Efficacy and Safety of First-Line Everolimus Therapy Alone or in Combination with Octreotide in Gastroenteropancreatic Neuroendocrine Tumors. A Hellenic Cooperative Oncology Group (HeCOG) Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Treatment Protocol
2.2. Laboratory and Imaging Studies
2.3. Pathology
2.4. Statistical Analysis
3. Results
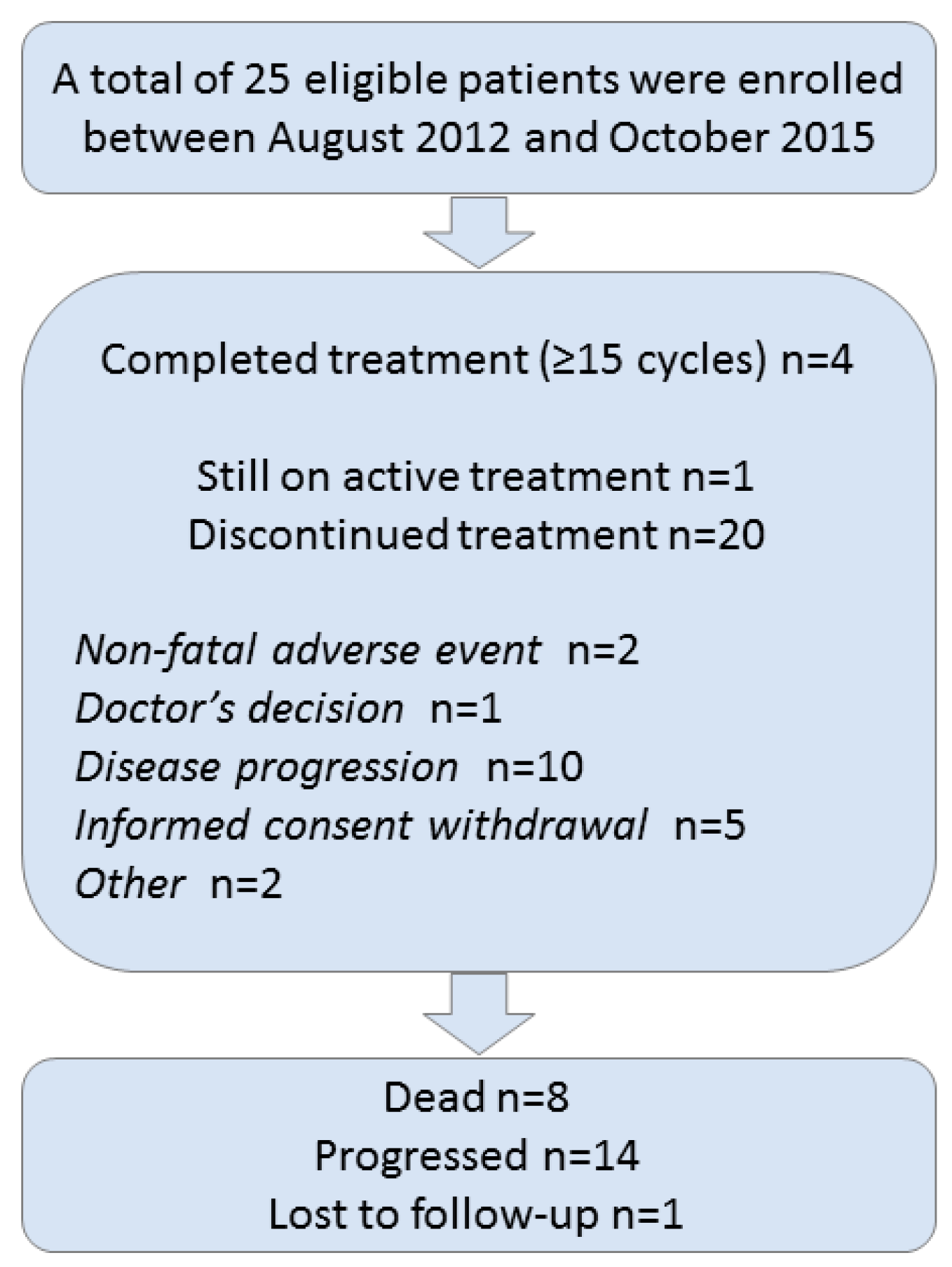
3.1. Patient Characteristics
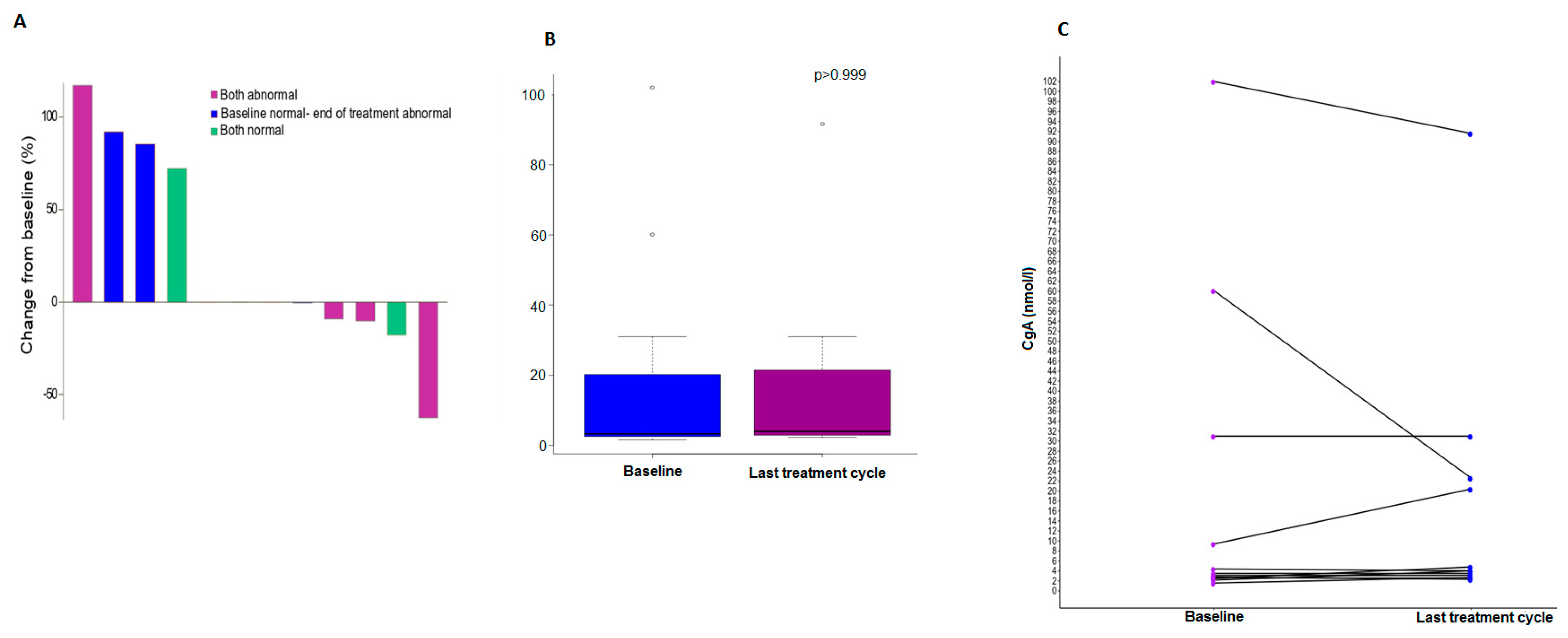
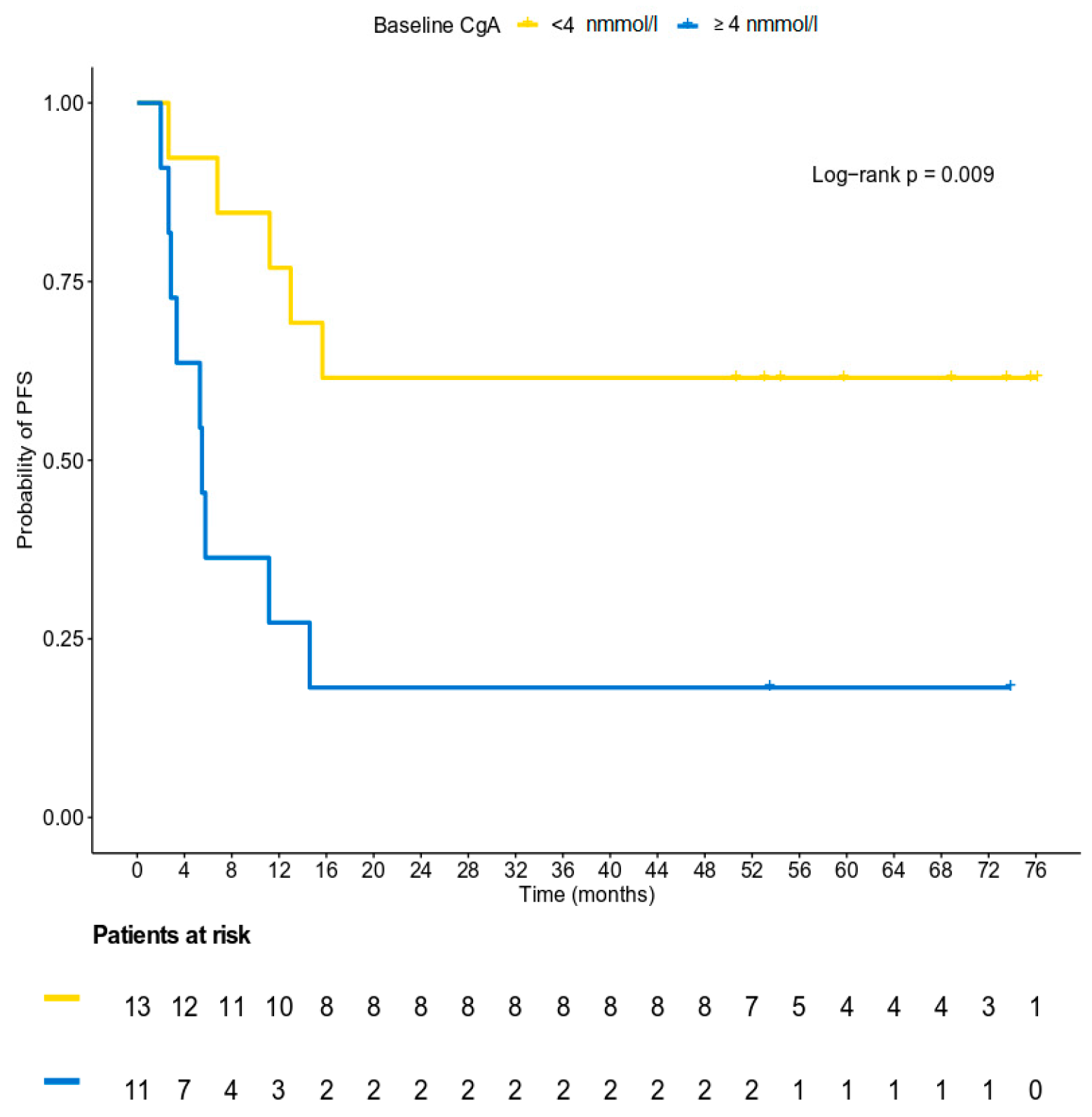
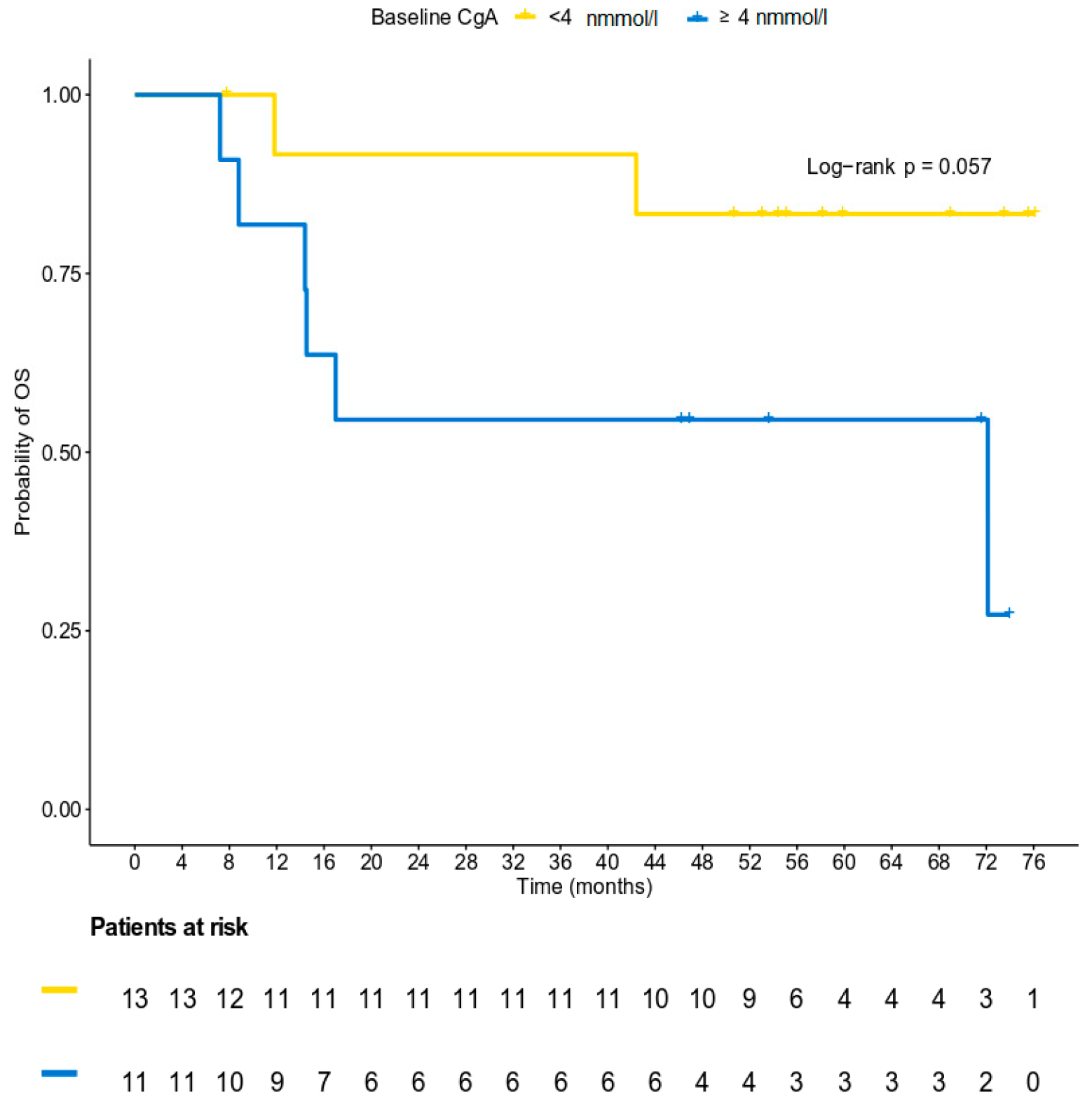
3.2. Chromogranin A Data
3.3. Treatment Cycles
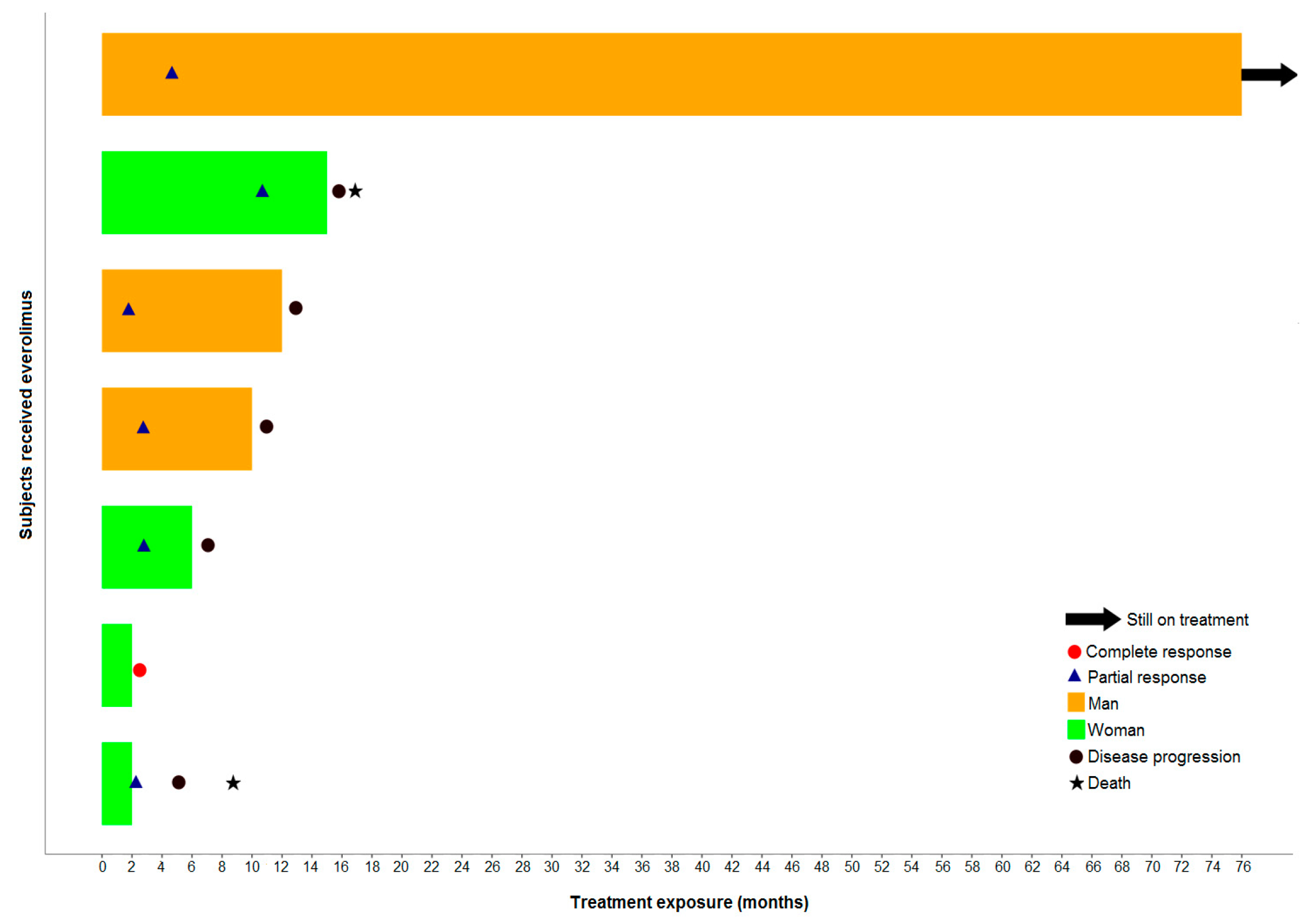
3.4. Response Assessment
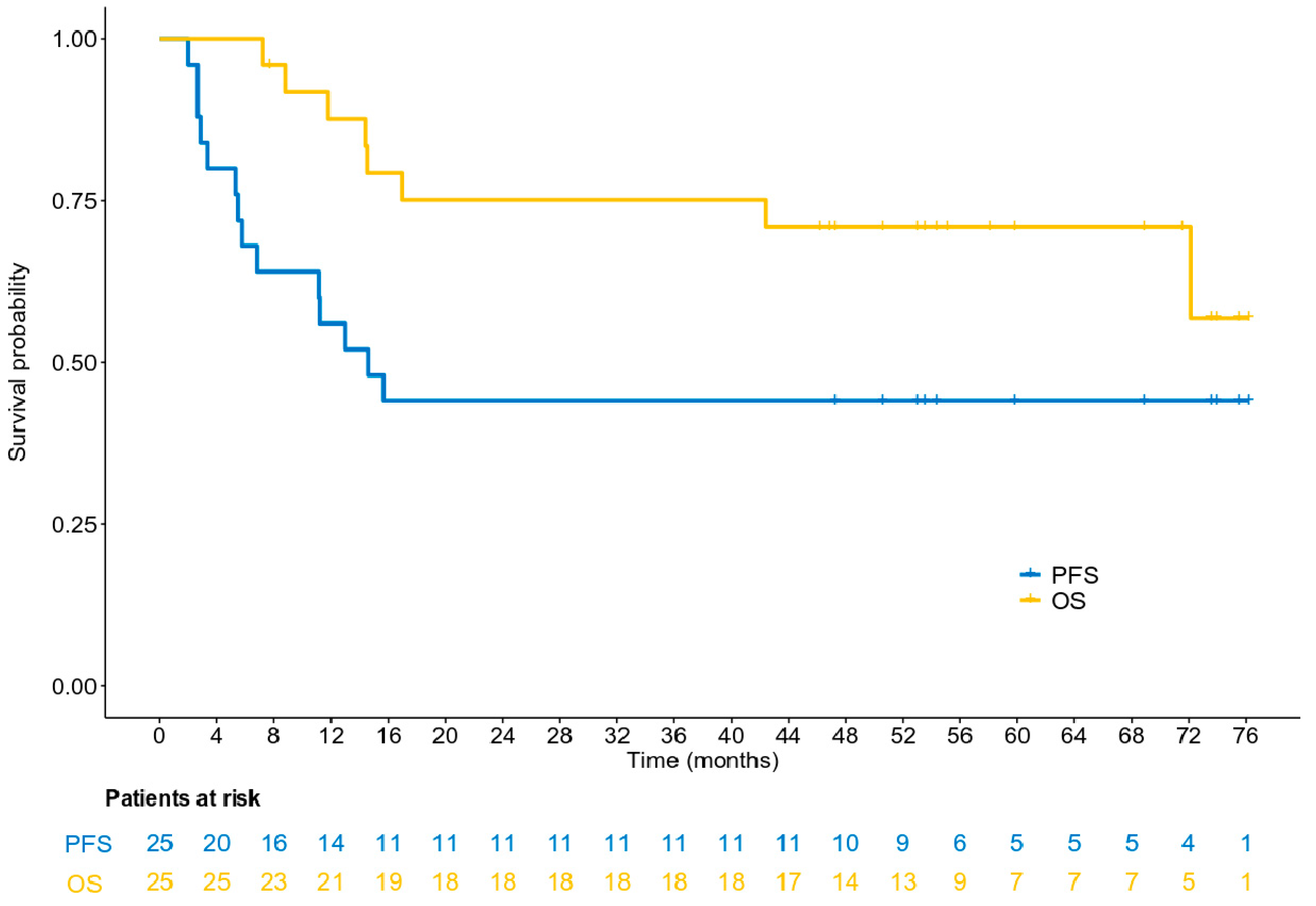
3.5. Survival Analysis
3.6. Safety Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef]
- Chauhan, A.; Yu, Q.; Ray, N.; Farooqui, Z.; Huang, B.; Durbin, E.B.; Tucker, T.; Evers, M.; Arnold, S.; Anthony, L.B. Global burden of neuroendocrine tumors and changing incidence in Kentucky. Oncotarget 2018, 9, 19245–19254. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Cwikla, J.B.; Phan, A.T.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Pyronnet, S.; Bousquet, C.; Najib, S.; Azar, R.; Laklai, H.; Susini, C. Antitumor effects of somatostatin. Mol. Cell Endocrinol. 2008, 286, 230–237. [Google Scholar] [CrossRef]
