Sustainable Antibiotic-Free Broiler Meat Production: Current Trends, Challenges, and Possibilities in a Developing Country Perspective
Abstract
Simple Summary
Abstract
1. Introduction
2. Economic and Clinical Importance of Antibiotic-Free Broiler Meat Production
3. Prospects of Antibiotic-Free Broiler Meat Production
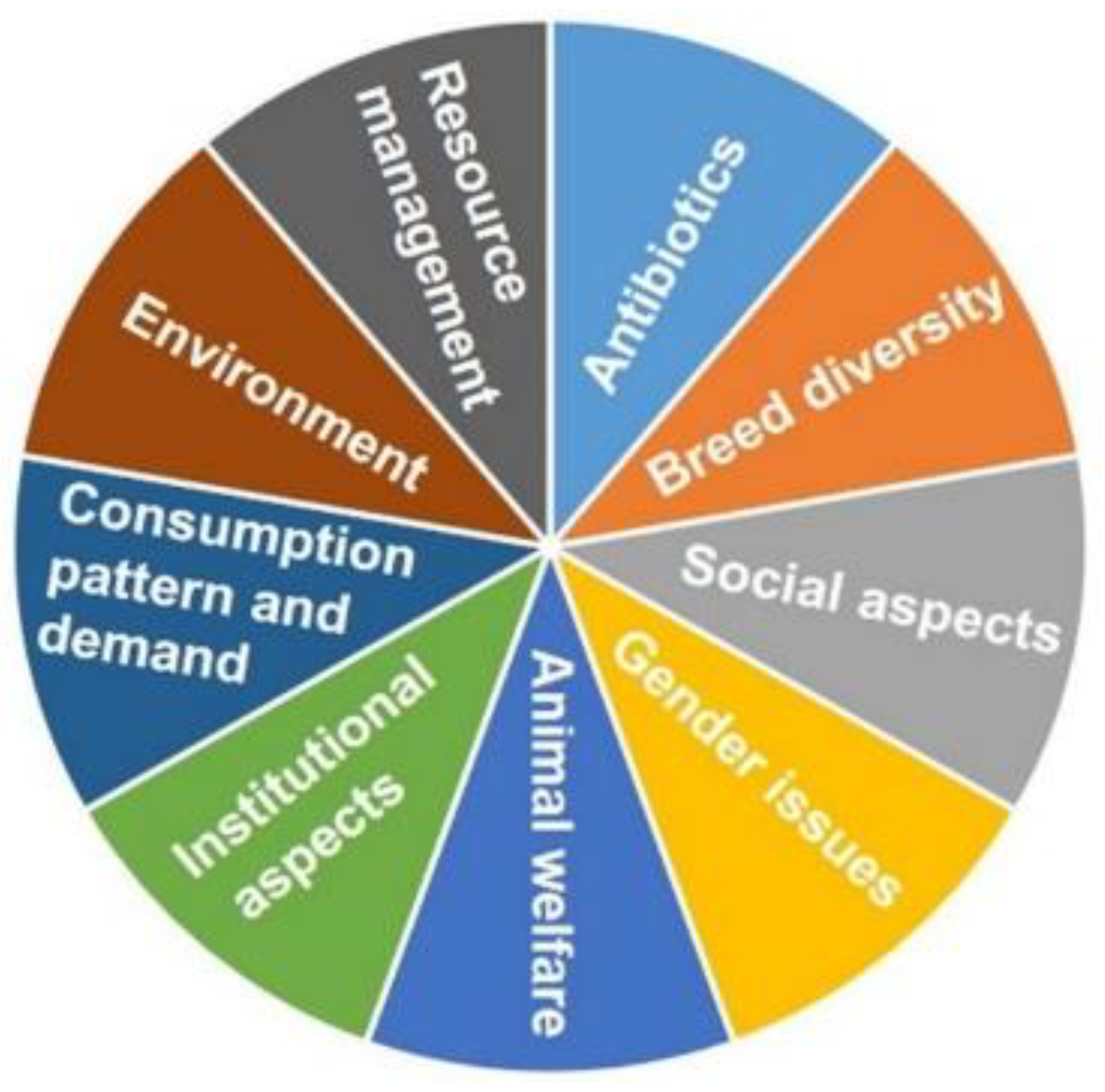
4. Significant Challenges for Sustainable Antibiotic-Free Broilers Production
4.1. Environment
4.2. Resource Management
4.3. Antibiotics
4.4. Breed Diversity
4.5. Social Aspects
4.6. Gender Issue
4.7. Animal Welfare
4.8. Institutional Aspects
4.9. Consumption Pattern and Demand for Poultry Products
5. Possibilities of Antibiotic-Free Broiler Production
5.1. Strict Downtime between Placements
5.2. Optimum Stocking Density
5.3. Good Litter Management
5.4. Control Environment Housing
5.5. Pre-Starter Feed
5.6. Water Quality and Sanitation
5.7. Antibiotic Alternatives
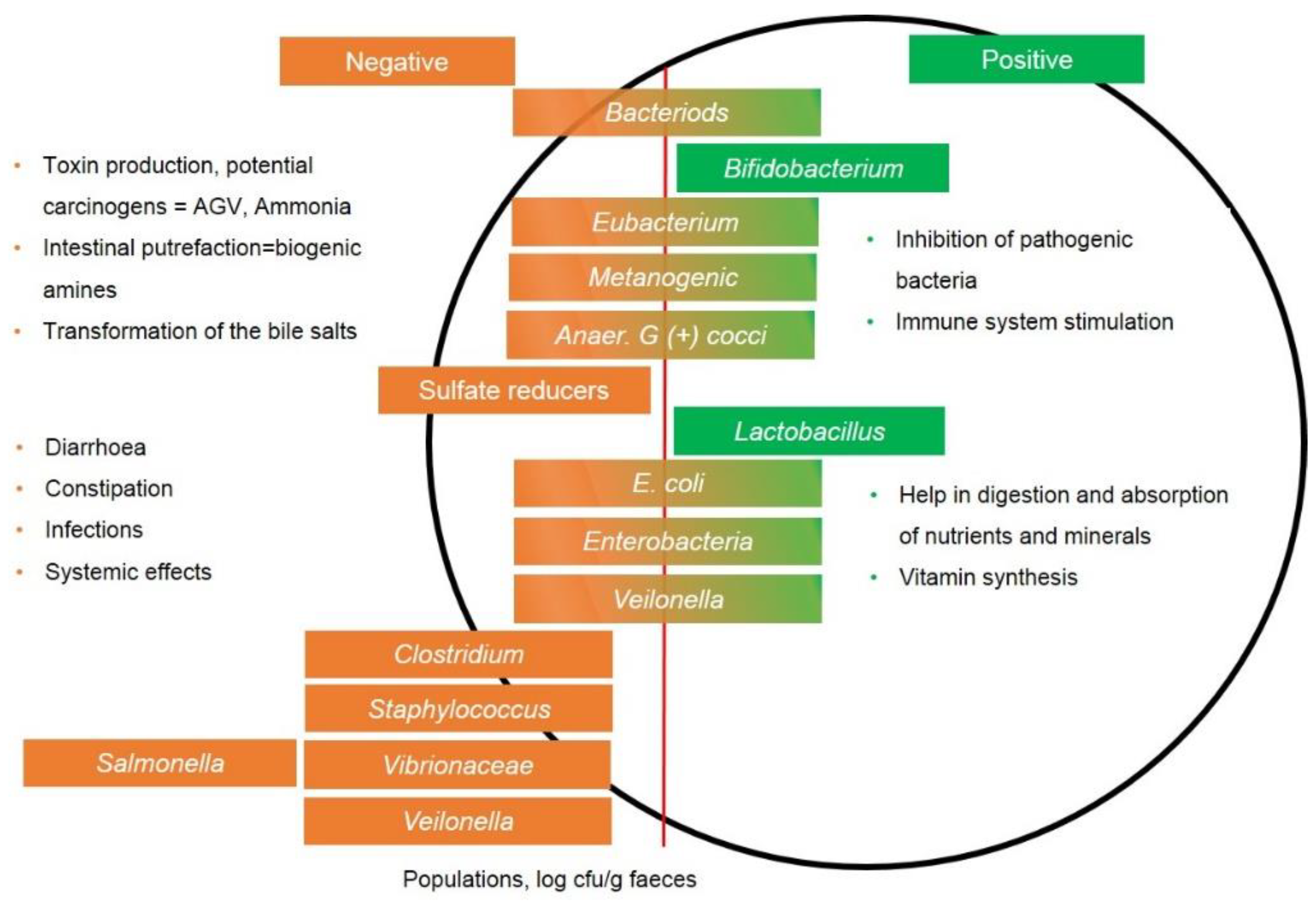
5.7.1. Probiotics and Prebiotics
5.7.2. Organic Acids
5.7.3. Amino Acids and Enzymes
5.7.4. Phytogenic Feed Additives
5.7.5. Nanoparticles as Feed Additives
6. Research Gap, Status, and Future Trends
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Masud, A.A.; Rousham, E.K.; Islam, M.A.; Alam, M.U.; Rahman, M.; Mamun, A.A.; Sarker, S.; Asaduzzaman, M.; Unicomb, L. Drivers of Antibiotic Use in Poultry Production in Bangladesh: Dependencies and Dynamics of a Patron-Client Relationship. Front. Vet. Sci. 2020, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Kryger, K.N.; Thomsen, K.; Whyte, M.; Dissing, M. Smallholder Poultry Production: Livelihoods, Food Security and Sociocultural Significance; Series FAO Smallholder Poultry Production; FAO: Rome, Italy, 2010; p. 4. [Google Scholar]
- Najeeb, A.; Mandal, P.; Pal, U. Efficacy of fruits (red grapes, gooseberry and tomato) powder as natural preservatives in restructured chicken slices. Int. Food Res. J. 2014, 21, 2431–2436. [Google Scholar]
- Tollefson, L.; Miller, M.A. Antibiotic use in food animals: Controlling the human health impact. J. AOAC Int. 2000, 83, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, H.; Collier, C.; Anderson, D. Antibiotics as growth promotants: Mode of action. Anim. Biotechnol. 2002, 13, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef]
