The Scarcity of Specific Nutrients in Wild Bee Larval Food Negatively Influences Certain Life History Traits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
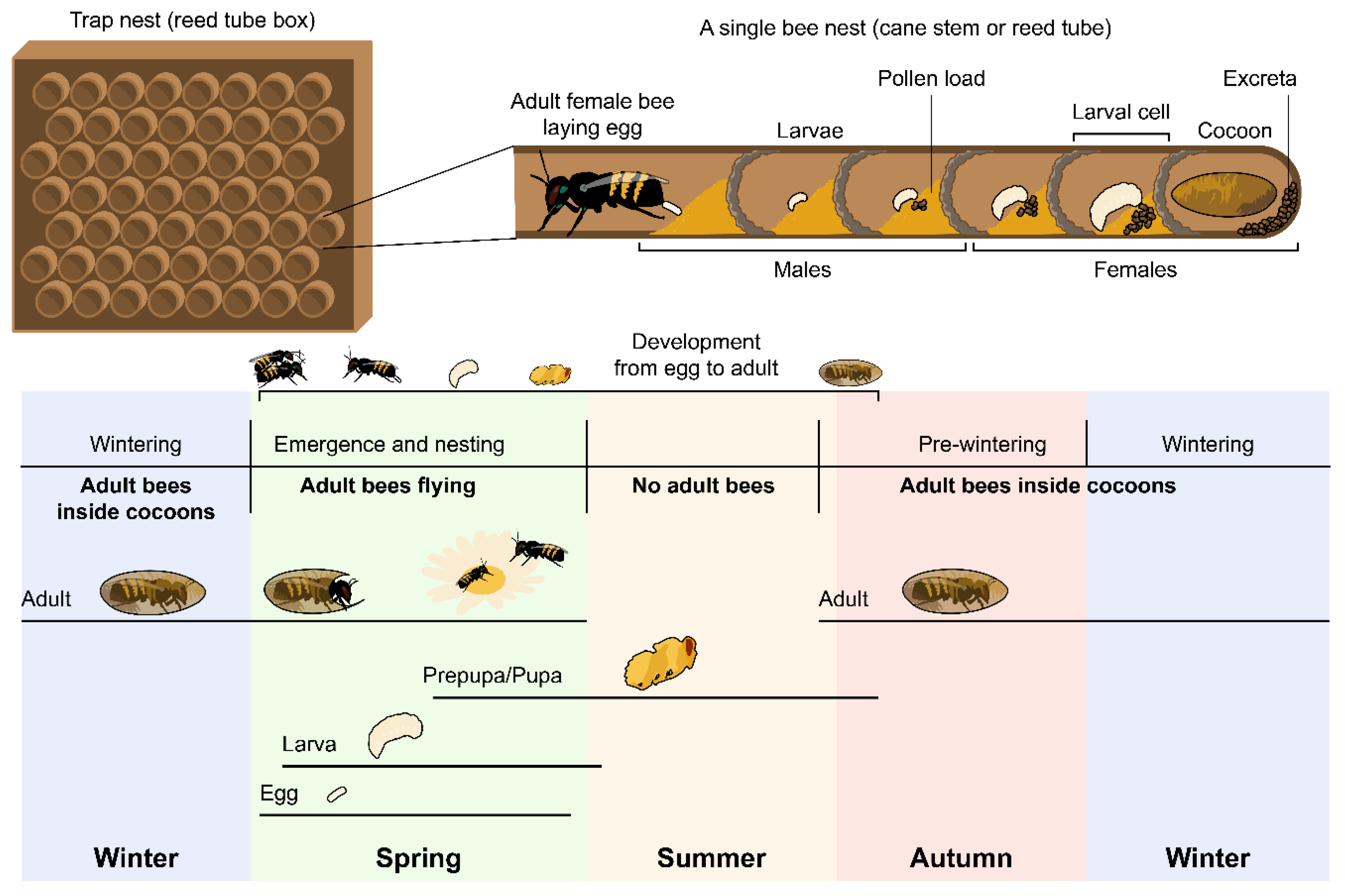
2.1. Model Organism
2.2. Experimental Design