- Rinke, A.; Muller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Blaker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Merola, E.; Panzuto, F.; Delle Fave, G. Antiproliferative effect of somatostatin analogs in advanced gastro-entero-pancreatic neuroendocrine tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 46624–46634. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.E.; Hainsworth, J.D.; Baudin, E.; Peeters, M.; Horsch, D.; Winkler, R.E.; Klimovsky, J.; Lebwohl, D.; Jehl, V.; Wolin, E.M.; et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet 2011, 378, 2005–2012. [Google Scholar] [CrossRef]
- Bousquet, C.; Lasfargues, C.; Chalabi, M.; Billah, S.M.; Susini, C.; Vezzosi, D.; Caron, P.; Pyronnet, S. Clinical review: Current scientific rationale for the use of somatostatin analogs and mTOR inhibitors in neuroendocrine tumor therapy. J. Clin. Endocrinol. Metab. 2012, 97, 727–737. [Google Scholar] [CrossRef]
- Yao, J.C.; Phan, A.T.; Chang, D.Z.; Wolff, R.A.; Hess, K.; Gupta, S.; Jacobs, C.; Mares, J.E.; Landgraf, A.N.; Rashid, A.; et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: Results of a phase II study. J. Clin. Oncol. 2008, 26, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Lombard-Bohas, C.; Baudin, E.; Kvols, L.K.; Rougier, P.; Ruszniewski, P.; Hoosen, S.; St Peter, J.; Haas, T.; Lebwohl, D.; et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: A phase II trial. J. Clin. Oncol. 