- Diaz-Sanchez, S.; Moscoso, S.; Solís de los Santos, F.; Andino, A.; Hanning, I. Antibiotic use in poultry; A driving force for organic poultry production. Food Prot. Trends 2015, 35, 440–447. [Google Scholar]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Hoque, R.; Ahmed, S.M.; Naher, N.; Islam, M.A.; Rousham, E.K.; Islam, B.Z.; Hassan, S. Tackling Antimicrobial Resistance in Bangladesh: A Scoping Review of Policy and Practice in Human, Animal and Environment Sectors. PLoS ONE 2020, 15, e0227947. [Google Scholar] [CrossRef]
- Saiful, I.K.B.M.; Shiraj-Um-Mahmuda, S.; Hazzaz-Bin-Kabir, M. Antibiotic Usage Patterns in Selected Broiler Farms of Bangladesh and their Public Health Implications. J. Public Health Dev. Ctries. 2016, 2, 276–284. [Google Scholar]
- Stutz, M.W.; Lawton, G.C. Effects of diet and antimicrobials on growth, feed efficiency, intestinal Clostridium perfringens, and ileal weight of broiler chicks. Poult. Sci. 1984, 63, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Sirdar, M.M.; Picard, J.; Bisschop, S.; Gummow, B. A questionnaire survey of poultry layer farmers in Khartoum State, Sudan, to study their antimicrobial awareness and usage patterns. Onderstepoort J. Vet. Res. 2012, 79, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.; Hassan, M.M.; Islam, S.K.M.A.; Alam, M.; Faruk, M.S.A.; Chowdhury, S.; Saifuddin, A.K.M. Antibiotic residues in broiler and layer meat in Chittagong district of Bangladesh. Vet. World 2014, 7, 738–743. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zatula, G.; Camels, F.; Debruyme, R.; et al. Antibiotic resistance is ancient. Nat. Lett. 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Van Boeckel, T.; Teillant, A. The Economic Costs of Withdrawing Antimicrobial Growth Promoters from the Livestock Sector. OECD Food Agric. Fish. 2015. [Google Scholar] [CrossRef]
- Islam, M.K.; Uddin, M.F.; Alam, M.H. Challenges and Prospects of Poultry Industry in Bangladesh. Eur. J. Bus. Manag. 2014, 6, 116–127. [Google Scholar]
- Akter, S.; Uddin, M. Bangladesh poultry industry. J. Bus. Technol. 2009, 4, 97–112. [Google Scholar]
- Al-Mamun, M.; Islam, M.; Rahman, M.M. The occurrence of poultry diseases at Kishoregonj district of Bangladesh. MOJ Proteom. Bioinform. 2019, 8, 7–12. [Google Scholar]
- Khaled, S.M.S. Poultry Industry: Realities and Prospects. The Financial Express, International Publication Limited. Available online: http://www.thefinancialexpress-bd.com (accessed on 26 April 2014).
