnt-11 and miR-21 Expression Trigger Epithelial Mesenchymal Transition in Aggressive Prostate Cancer Cells. Biology. 2020; 9(3):52. https://doi.org/10.3390/biology9030052
Chicago/Turabian StyleArisan, Elif Damla, Ozge Rencuzogullari, Ines Lua Freitas, Syanas Radzali, Buse Keskin, Archana Kothari, Antony Warford, and Pinar Uysal-Onganer. 2020. "Upregulated Wnt-11 and miR-21 Expression Trigger Epithelial Mesenchymal Transition in Aggressive Prostate Cancer Cells" Biology 9, no. 3: 52. https://doi.org/10.3390/biology9030052
APA StyleArisan, E. D., Rencuzogullari, O., Freitas, I. L., Radzali, S., Keskin, B., Kothari, A., Warford, A., & Uysal-Onganer, P. (2020). Upregulated Wnt-11 and miR-21 Expression Trigger Epithelial Mesenchymal Transition in Aggressive Prostate Cancer Cells. Biology, 9(3), 52. https://doi.org/10.3390/biology9030052