2010, 28, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litiere, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Kloppel, G.; La Rosa, S. Ki67 labeling index: Assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018, 472, 341–349. [Google Scholar] [CrossRef]
- Fleming, T.R. One-sample multiple testing procedure for phase II clinical trials. Biometrics 1982, 38, 143–151. [Google Scholar] [CrossRef]
- Pavel, M.E.; Baudin, E.; Oberg, K.E.; Hainsworth, J.D.; Voi, M.; Rouyrre, N.; Peeters, M.; Gross, D.J.; Yao, J.C. Efficacy of everolimus plus octreotide LAR in patients with advanced neuroendocrine tumor and carcinoid syndrome: Final overall survival from the randomized, placebo-controlled phase 3 RADIANT-2 study. Ann. Oncol. 2017, 28, 1569–1575. [Google Scholar] [CrossRef]
- Anthony, L.B.; Pavel, M.E.; Hainsworth, J.D.; Kvols, L.K.; Segal, S.; Horsch, D.; Van Cutsem, E.; Oberg, K.; Yao, J.C. Impact of Previous Somatostatin Analogue Use on the Activity of Everolimus in Patients with Advanced Neuroendocrine Tumors: Analysis from the Phase III RADIANT-2 Trial. Neuroendocrinology 2015, 102, 18–25. [Google Scholar] [CrossRef]
- Capdevila, J.; Sevilla, I.; Alonso, V.; Anton Aparicio, L.; Jimenez Fonseca, P.; Grande, E.; Reina, J.J.; Manzano, J.L.; Alonso Lajara, J.D.; Barriuso, J.; et al. Evaluation of the efficacy and safety of lanreotide in combination with targeted therapies in patients with neuroendocrine tumours in clinical practice: A retrospective cross-sectional analysis. BMC Cancer 2015, 15, 495. [Google Scholar] [CrossRef] [PubMed]