- Hao, H.; Sander, P.; Iqbal, Z.; Wang, Y.; Cheng, G. The Risk of Some Veterinary Antimicrobial Agents on Public Health Associated with Antimicrobial Resistance and their Molecular Basis. Front. Microbiol. 2016, 7, 1626. [Google Scholar] [CrossRef]
- Sarker, Y.A.; Hasan, M.M.; Paul, T.K.; Rashid, S.Z.; Alam, M.N.; Sikder, M.H. Screening of antibiotic residues in chicken meat in Bangladesh by thin layer chromatography. J. Adv. Vet. Anim. Res. 2018, 5, 140–145. [Google Scholar] [CrossRef]
- Sultan, S.; Begum, R.; Rahman, M.A.; Ahmed, M.J.U.; Islam, M.M.; Haque, S. Economic analysis of antibiotics use and vaccine program in commercial broiler farming of Tangail district in Bangladesh. Prog. Agric. 2016, 27, 490–501. [Google Scholar] [CrossRef]
- Faiz, M.A.; Bashe, A. Antimicrobial resistance: Bangladesh experience. Reg. Health Forum. 2011, 15, 1–8. [Google Scholar]
- Ahmed, I.; Rabbi, M.B.; Sultana, S. Antibiotic resistance in Bangladesh: A systematic review. Int. J. Infect. Dis. 2019, 80, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Saleque, M.A. Poultry Industry in Bangladesh: Current Status and Its Challenges and Opportunity in the Emerging Market Environment. In Poul Business. Directory 2007; Khamar Bichitra: Dhaka, Bangladesh, 2007. [Google Scholar]
- Sibi, G.; Shukla, A.; Dhananjaya, K.; Ravikumar, K.R.; Mallesha, H. In vitro antibacterial activities of Broccoli (Brassica oleracea L.var italica) against food borne bacteria. J. App. Pharm. Sci. 2013, 3, 100–103. [Google Scholar]
- Farhana, J.A.; Hossain, M.F.; Mowlah, A. Antibacterial effects of guava (Psidium guajava L.) extracts against food borne pathogens. Int. J. Nutr. Food Sci. 2017, 6, 1–5. [Google Scholar] [CrossRef]
- Carvalho, C.C.R.; Cruz, P.A.; Fonseca, M.M.R.; Xavier-Filho, L. Antibacterial properties of the extract of Abelmoschus esculentus. Biotechnol. Bioproc. Eng. 2011, 16, 971. [Google Scholar] [CrossRef]
- Tijjani, A.; Musa, D.D.; Aliyu, Y. Antibacterial Activity of Garlic (Allium sativum) on Staphylococcus aureus and Escherihia coli. Int. J. Curr. Sci. Stud. 2017, 1, 1410–1703. [Google Scholar]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; De Haan, C. Livestock’s Long Shadow—Environmental Issues and Options; FAO: Rome, Italy, 2006; p. 390. [Google Scholar]
- De Vries, M.; de Boer, I. Comparing environmental impacts for livestock products: A review of life cycle assessments. Livest. Sci. 2010, 128, 1–11. [Google Scholar] [CrossRef]
- Leinonen, I.; Williams, A.G.; Wiseman, J.; Guy, J.; Kyriazakis, I. Predicting the environmental impact of chicken systems in the UK through a life cycle assessment: Broiler production systems. Poult. Sci. 2012, 91, 8–25. [Google Scholar] [CrossRef]
- Leinonen, I.; Williams, A.G.; Wiseman, J.; Guy, J.; Kyriazakis, I. Comparing the environmental impact of alternative protein crops in poultry diets: The consequences of uncertainty. Agric. Syst. 2013, 121, 33–42. [Google Scholar] [CrossRef]
- Gerbens-Leenes, P.W.; Mekonnen, M.M.; Hoekstra, A.Y. The water footprint of poultry, pork and beef: A comparative study in different countries and production systems. Water Resour. Ind. 2013, 1–2, 25–36. [Google Scholar] [CrossRef]
- Magdelaine, P.; Riffard, C.; Berlier, C. Comparative survey of the organic poultry production in the European Union. In Proceedings of the XIIIth European Poultry Conference, Tours, France, 23–27 August 2010; p. 9. [Google Scholar]
- Sundrum, A. Possibilities and Limitations of Protein Supply in Organic Poultry and Pig Production. Report, EU Project No. SSPE-CT-2004-502397. 2005, p. 107. Available online: http://www.organic-revision.org/pub/Final_Report_EC_Revision.pdf (accessed on 25 June 2020).
