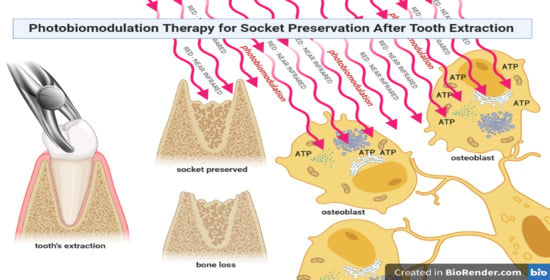
Interaction between Laser Light and Osteoblasts: Photobiomodulation as a Trend in the Management of Socket Bone Preservation—A Review
Abstract
Simple Summary
Abstract
1. Introduction
1.1. The Challenge of Socket Preservation
- Autogenous Grafts: Grafts transferred from one position to another within the same individual. This type of graft comprises cortical bone or cancellous bone and marrow, and is harvested either from intraoral or extraoral donor sites.
- Allogeneic grafts: Grafts transferred between genetically dissimilar members of the same species. Frozen cancellous bone and marrow and freeze-dried bone are used.
- Xenogenic grafts: Grafts taken from a donor of another species.
- Alloplastic materials: Synthetic or inorganic implant materials that are used as substitutes for bone grafts.
- Osteoproliferative action (osteogenetic): New bone is formed by bone-forming cells (osteoblasts) contained in the grafted material; this is typical of autogenous grafts.
- Osteoconductive action: The grafted material does not contribute to new bone formation per se but serves as a scaffold for bone formation originating from the adjacent host bone; this happens with xenogenic and alloplastic grafts.
- Osteoinductive action: Bone formation is induced in the surrounding soft tissue immediately adjacent to the grafted material; the most widely studied type of osteoinductive cell mediator is the bone morphogenetic protein (BMP) family.
1.2. Laser Light–Osteoblast Interaction
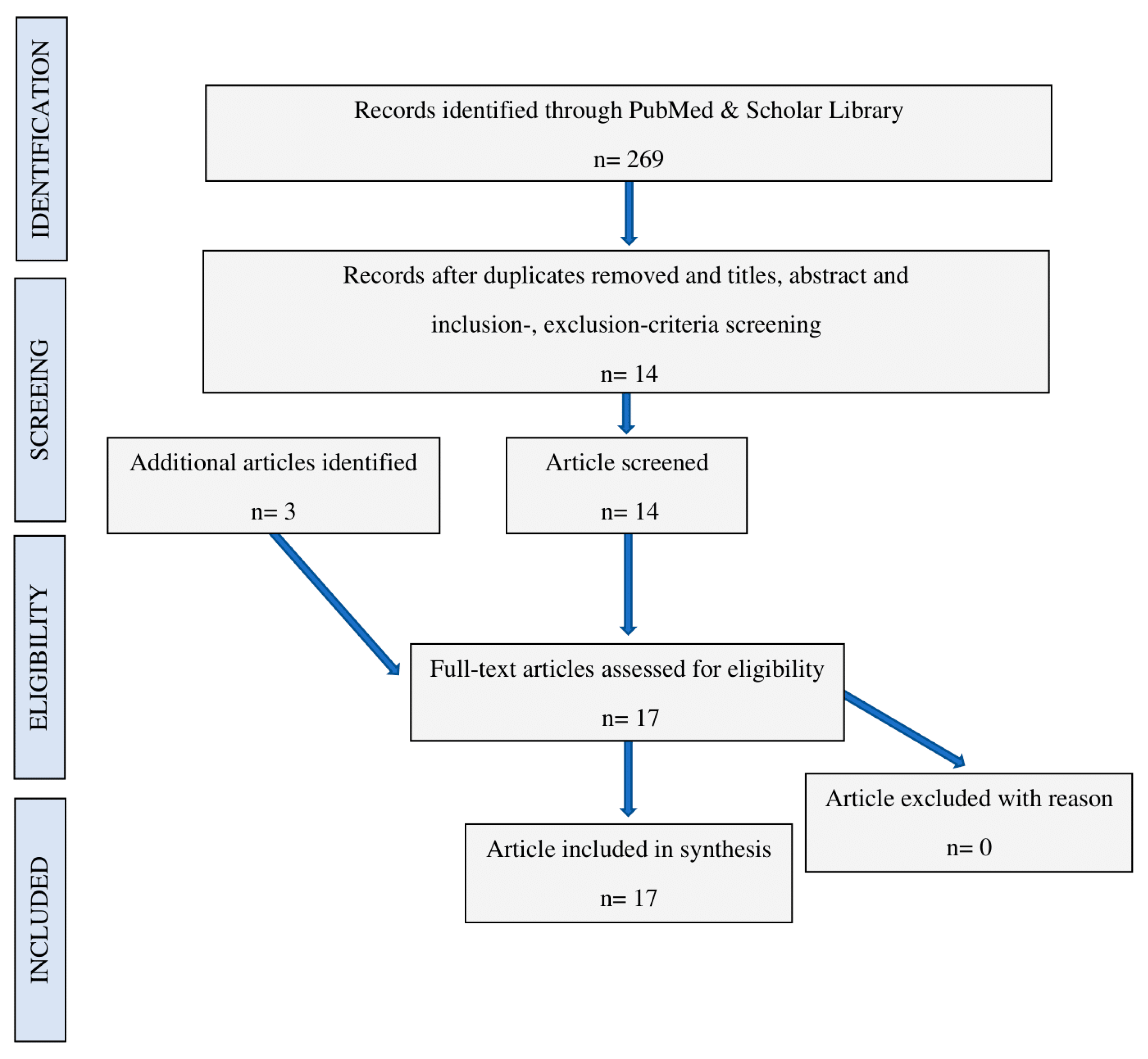
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hanna, R.; Agas, D.; Benedicenti, S.; Ferrando, S.; Laus, F.; Cuteri, V.; Lacava, G.; Sabbieti, M.G.; Amaroli, A. A Comparative Study between the Effectiveness of 980 nm Photobiomodulation Delivered by Hand-Piece with Gaussian vs. Flat-Top Profiles on Osteoblasts Maturation. Front. Endocrinol. 2019, 10, 92. [Google Scholar] [CrossRef]
- Czerwinski, M.; Hopper, R.A.; Gruss, J.; Fearon, J.A. Major Morbidity and Mortality Rates in Craniofacial Surgery: An Analysis of 8101 Major Procedures. Plast. Reconstr. Surg. 2010, 126, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, O.; Linero, I. Regenerative Medicine: A New Paradigm in Bone Regeneration. In Advanced Techniques in Bone Regeneration; IntechOpen: London, UK, 2016; pp. 253–274. [Google Scholar]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology-is it still a “gold standard”? A consutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.J. Sublingual hematoma formation during immediate placement of mandibular endosseous implants. J. Am. Dent. Assoc. 2004, 135, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of all Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Kim, D.M.; De Angelis, N.; Camelo, M.; Nevins, M.L.; Schupbach, P.; Nevins, M. Ridge Preservation with and Without Primary Wound Closure: A Case Series. Int. J. Periodontics Restor. Dent. 2013, 33, 71–78. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Chappuis, V.; Engel, O.; Reyes, M.; Shahim, K.; Nolte, L.-P.; Buser, D. Ridge Alterations Post-extraction in the Esthetic Zone. J. Dent. Res. 2013, 92, 195S–201S. [Google Scholar] [CrossRef]