- Bajetta, E.; Catena, L.; Fazio, N.; Pusceddu, S.; Biondani, P.; Blanco, G.; Ricci, S.; Aieta, M.; Pucci, F.; Valente, M.; et al. Everolimus in combination with octreotide long-acting repeatable in a first-line setting for patients with neuroendocrine tumors: An ITMO group study. Cancer 2014, 120, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Bajetta, E.; Catena, L.; Pusceddu, S.; Spada, F.; Iannacone, C.; Sarno, I.; Di Menna, G.; Dottorini, L.; Marte, A.M. Everolimus in Combination with Octreotide Long-Acting Repeatable in a First-Line Setting for Patients with Neuroendocrine Tumors: A 5-Year Update. Neuroendocrinology 2018, 106, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.H.; Ruszniewski, P.; Van Cutsem, E.; Lombard-Bohas, C.; Valle, J.W.; De Herder, W.W.; Pavel, M.; Degtyarev, E.; Brase, J.C.; Bubuteishvili-Pacaud, L.; et al. A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann. Oncol. 2017, 28, 1309–1315. [Google Scholar] [CrossRef]
| Parameter | Total |
|---|---|
| (N = 25) | |
| Median (min,max) | |
| Age (in years) ^ | 56.9 (37.6,79.9) |
| Tumor size (in mm) * | 35.0 (15.0,100.0) |
| Ki67 (% of positive nuclei per 2000 cells, central assessment) * | 0.0 (0.0–20.0) |
| N (%) | |
| Sex | |
| Man | 10 (40.0) |
| Woman | 15 (60.0) |