- Donoghue, D.J. Antibiotic residues in poultry tissues and eggs: Human health concern. Poult. Sci. 2003, 82, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Poole, T.; Sheffield, C. Use and misuse of antimicrobial drugs in poultry and livestock: Mechanisms of antimicrobial resistance. Pak. Vet. J. 2013, 33, 266–271. [Google Scholar]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Archawakulathep, A.; Kim, C.T.T.D.; Meunsene, D.; Handijatno, H.; Hassim, A.; Rovira, H.R.G.; Myint, K.S. Perspectives on antimicrobial resistance in livestock and livestock products in asean countries. Wetchasan Sattawaphaet. 2014, 44, 5–13. [Google Scholar]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Agare, C.; Boamah, V.E.; Zumbi, C.N.; Osei, F.B. Anti-biotic Use in Poultry Production and Its Effects on Bacterial Resis-Tance. In Antimicrobial Resistance—A Global Threa; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Rauw, W.M.; Kanis, E.; Noordhuzen-Stassen, E.N.; Grommers, F.J. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livest. Prod. Sci. 1998, 56, 15–33. [Google Scholar] [CrossRef]
- Coping with Climate Change—The Roles of Genetic Resources for Food and Agriculture. In Commision on Genetic Resources for Food and Agriculture. 2015, p. 110. Available online: http://www.fao.org/3/a-i3866e.pdf (accessed on 25 June 2020).
- Vaarst, M.; Steenfeldt, S.; Horsted, K. Sustainable development perspectives of poultry production. Worlds Poult. Sci. J. 2015, 71, 609–620. [Google Scholar] [CrossRef]
- Le Bouquin, S. Air Quality in Poultry Hatcheries. In Proceedings of the XIVth European Poultry Conference, Stavanger, Norway, 24–27 June 2014. [Google Scholar]
- Quandt, S.A.; Argury-Quandt, A.E.; Lawlor, E.J.; Carrillo, L.; Marín, A.J.; Grzywacz, J.G.; Arcury, T.A. 3-D Jobs and Health Disparities: The Health Implications of Latino Chicken Catchers’ Working Conditions. Am. J. Ind. Med. 2013, 56, 206–215. [Google Scholar] [CrossRef]
- World Livestock 2011–Livestock in Food Security; FAO: Rome, Italy, 2011; p. 115.
- Anonymous. Life Under Contract. 2015. Available online: https://www.farmaid.org/blog/farmer-heroes/life-under-contractpoultry-farming-in-arkansas/ (accessed on 15 July 2020).
- Dolberg, F. Poultry production for livelihood improvement and poverty alleviation. In Poultry in the 21st Century; FAO: Rome, Italy, 2007; p. 26. [Google Scholar]
- Gateway to Poultry Production and Products; FAO: Rome, Italy, 2020.
- Guèye, E.F. Gender issues in family poultry production systems in low-income food-deficit countries. Am. J. Altern. Agric. 2003, 18, 185–195. [Google Scholar]
- Thompson, P.B. Agricultural sustainability: What it is and what it is not. Int. J. Agric. Sustain. 2007, 5, 5–16. [Google Scholar] [CrossRef]
- Nielsen, B.L.; Thomsen, M.G.; Sorensen, P.; Young, J.F. Feed and strain effects on the use of outdoor areas by broilers. Br. Poult. Sci. 2003, 44, 161–169. [Google Scholar] [CrossRef] [PubMed]
- De Jong, I.C.; Enting, H.; Van Voorst, A.; Blokhuis, H.J. Do Low-Density Diets Improve Broiler Breeder Welfare During Rearing and Laying? Poult. Sci. 2005, 84, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Van De Weerd, H.A.; Keatinge, R.; Roderick, S. A review of key health-related welfare issues in organic poultry production. World’s Poult. Sci. J. 2009, 65, 649–684. [Google Scholar] [CrossRef]
- Report on the Expert Consultation on Rural Poultry Development in Asia, Dhaka, Bangladesh; Technology Report 274415; FAO: Rome, Italy, 1987.
- Bilgili, S.F.; Hess, J.B. Placement density influences broiler carcass grade and meat yields. J. Appl. Poult. Res. 1995, 4, 384–389. [Google Scholar] [CrossRef]
- Kristensen, H.H.; Wathes, C.M. Ammonia and poultry welfare: A Review. World’s Poult. Sci. J. 2000, 56, 235–245. [Google Scholar] [CrossRef]
- Miles, D.M.; Branton, S.L.; Lott, B.D. Atmospheric ammonia is detrimental to the performance of modern commercial broilers. Poult. Sci. 2004, 83, 1650–1654. [Google Scholar] [CrossRef]
- Payne, J. Litter management strategies impact nutrient content. Poult. Pract. 2012, 2, 1–3. [Google Scholar]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Verma, K.K.; Singh, V.; Gupta, S.L.; Yadav, J.; Verma, A.K. Environmentally Controlled House-In Poultry Production. Poultry Line. 2014. Available online: https://www.researchgate.net/publication/324483130_Environmentally_Controlled_House-In_Poultry_Production (accessed on 16 October 2020).