2.3. Pollen Diets
2.4. Chemical Analysis
2.5. Data Handling and Statistical Analysis
3. Results
3.1. Pollen
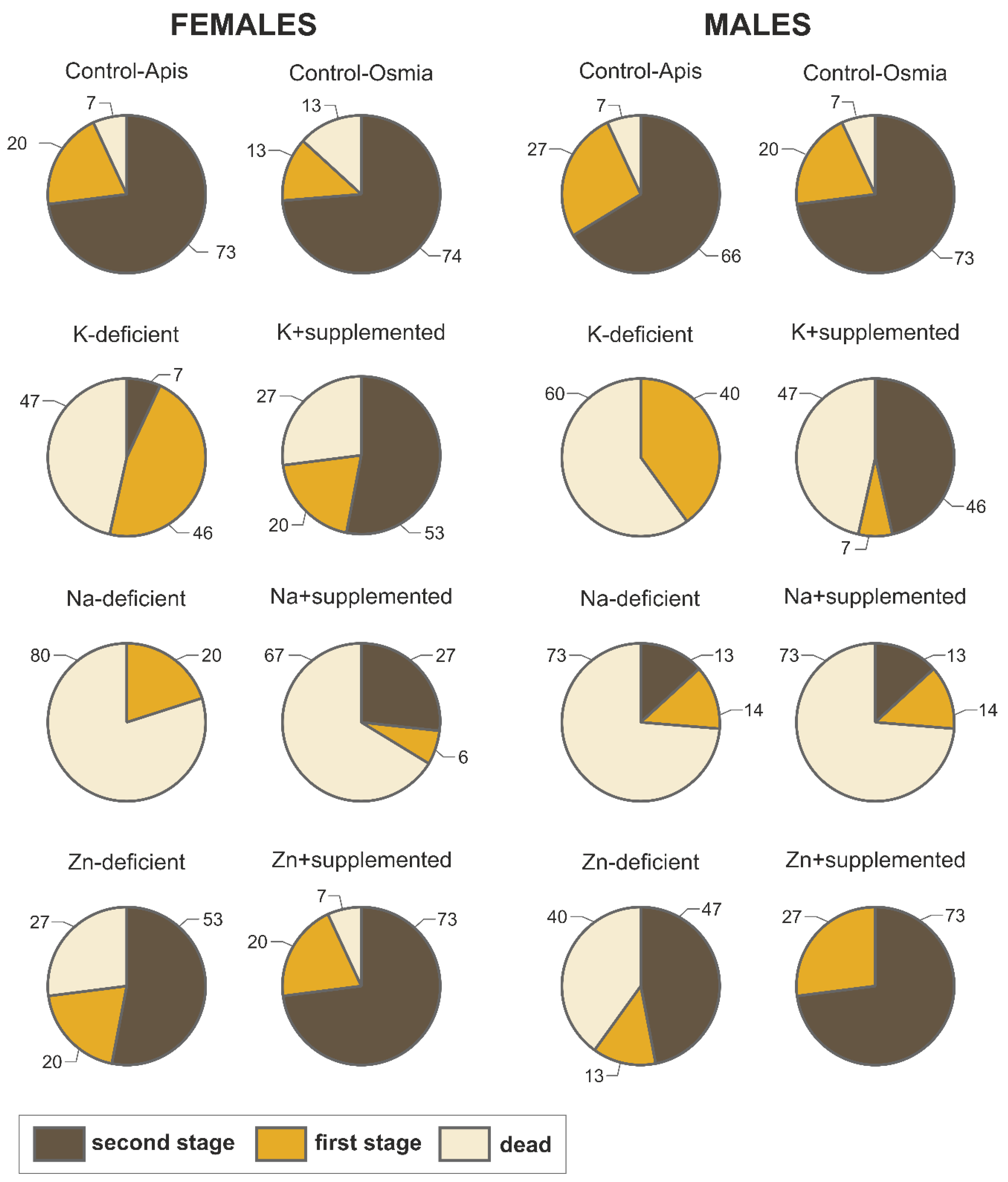
3.2. Mortality
3.3. Cocoon Development
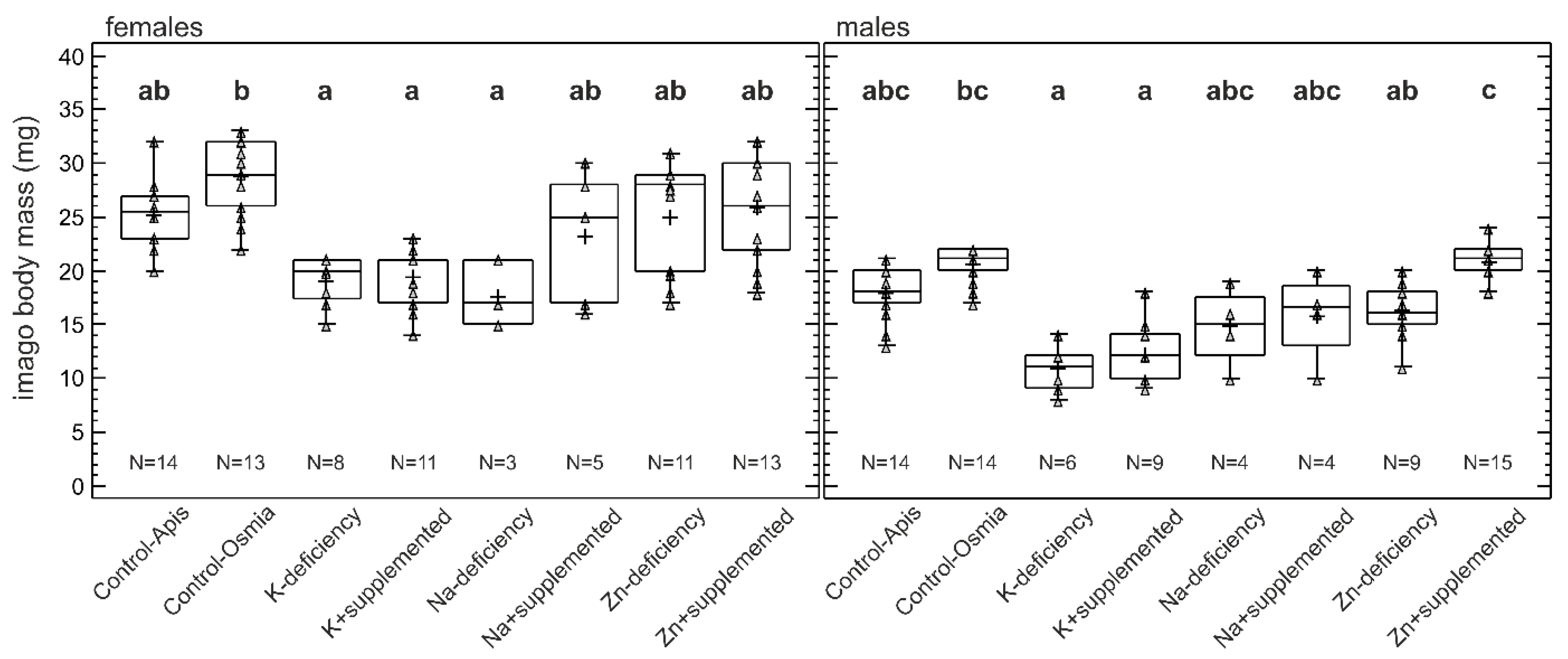
3.4. Imago Body Mass