| PS | |
| 0 | 19 (76.0) |
| 1 | 6 (24.0) |
| Sporadic | |
| No | 4 (16.0) |
| Yes | 21 (84.0) |
| MEN testing | |
| No | 24 (96.0) |
| Yes | 1 (4.0) |
| Initial surgery | |
| No | 12 (48.0) |
| Yes | 13 (52.0) |
| Type of surgery | |
| Laparotomy | 1 (7.7) |
| Open surgery | 12 (92.3) |
| Type of open surgery | |
| Total resection | 4 (33.3) |
| Subtotal resection | 5 (41.7) |
| Other ** | 3 (25.0) |
| Tumor localization | |
| Jejunum | 2 (8.0) |
| Ileus | 4 (16.0) |
| Colon | 2 (8.0) |
| Pancreas | 10 (40.0) |
| Unknown primary | 2 (8.0) |
| Other *** | 5 (20.0) |
| Tumor grade | |
| G1 [Low grade] | 11 (44.0) |
| G2 [Intermediate grade] | 14 (56.0) |
| Ki67 (local assessment) | |
| <3% | 11(44.0) |
| 3–20% | 14 (56.0) |
| Ki67 (central assessment) * | |
| <3% | 10 (58.8) |
| 3–20% | 7 (41.2) |
| Locally advanced ^ | |
| No | 21 (84.0) |
| Yes | 4 (16.0) |
| Number of metastatic sites ^ | |
| 0 | 4 (16.0) |
| 1 | 17 (68.0) |
| 2 | 4 (16.0) |
| Site of metastases ^ | |
| Lung | 0 (0.0) |
| Liver | 19 (76.0) |
| Bones | 0 (0.0) |
| LNS | 5 (20.0) |
| Other **** | 1 (4.0) |
| CgA | Time point | ||
|---|---|---|---|
| Baseline | Last Cycle | p-Value | |
| N | 24 | 12 | >0.999 ^ |
| mean (std) | 21.22 (30.36) | 16.05 (25.73) | |
| median | 3.50 | 4 | |
| min-max | 1.57–102.0 | 2.30–91.60 | |
| p25–p75 | 2.55–27.60 | 2.90-21.50 | |
| Normal (N,%) | 13 (54.2) | 5 (41.7) | 0.16 ^^ |