- De’Angelis, N.; Felice, P.; Pellegrino, G.; Camurati, A.; Gambino, P.; Esposito, M. Guided bone regeneration with and without a bone substitute at single post-extractive implants: 1-year post-loading results from a pragmatic multicentre randomised controlled trial. Eur. J. Oral Implant. 2011, 4, 313–325. [Google Scholar]
- Horváth, A.; Mardas, N.; Mezzomo, L.A.; Needleman, I.G.; Donos, N. Alveolar ridge preservation. A systematic review. Clin. Oral Investig. 2013, 17, 341–363. [Google Scholar] [PubMed]
- Ten Heggeler, J.M.A.G.; Slot, D.E.; Van Der Weijden, F. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: A systematic review. Clin. Oral Implant. Res. 2010, 22, 779–788. [Google Scholar] [CrossRef]
- Lindhe, J.; Lang, N.P. Concept in Periodontal Tissue Regeneration. In Clinical Periodontology and Implant Dentistry, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 536–552. [Google Scholar]
- Ellegaard, B. Bone grafts in periodontal attachment procedures. J. Clin. Periodontol. 1976, 3, 1–54. [Google Scholar] [PubMed]
- Nielsen, I.M.; Ellegaard, B.; Karring, T. KielboneR in healing interradicular lesions in monkeys. J. Periodontal Res. 1980, 15, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Fiorellini, J.P.; Howell, T.H.; Cochran, D.; Malmquist, J.; Lilly, L.C.; Spagnoli, D.; Toljanic, J.; Jones, A.; Nevins, M. Randomized Study Evaluating Recombinant Human Bone Morphogenetic Protein-2 for Extraction Socket Augmentation. J. Periodontol. 2005, 76, 605–613. [Google Scholar] [CrossRef]
- Simpson, B.B.; Niklas, K.J. The Evolutionary Biology of Plants. Syst. Bot. 1997, 22, 727. [Google Scholar] [CrossRef]
- Kream, R.M.; Stefano, G.B.; Snyder, C. Mitochondria, Chloroplasts in Animal and Plant Cells: Significance of Conformational Matching. Med. Sci. Monit. 2015, 21, 2073–2078. [Google Scholar] [CrossRef]
- Karu, T.I. Molecular mechanism of the therapeutic effect of low-intensity laser irradiation. Dokl. Akad. Nauk. SSSR 1986, 291, 1245–1249. [Google Scholar]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. An 808-nm Diode Laser with a Flat-Top Handpiece Positively Photobiomodulates Mitochondria Activities. Photomed. Laser Surg. 2016, 34, 564–571. [Google Scholar] [CrossRef]
- Ravera, S.; Ferrando, S.; Agas, D.; De Angelis, N.; Raffetto, M.; Sabbieti, M.G.; Signore, A.; Benedicenti, S.; Amaroli, A. 1064 nm Nd:YAG laser light affects transmembrane mitochondria respiratory chain complexes. J. Biophotonics 2019, 12, e201900101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, D.; Schwarz, W. TRPV Channels in Mast Cells as a Target for Low-Level-Laser Therapy. Cells 2014, 3, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, S.; Agas, D.; Mirata, S.; Signore, A.; De Angelis, N.; Ravera, S.; Utyuzh, A.S.; Parker, S.; Sabbieti, M.G.; Benedicenti, S.; et al. The 808 nm and 980 nm infrared laser irradiation affects spore germination and stored calcium homeostasis: A comparative study using delivery hand-pieces with standard (Gaussian) or flat-top profile. J. Photochem. Photobiol. B Biol. 2019, 199, 111627. [Google Scholar] [CrossRef]