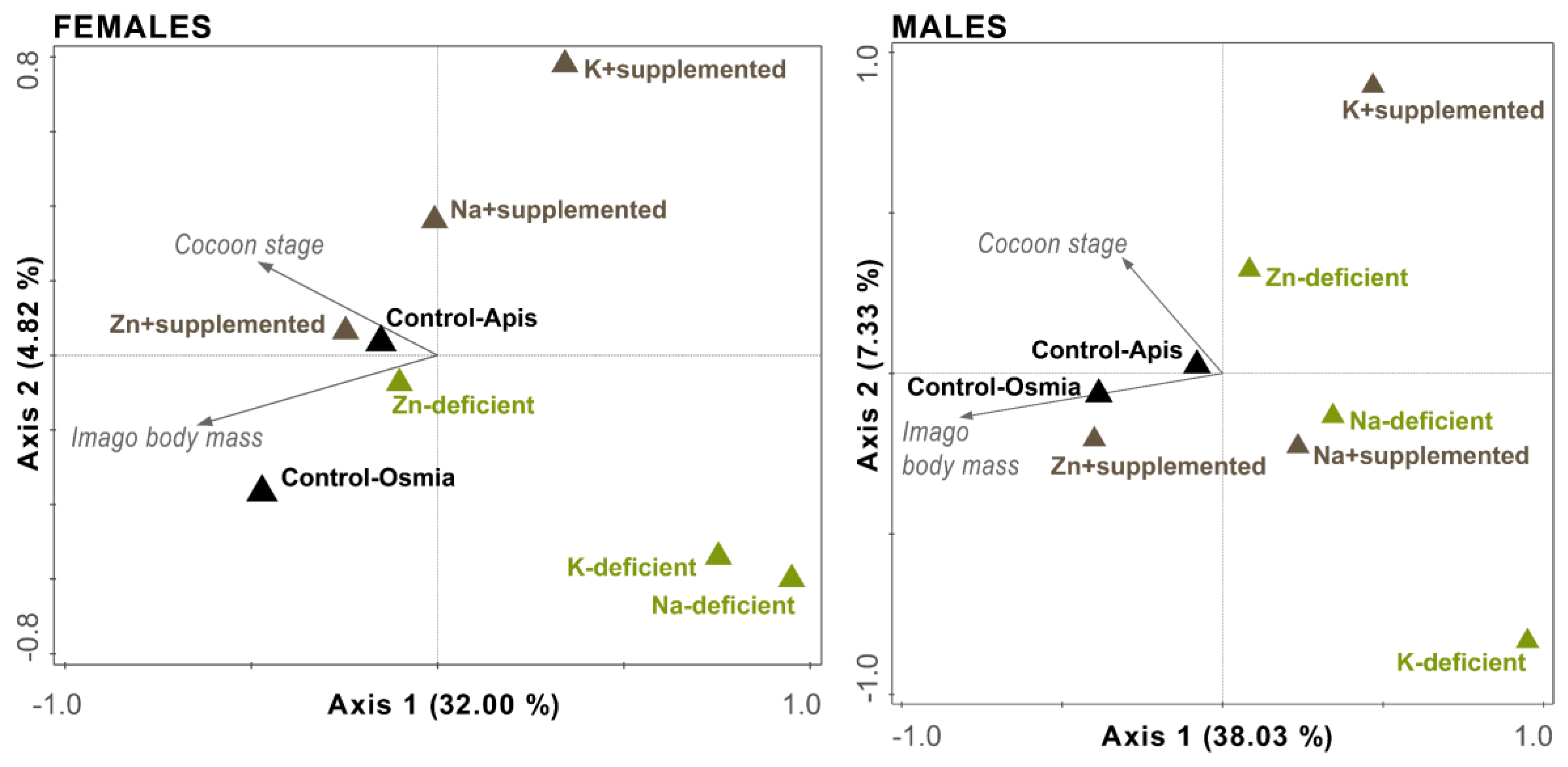
3.5. Simultaneous Redundancy Analysis (Body Mass Plus Cocoon Stage)
4. Discussion
5. Conclusions
- O. bicornis life history traits and fitness are shaped by the availability of atoms of specific chemical elements in larval food.
- Some of these traits might be shaped by the availability of specific elements in a sex-specific manner: Na might influence female body mass, whereas Zn might influence the mortality and body mass of males.