| Abnormal (N,%) | 11 (45.8) | 7 (58.3) | |
| Adverse Event | Grade 1 | Grade 2 | ||||
|---|---|---|---|---|---|---|
| System Organ Class | N of evts | N of pts | % pts | N of evts | N of pts | % pts |
| Preferred Term | ||||||
| Anemia | 5 | 5 | 20.00 | 3 | 3 | 12.00 |
| Diarrhea | 2 | 2 | 8.00 | 6 | 6 | 24.00 |
| Edema limbs | 4 | 4 | 16.00 | 1 | 1 | 4.00 |
| Fatigue | 4 | 4 | 16.00 | 2 | 2 | 8.00 |
| Fever | 4 | 4 | 16.00 | 1 | 1 | 4.00 |
| Alanine aminotransferase increased | 6 | 6 | 24.00 | 4 | 4 | 16.00 |
| Alkaline phosphatase increased | 7 | 7 | 28.00 | 0 | 0 | 0.00 |
| Aspartate aminotransferase increased | 6 | 6 | 24.00 | 4 | 4 | 16.00 |
| Cholesterol high | 6 | 6 | 24.00 | 2 | 2 | 8.00 |
| Creatinine increased | 3 | 3 | 12.00 | 1 | 1 | 4.00 |
| GGT increased | 2 | 2 | 8.00 | 5 | 5 | 20.00 |
| White blood cell decreased | 4 | 4 | 16.00 | 1 | 1 | 4.00 |
| Hyperglycemia | 8 | 8 | 32.00 | 8 | 8 | 32.00 |
| Hypertriglyceridemia | 5 | 5 | 20.00 | 1 | 1 | 4.00 |
| Hypocalcemia | 4 | 4 | 16.00 | 1 | 1 | 4.00 |
| Hypokalemia | 3 | 3 | 12.00 | 1 | 1 | 4.00 |
| Hypophosphatemia | 2 | 2 | 8.00 | 3 | 3 | 12.00 |
| Rash maculo-papular | 4 | 4 | 16.00 | 2 | 2 | 8.00 |
| Adverse Event | Grade 3 | Grade 4 | ||||
|---|---|---|---|---|---|---|
| System Organ Class | N of evts | N of pts | % pts | N of evts | N of pts | % pts |
| Preferred Term | ||||||
| Total | 16 | 11 | 44.00 | 2 | 2 | 8.00 |