- Barekatain, M.R.; Swick, R.A. Composition of more specialised pre-starter and starter diets for young broiler chickens: A review. Anim. Prod. Sci. 2016, 56, 1239–1247. [Google Scholar] [CrossRef]
- Hancock, A.; Hughes, J.; Watkins, S. In search of the ideal water line cleaner. Avian Adv. 2007, 9, 1–3. [Google Scholar]
- Jacobs, L.; Persia, M.E.; Siman-Tov, N.; McCoy, J.; Ahmad, M.; Lyman, J.; Good, L. Impact of water sanitation on broiler chicken production and welfare parameters. J. Appl. Poult. Res. 2020, 29, 258–268. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Kluenter, A. Contribution of exogenous enzymes to potentiate the removal of antibiotic growth promoters in poultry production. Anim. Feed. Sci. Technol. 2019, 250, 81–92. [Google Scholar] [CrossRef]
- Cardinal, K.; Kipper, M.; Andretta, I.; Ribeiro, A.M.L. Withdrawal of antibiotic growth promoters from broiler diets: Performance indexes and economic impact. Poult. Sci. 2019, 98, 6659–6667. [Google Scholar] [CrossRef]
- Engster, H.M.; Marvil, D.; Stewart-Brown, B. The Effect of Withdrawing Growth Promoting Antibiotics from Broiler Chickens: A Long-Term Commercial Industry Study. J. Appl. Poult. Res. 2002, 11, 431–436. [Google Scholar] [CrossRef]
- Dela Cruz, P.J.D.; Dagaas, C.T.; Mangubat, K.M.M.; Angeles, A.A.; Abanto, O.D. Dietary effects of commercial probiotics on growth performance, digestibility, and intestinal morphometry of broiler chickens. Trop. Anim. Health Prod. 2019, 51, 1105–1115. [Google Scholar] [CrossRef]
- Ghasemi, H.A.; Kasani, N.; Taherpour, K. Effects of black cumin seed (Nigella sativa L.), a probiotic, a prebiotic and a synbiotic on growth performance, immune response and blood characteristics of male broilers. Livest. Sci. 2014, 164, 128–134. [Google Scholar] [CrossRef]
- Giannenas, I.; Papadopoulos, E.; Tsalie, E.; Triantafillou, E.; Henikl, S.; Teichmann, K.; Tontis, D. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet. Parasitol. 2012, 188, 31–40. [Google Scholar] [CrossRef]
- Hassanein, S.M.; Soliman, N.K. Effect of probiotic (Saccharomyces cerevisiae) adding to diets on intestinal microflora and performance of hy-line layers hens. J. Am. Sci. 2010, 6, 159–169. [Google Scholar]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microb. 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Levkut, M.; Revajova, V.; Laukova, A.; Sevcikova, Z.; Spisakova, V.; Faixova, Z. Leukocytic Responses and Intestinal Mucin Dynamics of Broilers Protected with Enterococcus Faecium Ef55 and Challenged with Salmonella Enteritidis. Res. Vet. Sci. 2012, 93, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Latha, S.; Vinothini, G.; John Dickson Calvin, D.; Dhanasekaran, D. In vitro probiotic profile based selection of indigenous actinobacterial probiont Streptomyces sp. Jd9 for enhanced broiler production. J. Biosci. Bioeng. 2016, 121, 124–131. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Cho, J.H.; Kim, I.H. Effects of Bacillus subtilis Ubt-Mo2 on growth performance, relative immune Organ weight, gas concentration in excreta, and intestinal microbial shedding in broiler chickens. Livest. Sci. 2013, 155, 343–347. [Google Scholar] [CrossRef]
- Popova, T. Effect of probiotics in poultry for improving meat quality. Curr. Opin. Food Sci. 2017, 14, 72–77. [Google Scholar] [CrossRef]
- Liu, X.; Yan, H.; Lv, L.; Xu, Q.; Yin, C.; Zhang, K.; Wang, P.; Hu, J. Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas J. Anim. Sci. 2012, 25, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Létourneau-Montminy, M.P.; Gaucher, M.L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Brar, S.K.; Côté, C.; Ramirez, A.A.; Godbout, S. Use of antibiotics in broiler production: Global impacts and Alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Jozefiak, D.; Kaczmarek, S.; Rutkowski, A. A note on the effects of selected prebiotics on the performance and ileal microbiota of broiler chickens. J. Anim. Feed Sci. 2008, 17, 392–397. [Google Scholar] [CrossRef]
- Morales-Lopez, R.; Auclair, E.; Garcia, F.; Esteve-Garcia, E.; Brufau, J. Use of yeast cell walls; Beta-1, 3/1, 6-glucans; and mannoproteins in broiler chicken diets. Poult. Sci. 2009, 88, 601–607. [Google Scholar] [CrossRef]
- Zhang, A.W.; Lee, B.D.; Lee, S.K.; Lee, K.W.; An, G.H.; Song, K.B.; Lee, C.H. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult. Sci. 2005, 84, 1015–1021. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.I.; Ricke, S.C. Microbial populations in naked neck chicken ceca raised on pasture flock fed with commercial yeast cell wall prebiotics via an illuminamiseq platform. PLoS ONE 2016, 11, 0151944. [Google Scholar] [CrossRef]
- Baurhoo, B.; Phillip, L.; Ruiz-Feria, C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007, 86, 1070–1078. [Google Scholar] [CrossRef]
- Baurhoo, B.; Ferket, P.R.; Zhao, X. Effects of diets containing different concentrations of manna oligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poult. Sci. 2009, 88, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Kum, S.; Eren, U.; Onol, A.; Sandikci, M. Effects of dietary organic acid supplementation on the intestinal mucosa in broilers. Rev. Med. Vet. 2010, 10, 463–468. [Google Scholar]
- Cherrington, C.A.; Hinton, M.; Mead, G.C.; Chopra, I. Organic acids: Chemistry, antibacterial activity and practical applications. Adv. Microb. Physiol. 1991, 32, 87–108. [Google Scholar]