- A trade-off between the K allocation to cocoons and the adult body may exist and might influence the development of cocoons and the body mass of adult bees.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belsky, J.; Joshi, N.K. Impact of biotic and abiotic stressors on managed and feral bees. Insects 2019, 10, 233. [Google Scholar] [CrossRef]
- Drossart, M.; Gerard, M. Beyond the decline of wild bees: Optimizing conservation measures and bringing together the actors. Insects 2020, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Gresty, C.E.A.; Clare, E.; Devey, D.S.; Cowan, R.S.; Csiba, L.; Malakasi, P.; Lewis, O.T.; Willis, K.J. Flower preferences and pollen transport networks for cavity-nesting solitary bees: Implications for the design of agri-environment schemes. Ecol. Evol. 2018, 8, 7574–7587. [Google Scholar] [CrossRef] [PubMed]
- Woodard, S.H.; Jha, S. Wild bee nutritional ecology: Predicting pollinator population dynamics, movement, and services from floral resources. Curr. Opin. Insect Sci. 2017, 21, 83–90. [Google Scholar] [CrossRef]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Power, E.F.; Wright, G.A.; Wilson, K. Nutritional composition of honey bee food stores vary with floral composition. Oecologia 2017, 185, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.E.; Drummond, F. A review of native wild bee nutritional health. Int. J. Ecol. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Danforth, B.N.; Minckley, R.L.; Neff, J.L.; Fawcett, F. The Solitary Bees: Biology, Evolution, Conservation; Princeton University Press: Priceton, NJ, USA; Oxford, UK, 2019. [Google Scholar]
- Filipiak, M.; Weiner, J. Plant–insect interactions: The role of ecological stoichiometry. Acta Agrobot. 2017, 70, 1710. [Google Scholar] [CrossRef]
- Filipiak, M. A better understanding of bee nutritional ecology is needed to optimize conservation strategies for wild bees-the application of ecological stoichiometry. Insects 2018, 9, 85. [Google Scholar] [CrossRef]
- Austin, A.J.; Gilbert, J.D.J. The geometry of dependence: Solitary bee larvae prioritize carbohydrate over protein in parentally provided pollen. bioRxiv 2020, 397802. [Google Scholar] [CrossRef]
- Trinkl, M.; Kaluza, B.F.; Wallace, H.; Heard, T.A.; Keller, A.; Leonhardt, S.D. Floral species richness correlates with changes in the nutritional quality of larval diets in a stingless bee. Insects 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, A.D.; Tooker, J.F.; Patch, H.M.; Biddinger, D.J.; Coccia, M.; Crone, M.K.; Fiely, M.; Francis, J.S.; Hines, H.M.; Hodges, M.; et al. Pollen protein: Lipid macronutrient ratios may guide broad patterns of bee species floral preferences. Insects 2020, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Kriesell, L.; Hilpert, A.; Leonhardt, S.D. Different but the same: Bumblebee species collect pollen of different plant sources but similar amino acid profiles. Apidologie 2017, 48, 102–116. [Google Scholar] [CrossRef]
- Bukovinszky, T.; Rikken, I.; Evers, S.; Wäckers, F.L.; Biesmeijer, J.C.; Prins, H.H.T.; Kleijn, D. Effects of pollen species composition on the foraging behaviour and offspring performance of the mason bee Osmia bicornis (L.). Basic Appl. Ecol. 2017, 18, 21–30. [Google Scholar] [CrossRef]
- Lawson, S.P.; Kennedy, K.B.; Rehan, S.M. Pollen composition significantly impacts the development and survival of the native small carpenter bee, Ceratina calcarata. Ecol. Entomol. 2020. [Google Scholar] [CrossRef]
- Klein, S.; Cabirol, A.; Devaud, J.M.; Barron, A.B.; Lihoreau, M. Why bees are so vulnerable to environmental stressors. Trends Ecol. Evol. 2017, 32, 268–278. [Google Scholar] [CrossRef]