| Blood and lymphatic system disorders | 1 | 1 | 4.00 | 0 | 0 | 0.00 |
| Anemia | 1 | 1 | 4.00 | 0 | 0 | 0.00 |
| Gastrointestinal disorders | 5 | 5 | 20.00 | 0 | 0 | 0.00 |
| Diarrhea | 2 | 2 | 8.00 | 0 | 0 | 0.00 |
| Mucositis oral | 3 | 3 | 12.00 | 0 | 0 | 0.00 |
| Infections and infestations | 2 | 2 | 8.00 | 0 | 0 | 0.00 |
| Infections and infestations - Other, specify * | 2 | 2 | 8.00 | 0 | 0 | 0.00 |
| Investigations | 4 | 4 | 16.00 | 2 | 2 | 8.00 |
| Alanine aminotransferase increased | 1 | 1 | 4.00 | 0 | 0 | 0.00 |
| CPK increased | 1 | 1 | 4.00 | 1 | 1 | 4.00 |
| GGT increased | 1 | 1 | 4.00 | 1 | 1 | 4.00 |
| Neutrophil count decreased | 1 | 1 | 4.00 | 0 | 0 | 0.00 |
| Metabolism and nutrition disorders | 3 | 3 | 12.00 | 0 | 0 | 0.00 |
| Anorexia | 1 | 1 | 4.00 | 0 | 0 | 0.00 |
| Hyperglycemia | 1 | 1 | 4.00 | 0 | 0 | 0.00 |
| Hypokalemia | 1 | 1 | 4.00 | 0 | 0 | 0.00 |
| Vascular disorders | 1 | 1 | 4.00 | 0 | 0 | 0.00 |
| Hypertension | 1 | 1 | 4.00 | 0 | 0 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koumarianou, A.; Pectasides, D.; Koliou, G.-A.; Dionysopoulos, D.; Kolomodi, D.; Poulios, C.; Skondra, M.; Sgouros, J.; Pentheroudakis, G.; Kaltsas, G.; et al. Efficacy and Safety of First-Line Everolimus Therapy Alone or in Combination with Octreotide in Gastroenteropancreatic Neuroendocrine Tumors. A Hellenic Cooperative Oncology Group (HeCOG) Study. Biology 2020, 9, 51. https://doi.org/10.3390/biology9030051