- Hassan, H.M.A.; Mohamed, M.A.; Youssef, A.W.; Hassan, E.R. Effect of using organic acids to substitute antibiotic growth promoters on performance and intestinal microflora of broilers. Asian-Australas J. Anim. Sci. 2010, 23, 1348–1353. [Google Scholar] [CrossRef]
- Nava, G.M.; Attene-Ramos, M.S.; Gaskins, H.R.; Richards, J.D. Molecular analysis of microbial community structure in the chicken ileum following organic acid supplementation. Vet. Microbiol. 2009, 137, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Adil, S.; Banday, T.; Bhat, G.A.; Mir, M.S.; Rehman, M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2020, 2010, 479485. [Google Scholar] [CrossRef] [PubMed]
- Chaveerach, P.; Keuzenkamp, D.A.; Lipman, L.J.; Van Knapen, F. Effect of organic acids in drinking water for young broilers on Campylobacter infection, volatile fatty acid production, gut microflora and histological cell changes. Poult. Sci. 2004, 83, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Izat, A.L.; Tidwell, N.M.; Thomas, R.A.; Reiber, M.A.; Adams, M.H.; Colberg, M.; Waldroup, P.W. Effects of a buffered propionic acid in diets on the performance of broiler chickens and on microflora of the intestine and carcass. Poult. Sci. 1990, 69, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Mohammadagheri, N.; Najafi, R.; Najafi, G. Effects of dietary supplementation of organic acids and phytase on performance and intestinal histomorphology of broilers. Vet. Res. Forum 2016, 7, 189–195. [Google Scholar]
- Ricke, S.C.; Dittoe, D.K.; Richardson, K.E. Formic Acid as an Antimicrobial for Poultry Production: A Review. Front. Vet. Sci. 2020, 7, 563. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Guo, Y. Effects of dietary sodium butyrate supplementation on the intestinal morphological structure, absorptive function and gut flora in chickens. Anim. Feed Sci. Technol. 2007, 132, 240–249. [Google Scholar] [CrossRef]
- Qaisrani, S.; Van Krimpen, M.; Kwakkel, R.; Verstegen, M.; Hendriks, W. Diet structure, butyric acid, and fermentable carbohydrates influence growth performance, gut morphology, and cecal fermentation characteristics in broilers. Poult. Sci. 2015, 94, 2152–2164. [Google Scholar] [CrossRef] [PubMed]
- Cowieson, A.J.; Hruby, M.; Pierson, E.E. Evolving enzyme technology: Impact on commercial poultry nutrition. Nutr. Res. Rev. 2006, 19, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.; Ross, P.; McAuliffe, O.; O’Mahony, J.; Coffey, A. Recombinant bacteriophage lysins as antibacterials. Bioeng. Bugs 2010, 1, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Del Fiol, F.S.; Jozala, A.; Chaud, M.V. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Volozhantsev, N.V.; Verevkin, V.V.; Bannov, V.A.; Krasilnikova, V.M.; Myakinina, V.P.; Zhilenkov, E.L.; Svetoch, E.A.; Stern, N.J.; Oakley, B.B.; Seal, B.S. The genome sequence and proteome of bacteriophage ΦCPV1 virulent for Clostridium perfringens. Virus Res. 2011, 155, 433–439. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef]
- Frankic, T.; Voljc, M.; Salobir, J.; Rezar, V. Use of herbs and spices and their extracts in animal nutrition. Acta Agric. Slov. 2009, 94, 95–102. [Google Scholar]
- Toghyani, M.; Toghyani, M.; Gheisari, A.; Ghalamkari, G.; Eghbalsaied, S. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immune responses, serum biochemical and haematological parameters in broiler chicks. Livest. Sci. 2011, 138, 167–173. [Google Scholar] [CrossRef]
- Li, H.L.; Zhao, P.Y.; Lei, Y.; Hossain, M.M.; Kim, I.H. Phytoncide, phytogenic feed additive as an alternative to conventional antibiotics, improved growth performance and decreased excreta gas emission without adverse effect on meat quality in broiler chickens. Livest. Sci. 2015, 181, 1–6. [Google Scholar] [CrossRef]
- Mpofu, D.A.; Marume, U.; Mlambo, V.; Hugo, A. The effects of Lippia javanica dietary inclusion on growth performance, carcass characteristics and fatty acid profiles of broiler chickens. Anim. Nutr. 2016, 2, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, A.; Moorthy, M.; Chitra, R.; Prabakar, G. Influence of combinations of fenugreek, garlic, and black pepper powder on production traits of the broilers. Vet. World 2016, 9, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Khattak, F.; Ronchi, A.; Castelli, P.; Sparks, N. Effects of natural blend of essential Oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poult. Sci. 2014, 93, 132–137. [Google Scholar] [CrossRef]
- Peng, Q.Y.; Li, J.D.; Li, Z.; Duan, Z.Y.; Wu, Y.P. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed Sci. Technol. 2016, 214, 148–153. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Bravo, D.; Mirza, M.W.; Rose, S.P. Growth performance and endogenous losses of broilers fed wheat-based diets with and without essential oils and xylanase supplementation. Poult. Sci. 2015, 94, 1227–1232. [Google Scholar] [CrossRef]
- Jerzsele, A.; Szeker, K.; Csizinszky, R.; Gere, E.; Jakab, C.; Mallo, J.J.; Galfi, P. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult. Sci. 2012, 91, 837–843. [Google Scholar] [CrossRef]
- Mahmoud, U.T. Silver Nanoparticles in Poultry Production. J. Adv. Vet. Res. 2012, 2, 303–306. [Google Scholar]
- Gangadoo, S.; Stanley, D.; Hughes, R.J.; Moore, R.J.; Chapman, J. Nanoparticles in feed: Progress and prospects in poultry research. Trends Food Sci Technol. 2016, 58, 115–126. [Google Scholar] [CrossRef]