- Requier, F.; Odoux, J.F.; Tamic, T.; Moreau, N.; Henry, M.; Decourtye, A.; Bretagnolle, V. Honey bee diet in intensive farmland habitats reveals an unexpectedly high flower richness and a major role of weeds. Ecol. Appl. 2015, 25, 881–890. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Biddinger, D.J.; Sickel, W.; Keller, A.; López-Uribe, M.M. Introduced bees (Osmia cornifrons) collect pollen from both coevolved and novel host-plant species within their family-level phylogenetic preferences. R. Soc. Open Sci. 2020, 7, 200225. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Ptacnik, R.; Jenerette, G.D.; Verschoor, A.M.; Huberty, A.F.; Solimini, A.G.; Brookes, J.D. Applications of ecological stoichiometry for sustainable acquisition of ecosystem services. Oikos 2005, 109, 52–62. [Google Scholar] [CrossRef]
- Bärlocher, F.; Rennenberg, H. Food chains and nutrient cycles. In Ecological Biochemistry; Bärlocher, F., Rennenberg, H., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 92–122. [Google Scholar]
- DeAngelis, D.L. Dynamics of Nutrient Cycling and Food Webs; Springer: Dordrecht, The Netherland, 1992. [Google Scholar]
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Jeyasingh, P.D.; Cothran, R.D.; Tobler, M. Testing the ecological consequences of evolutionary change using elements. Ecol. Evol. 2014, 4, 528–538. [Google Scholar] [CrossRef]
- Paseka, R.E.; Bratt, A.R.; MacNeill, K.L.; Burian, A.; See, C.R. Elemental ratios link environmental change and human health. Front. Ecol. Evol. 2019, 7, 1–17. [Google Scholar] [CrossRef]
- Kaspari, M. The seventh macronutrient: How sodium shortfall ramifies through populations, food webs and ecosystems. Ecol. Lett. 2020, 23, 1153–1168. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, M. Key pollen host plants provide balanced diets for wild bee larvae: A lesson for planting flower strips and hedgerows. J. Appl. Ecol. 2019, 56, 1410–1418. [Google Scholar] [CrossRef]
- Blanco, G.; Mercer, R.W. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am. J. Physiol. Ren. Physiol. 1998, 275, F633–F650. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.A.T. The essential roles of metal ions in insect homeostasis and physiology. Curr. Opin. Insect Sci. 2017, 23, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Muzzioli, M.; Giacconi, R. Zinc, metallothioneins, immune responses, survival and ageing. Biogerontology 2000, 1, 133–143. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Ivanov, S.P. The nesting of Osmia rufa (L.) (Hymenoptera, Megachilidae) in the Crimea: Structure and composition of nests. Entomol. Rev. 2006, 86, 524–533. [Google Scholar] [CrossRef]
- Bosch, J.; Sgolastra, F.; Kemp, W.P. Life cycle ecophysiology of Osmia mason bees used as crop pollinators. In Bee Pollination in Agricultural Eco-Systems; James, R.R., Pitts-Singer, T.L., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 83–105. [Google Scholar]
- Giejdasz, K.; Wilkaniec, Z. Individual development of the red mason bee (Osmia rufa L., Megachilidae) under natural and laboratory conditions. J. Apic. Sci. 2002, 46, 51–57. [Google Scholar]
- Kim, J.Y. Female size and fitness in the leaf-cutter bee Megachile apicalis. Ecol. Entomol. 1997, 22, 275–282. [Google Scholar] [CrossRef]
- Seidelmann, K. Optimal progeny body size in a solitary bee, Osmia bicornis (Apoidea: Megachilidae). Ecol. Entomol. 2014, 39, 656–663. [Google Scholar] [CrossRef]
- Kozlowski, J. Why life histories are diverse. Pol. J. Ecol. 2006, 54, 585–605. [Google Scholar]