Koumarianou A, Pectasides D, Koliou G-A, Dionysopoulos D, Kolomodi D, Poulios C, Skondra M, Sgouros J, Pentheroudakis G, Kaltsas G, et al. Efficacy and Safety of First-Line Everolimus Therapy Alone or in Combination with Octreotide in Gastroenteropancreatic Neuroendocrine Tumors. A Hellenic Cooperative Oncology Group (HeCOG) Study. Biology. 2020; 9(3):51. https://doi.org/10.3390/biology9030051
Chicago/Turabian StyleKoumarianou, Anna, Dimitrios Pectasides, Georgia-Angeliki Koliou, Dimitrios Dionysopoulos, Dionysia Kolomodi, Christos Poulios, Maria Skondra, Joseph Sgouros, George Pentheroudakis, Gregory Kaltsas, and et al. 2020. "Efficacy and Safety of First-Line Everolimus Therapy Alone or in Combination with Octreotide in Gastroenteropancreatic Neuroendocrine Tumors. A Hellenic Cooperative Oncology Group (HeCOG) Study" Biology 9, no. 3: 51. https://doi.org/10.3390/biology9030051
APA StyleKoumarianou, A., Pectasides, D., Koliou, G.-A., Dionysopoulos, D., Kolomodi, D., Poulios, C., Skondra, M., Sgouros, J., Pentheroudakis, G., Kaltsas, G., & Fountzilas, G. (2020). Efficacy and Safety of First-Line Everolimus Therapy Alone or in Combination with Octreotide in Gastroenteropancreatic Neuroendocrine Tumors. A Hellenic Cooperative Oncology Group (HeCOG) Study. Biology, 9(3), 51. https://doi.org/10.3390/biology9030051