- Fuxiang, W.; Huiying, R.; Fenghua, Z.; Jinquan, S.; Jianyang, J.; Wenli, L. Effects of Nano-Selenium on the Immune Functions and Antioxidant Abilities of Broiler Chickens. Chin Agric. Sci. Bull. 2008, 2, 831–835. [Google Scholar]
- Hu, C.H.; Li, Y.L.; Xiong, L.; Zhang, H.; Song, J.; Xia, M.S. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim. Feed Sci. Technol. 2012, 177, 204–210. [Google Scholar] [CrossRef]
- Cai, S.J.; Wu, C.X.; Gong, L.M.; Song, T.; Wu, H.; Zhang, L.Y. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult. Sci. 2012, 91, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.; Swain, R.; Mishra, S.; Behera, T.; Swain, P.; Mishra, S.; Behura, N.; Sabat, S.; Sethy, K.; Dhama, K. Effects of dietary nano-selenium on tissue selenium deposition, antioxidant status and immune functions in layer chicks. Int. J. Pharmacol. 2014, 10, 160–167. [Google Scholar] [CrossRef]
- Bagheri, M.; Golchin-Gelehdooni, S.; Mohamadi, M.; Tabidian, A. Comparative effects of nano, mineral and organic selenium on growth performance, immunity responses and total antioxidant activity in broiler chickens. Int. J. Biol. Pharm. Allied Sci. 2015, 4, 583–595. [Google Scholar]
- Selim, N.; Radwan, N.; Youssef, S.; Eldin, T.S.; Elwafa, S.A. Effect of Inclusion Inorganic, Organic or Nano Selenium Forms in Broiler Diets On: 2-Physiological, Immunological and Toxicity Statuses of Broiler Chicks. Int. J. Poult. Sci. 2015, 14, 144. [Google Scholar] [CrossRef]
- Mroczek-Sosnowska, N.; Lukasiewicz, M.; Wnuk, A.; Sawosz, E.; Niemiec, T.; Skot, A.; Jaworski, S.; Chwalibog, A. In ovo administration of copper nanoparticles and copper sulphate positively influences chicken performance. J. Sci. Food Agric. 2015, 96, 3058–3062. [Google Scholar] [CrossRef]
- Akbar, A.; Anal, A.K. Zinc Oxide nanoparticles loaded active packaging, a challenge study against Salmonellas typhymurium and Staphylococcus aureus in ready-to-eat poultry. Food Control. 2013, 38, 88–95. [Google Scholar] [CrossRef]
- Ravikumar, S.; Gokulakrishnan, R. The Inhibitory Effect of Metal Oxide Nanoparticles against Poultry Pathogens. Int. J. Pharm. Sci. Drug Res. 2012, 4, 157–159. [Google Scholar]
- Prasek, M.; Sawosz, E.; Jaworski, S.; Grodzik, M.; Ostaszewska, T.; Kamaszewski, M.; Wierzbicki, M.; Chwalibog, A. Influence of nanoparticles of platinum on chicken embryo development and brain morphology. Nanoscale Res. Lett. 2013, 8, 251. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, G.; Li, L.; Wen, C.; Wang, T.; Zhou, Y. Effect of zinc-bearing zeolite clinoptilolite on growth performance, zinc accumulation, and gene expression of zinc transporters in broilers. J. Anim. Sci. 2015, 93, 620–626. [Google Scholar] [CrossRef]
- Elshuraydeh, K.N.; Al-Beitawi, N.A.; Al-Faqieh, M.A. Effect of Aqueous Nanosuspensions of Clay Minerals on Broilers’ Performance and Some Selected Antibody Titers. J. Nanotechnol. Eng. Med. 2014, 5, 011003. [Google Scholar] [CrossRef]
- Ghasemzadeh, A. Global Issues of Food Production. Agrotechnol 2012, 1, e102. [Google Scholar] [CrossRef]
- Roy, K.J.; Rahman, A.; Hossain, K.S.; Rahman, M.B.; Kafi, M.A. Antibacterial Investigation of Silver Nanoparticle against Staphylococcus, E. coli and Salmonella Isolated from Selected Live Bird Markets. Appl. Microbiol. 2020, 6, 170. [Google Scholar] [CrossRef]
- Andrew Selaledi, L.; Mohammed Hassan, Z.; Manyelo, T.G.; Mabelebele, M. The Current Status of the Alternative Use to Antibiotics in Poultry Production: An African Perspective. Antibiotics 2020, 9, 594. [Google Scholar] [CrossRef]
| Strategy | Specific Action |
|---|---|
| Apply minimum 14 day interval for new flock entry | Reduce the frequency of pathogens |
| Treat feed to reduce bacterial pathogens | Maintain bacterial CFU (colony-forming unit) to <10 after converting finely ground mash feed to capsule or <103 CFU at the farm |
| Process vegetable formulated feed in diet | Obtain low fat and high protein and reduce the likelihood of clostridia contamination |
| Maintain dry litter | Reduce ammonia level and stocking thickness, improve ventilation, increase the distance of shavings, etc. |
| Apply good sanitation program for drinking water | Reduce contamination of bacteria and remove biofilm from the pipelines, regulators, and nipple drinkers |
| Dispose of dead birds regularly | Prevent cannibalism and minimize bacterial contamination |
| Use probiotic supplements for early stages of broiler feed | Establish healthy gut microorganisms and increase growth performance |
| Grind coarser grain to finest | Upgrade the role of gizzard and digestion |
| Supplement with whole grain or grits | Reduce the temporary loss of growth rate and feed efficiency and progress the function of gizzard and digestion |
| Add essential oil extracts to feed | Maintain bacteria at safe levels and improve intestinal health |
| Reduce nonprotein nitrogen by preparing feeds based on digestible amino acids | Inhibit the proliferation of bacteria |
| Utilize ingredients with more soluble fiber | Avoid the deposit of insoluble fiber in the hindgut |
| Use digestible fats and starches | Help suitable digestion, prevent non-starch polysaccharides from getting into the hindgut |
| Lessen the addition of ingredients like wheat, barley, and oats | Minimize gut damage and subsequent enteritis |
| Maintain proper electrolyte balance | Decrease flushing and feed passage |
| Limit feed changes | Reduce disturbances of the gut microflora |
| Add exogenous enzymes | Exploit extraction and digestion of nutrients and minimize the viscosity of digesta |