- Filipiak, M.; Kuszewska, K.; Asselman, M.; Denisow, B.; Stawiarz, E.; Woyciechowski, M.; Weiner, J. Ecological stoichiometry of the honeybee: Pollen diversity and adequate species composition are needed to mitigate limitations imposed on the growth and development of bees by pollen quality. PLoS ONE 2017, 12, e0183236. [Google Scholar] [CrossRef] [PubMed]
- Smilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Eckhardt, M.; Haider, M.; Dorn, S.; Müller, A. Pollen mixing in pollen generalist solitary bees: A possible strategy to complement or mitigate unfavourable pollen properties? J. Anim. Ecol. 2014, 83, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Sedivy, C.; Müller, A.; Dorn, S. Closely related pollen generalist bees differ in their ability to develop on the same pollen diet: Evidence for physiological adaptations to digest pollen. Funct. Ecol. 2011, 25, 718–725. [Google Scholar] [CrossRef]
- Koštál, V.; Renault, D.; Mehrabianová, A.; Bastl, J. Insect cold tolerance and repair of chill-injury at fluctuating thermal regimes: Role of ion homeostasis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 231–238. [Google Scholar] [CrossRef]
- Horn, H. Die waldtrachtkrankheit der honigbiene. I. Der einfluß des mineralstoffgehaltes in honigtauhonigen [The causes of paralysis in honeybees during a honeydew flow. 1. The effect of mineral content in honeydew honeys]. Apidologie 1985, 16, 139–156. [Google Scholar] [CrossRef]
- Bonoan, R.E.; Tai, T.M.; Rodriguez, M.T.; Feller, L.; Daddario, S.R.; Czaja, R.A.; O’Connor, L.D.; Burruss, G.; Starks, P.T. Seasonality of salt foraging in honey bees (Apis mellifera). Ecol. Entomol. 2017, 42, 195–201. [Google Scholar] [CrossRef]
- Dorian, N.N.; Bonoan, R.E. Stingless bees (Apidae: Meliponini) seek sodium at carrion baits in Costa Rica. Ecol. Entomol. 2020. [Google Scholar] [CrossRef]
- Lau, P.W.; Nieh, J.C. Salt preferences of honey bee water foragers. J. Exp. Biol. 2016, 219, 790–796. [Google Scholar] [CrossRef]
- Beanland, L.; Phelan, P.L.; Salminen, S. Micronutrient interactions on soybean growth and the developmental performance of three insect herbivores. Environ. Entomol. 2003, 32, 641–651. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Cui, X.; Xu, B. Zinc nutrition increases the antioxidant defenses of honey bees. Entomol. Exp. Appl. 2015, 156, 201–210. [Google Scholar] [CrossRef]
- Roswell, M.; Dushoff, J.; Winfree, R. Male and female bees show large differences in floral preference. PLoS ONE 2019, 14, e0214909. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.H. Adult pollen diet essential for egg maturation by a solitary Osmia bee. J. Insect Physiol. 2016, 95, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, O.; Giejdasz, K.; Wojciechowicz, T.; Skrzypski, M. Ovary growth and protein levels in ovary and fat body during adult-wintering period in the red mason bee, Osmia rufa. Apidologie 2011, 42, 749–758. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, K.S.; Yoon, H.J.; Jin, B.R. Ovarian development and secretion of vitellogenin protein during the wintering period and after emergence in the hornfaced bee, Osmia cornifrons. J. Asia Pac. Entomol. 2015, 18, 515–523. [Google Scholar] [CrossRef]
- Falchuk, K.H. The molecular basis for the role of zinc in developmental biology. Mol. Cell. Biochem. 1998, 188, 41–48. [Google Scholar] [CrossRef]
- Amdam, G.V.; Simões, Z.L.P.; Hagen, A.; Norberg, K.; Schrøder, K.; Mikkelsen, Ø.; Kirkwood, T.B.L.; Omholt, S.W. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef]
- Guidugli, K.R.; Nascimento, A.M.; Amdam, G.V.; Barchuk, A.R.; Omholt, S.; Simões, Z.L.P.; Hartfelder, K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 2005, 579, 4961–4965. [Google Scholar] [CrossRef]