| Maintain good management practices | Minimize stress |
| Follow good biosecurity practices | Reduce the opportunity for disease |
| Antibiotic Alternatives | Active Ingredients | Basic Functions | Advantages | Disadvantages | Effects on Broiler | References | |
|---|---|---|---|---|---|---|---|
| Probiotics | Bacillus subtilis, Enterococcus faecium, Lactobacillus acidophilus, Bacillus licheniformis, Bifidobacterium bifidum | Appetite and digestion, stimulant, antioxidant | Modulation of immunity Proliferation of beneficial bacteria Increased nutrient absorption No development of resistance Stable | No antibacterial properties | Increased body weight and FCR Improved absorptive surface of duodenum and ileum Increase nutrient retention | Ghasemi et al. [72]; Giannenas et al. [73]; Levkut et al. [76]; Latha et al. [77]; Zhang et al. [78]; Popova [79]; Liu et al. [80] | |
| Prebiotics | Fructo-oligosaccharides (FOS), inulin, galacto-oligosaccharides (GOS), trans-galacto-oligosaccharides (TOS) | Digestion, stimulant | Modulation of immunity Proliferation of beneficial bacteria No development of resistance Stable | No antibacterial properties Unknown nutrient absorption | Increased growth performance Stimulation of metabolic activity in intestine | Jozefiak et al. [82]; Morales-Lopez et al. [83]; Zhang et al. [84]; Baurhoo et al. [86]; Baurhoo et al. [87] | |
| Organic acids | Citric acid | Digestion, stimulant, increased feed efficiency | Antibacterial properties Modulation of immunity Increased nutrient absorption Stable | Development of resistance is rare | Increased body weight Improved ileal nutrient digestibility, cell proliferation, and epithelial and villi height | Kum et al. [88]; Hassan et al. [90]; Nava et al. [91]; Adil et al. [92]; Chaveerach et al. [93]; Izat et al. [94]; Mohammadagheri et al. [95]; Hu and Guo [97]; Qaisrani et al. [98] | |
| Ascorbic acid | |||||||
| Propionic acid and sodium bentonite | |||||||
| Butyrate | |||||||
| Amino acids and enzymes | Phytase, lysins | Digestion, stimulant | Antibacterial properties Modulation of immunity | Improved growth performance | Cowieson et al. [99]; Fenton et al. [100]; Rios et al. [101]; Volozhantsev et al. [102] | ||
| Phytogenic feed additives | Pepper | Piperine | Digestion, stimulant | Modulation of immunity Antibacterial properties Proliferation of beneficial bacteria Increased nutrient absorption No development of resistance Stable | No effect on live performance | Windisch et al. [103]; Frankic et al. [104]; Toghyani et al. [105]; Li et al. [106]; Mpofu et al. [107]; Kirubakaran et al. [108]; Khattak et al. [109]; Peng et al. [110]; Pirgozliev et al. [111]; Jerzsele et al. [112] | |
| Garlic | Allicin | Digestion, stimulant, antiseptic | Higher body weight | ||||
| Ginger | Zingerone | Gastric stimulant | No effects on performance | ||||
| Rosemary | Cineol | Digestion, stimulant, antiseptic, antioxidant | Improved live weight and feed efficiency | ||||
| Thyme | Thymol | Digestion, stimulant, antiseptic, antioxidant | No significant effect on BW/FCR | ||||
| Mint | Menthol | Appetite, digestion, stimulant, antiseptic | Decreased serum total cholesterol, triglycerides, and low-density lipoprotein concentration | ||||
| Nanoparticles (NPs) | Silver NPs | Digestion, Stimulant | Modulation of immunity Antibacterial properties Proliferation of beneficial bacteria Increased nutrient absorption No development of resistance Stable | Some toxicity in broilers | Increased body weight and FCR | Gangadoo et al. [114] | |
| Selenium NPs | Fuxiang et al. [115]; Hu et al. [116]; Cai et al. [117]; Mohapatra et al. [118]; Bagheri et al. [119]; Selim et al. [120] | ||||||
| Copper NPs | Gangadoo et al. [114]; Mroczek-Sosnowska et al. [121] | ||||||
| Metal NPs such as zinc oxide, zirconium dioxide, and platinum | Akbar and Anal [122]; Ravikumar and Gokulakrishnan [123]; Prasek et al. [124] | ||||||
| Zn-bearing zeolite clinoptilolite NPs | Tang et al. [125] | ||||||
| Nanosuspensions of clay minerals | Elshuraydeh [126] | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, M.H.; Sarker, S.; Islam, M.S.; Islam, M.A.; Karim, M.R.; Kayesh, M.E.H.; Shiddiky, M.J.A.; Anwer, M.S. Sustainable Antibiotic-Free Broiler Meat Production: Current Trends, Challenges, and Possibilities in a Developing Country Perspective. Biology 2020, 9, 411. https://doi.org/10.3390/biology9110411
Haque MH, Sarker S, Islam MS, Islam MA, Karim MR, Kayesh MEH, Shiddiky MJA, Anwer MS. Sustainable Antibiotic-Free Broiler Meat Production: Current Trends, Challenges, and Possibilities in a Developing Country Perspective. Biology. 2020; 9(11):411. https://doi.org/10.3390/biology9110411
Chicago/Turabian StyleHaque, Md. Hakimul, Subir Sarker, Md. Shariful Islam, Md. Aminul Islam, Md. Rezaul Karim, Mohammad Enamul Hoque Kayesh, Muhammad J. A. Shiddiky, and M. Sawkat Anwer. 2020. "Sustainable Antibiotic-Free Broiler Meat Production: Current Trends, Challenges, and Possibilities in a Developing Country Perspective" Biology 9, no. 11: 411. https://doi.org/10.3390/biology9110411
APA StyleHaque, M. H., Sarker, S., Islam, M. S., Islam, M. A., Karim, M. R., Kayesh, M. E. H., Shiddiky, M. J. A., & Anwer, M. S. (2020). Sustainable Antibiotic-Free Broiler Meat Production: Current Trends, Challenges, and Possibilities in a Developing Country Perspective. Biology, 9(11), 411. https://doi.org/10.3390/biology9110411