- Levin, M.D.; Bohart, G.E. Selection of pollens by honey bees. Am. Bee J. 1955, 95, 392–393. [Google Scholar]
- Ruszkowski, A.; Biliński, M. Rośliny pokarmowe oraz znaczenie gospodarcze murarek [Food plants and economical importnace of mason bees]. Pszczel. Zesz. Nauk. [J. Apic. Sci.] 1986, 30, 63–87. [Google Scholar]
- Vaudo, A.D.; Patch, H.M.; Mortensen, D.A.; Tooker, J.F.; Grozinger, C.M. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proc. Natl. Acad. Sci. USA 2016, 113, E4035–E4042. [Google Scholar] [CrossRef] [PubMed]
- Hendriksma, H.P.; Shafir, S. Honey bee foragers balance colony nutritional deficiencies. Behav. Ecol. Sociobiol. 2016, 70, 509–517. [Google Scholar] [CrossRef]
- Ruedenauer, F.A.; Raubenheimer, D.; Kessner-Beierlein, D.; Grund-Mueller, N.; Noack, L.; Spaethe, J.; Leonhardt, S.D. Best be(e) on low fat: Linking nutrient perception, regulation and fitness. Ecol. Lett. 2020, 23, 545–554. [Google Scholar] [CrossRef]
- Prather, R.M.; Castillioni, K.; Kaspari, M.; Souza, L.; Prather, C.M.; Reihart, R.W.; Welti, E.A.R. Micronutrients enhance macronutrient effects in a meta-analysis of grassland arthropod abundance. Glob. Ecol. Biogeogr. 2020, 29, 1–16. [Google Scholar] [CrossRef]
- Tamburini, G.; Berti, A.; Morari, F.; Marini, L. Degradation of soil fertility can cancel pollination benefits in sunflower. Oecologia 2016, 180, 581–587. [Google Scholar] [CrossRef]
- Filipiak, M.; Woyciechowski, M.; Czarnoleski, M. Stoichiometric niche, nutrient partitioning and resource allocation in a solitary bee are sex-specific and phosphorous is allocated mainly to the cocoon. Sci. Rep. 2020. In Press. [Google Scholar]

| Treatment | K | Na | Zn | |||
|---|---|---|---|---|---|---|
| Concentration (ppm) | % | Concentration (ppm) | % | Concentration (ppm) | % | |
| Control-Osmia | 6086.2 ± 177.6 | 100 | 100.2 ± 47.6 | 100 | 57.0 ± 3.3 | 100 |
| Control-Apis | 6144.2 ± 111.0 | 101 | 116.4 ± 45.1 | 116 | 55.6 ± 2.1 | 98 |
| K-deficient | 4481.2 ± 131.1 | 74 | 100.8 ± 27.3 | 101 | 53.8 ± 2.6 | 94 |
| Na-deficient | 6127.4 ± 174.2 | 101 | 61.4 ± 26.0 | 61 | 52.8 ± 3.4 | 93 |
| Zn-deficient | 5914.8 ± 376.1 | 97 | 101.0 ± 28.2 | 101 | 34.6 ± 1.1 | 61 |
| Parameter | Sex | Control-Apis | Control-Osmia | K-Deficient | K+ Supplemented | Na-Deficient | Na+ Supplemented | Zn-Deficient | Zn+ Supplemented |
|---|---|---|---|---|---|---|---|---|---|
| Mortality (%) | Female | 7 | 13 | 47 * A | 27 A | 80 * A | 67 * A | 27 A | 7 A |
| Male | 7 | 7 | 60 * A | 47 A | 73 * A | 73 * A | 40 A | 0 B | |
| 2nd cocoon stage (%) | Female | 73 | 74 | 7 * A | 53 B | 0 * A | 27 * A | 53 A | 73 A |
| Male | 66 | 73 | 0 * A | 46 B | 13 * A | 13 * A | 47 A | 73 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipiak, Z.M.; Filipiak, M. The Scarcity of Specific Nutrients in Wild Bee Larval Food Negatively Influences Certain Life History Traits. Biology 2020, 9, 462. https://doi.org/10.3390/biology9120462
Filipiak ZM, Filipiak M. The Scarcity of Specific Nutrients in Wild Bee Larval Food Negatively Influences Certain Life History Traits. Biology. 2020; 9(12):462. https://doi.org/10.3390/biology9120462
Chicago/Turabian StyleFilipiak, Zuzanna M., and Michał Filipiak. 2020. "The Scarcity of Specific Nutrients in Wild Bee Larval Food Negatively Influences Certain Life History Traits" Biology 9, no. 12: 462. https://doi.org/10.3390/biology9120462
APA StyleFilipiak, Z. M., & Filipiak, M. (2020). The Scarcity of Specific Nutrients in Wild Bee Larval Food Negatively Influences Certain Life History Traits. Biology, 9(12), 462. https://doi.org/10.3390/biology9120462