- Zein, R.; Selting, W.; Hamblin, M.R. Review of light parameters and photobiomodulation efficacy: Dive into complexity. J. Biomed. Opt. 2018, 23, 120901. [Google Scholar] [CrossRef]
- Stein, A.; Benayahu, D.; Maltz, L.; Oron, U. Low-Level Laser Irradiation Promotes Proliferation and Differentiation of Human Osteoblasts in Vitro. Photomed. Laser Surg. 2005, 23, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Saygun, I.; Nizam, N.; Ural, A.U.; Serdar, M.A.; Avcu, F.; Tözüm, T.F. Low-Level Laser Irradiation Affects the Release of Basic Fibroblast Growth Factor (bFGF), Insulin-Like Growth Factor-I (IGF-I), and Receptor of IGF-I (IGFBP3) from Osteoblasts. Photomed. Laser Surg. 2012, 30, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bloise, N.; Ceccarelli, G.; Minzioni, P.; Vercellino, M.; Benedetti, L.; De Angelis, M.G.C.; Imbriani, M.; Visai, L. Investigation of low-level laser therapy potentiality on proliferation and differentiation of human osteoblast-like cells in the absence/presence of osteogenic factors. J. Biomed. Opt. 2013, 18, 128006. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Suzuki, H.; Kitayama, M.; Matsumoto, K.; Kimoto, A.; Shigeoka, M.; Komori, T. The long-term effects of red light-emitting diode irradiation on the proliferation and differentiation of osteoblast-like MC3T3-E1 cells. Kobe J. Med. Sci. 2014, 60, E12–E18. [Google Scholar]
- Oliveira, F.A.; Matos, A.A.; Santesso, M.R.; Tokuhara, C.K.; Leite, A.L.; Bagnato, V.S.; Machado, M.A.; Peres-Buzalaf, C.; De Oliveira, R.C. Low intensity lasers differently induce primary human osteoblast proliferation and differentiation. J. Photochem. Photobiol. B Biol. 2016, 163, 14–21. [Google Scholar] [CrossRef]
- Renno, A.C.M.; McDonnell, P.; Parizotto, N.; Laakso, E.-L. The Effects of Laser Irradiation on Osteoblast and Osteosarcoma Cell Proliferation and Differentiation in Vitro. Photomed. Laser Surg. 2007, 25, 275–280. [Google Scholar] [CrossRef]
- Filho, H.O.S.; Reimer, A.C.; Marcantonio, C.; Marcantonio, É.; Marcantonio, R.A.C. Effects of low-level laser therapy (685 nm) at different doses in osteogenic cell cultures. Lasers Med. Sci. 2011, 26, 539–543. [Google Scholar] [CrossRef]
- Pacheco, P.S.; De Oliveira, F.A.; Oliveira, R.C.; Sant’Ana, A.C.P.; De Rezende, M.L.R.; Greghi, S.L.A.; Damante, C.A. Laser Phototherapy at High Energy Densities Do Not Stimulate Pre-Osteoblast Growth and Differentiation. Photomed. Laser Surg. 2013, 31, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Ateş, G.B.; Ak, A.; Garipcan, B.; Yuksel, S.; Gulsoy, M. Controversial effects of low level laser irradiation on the proliferation of human osteoblasts. In Mechanisms for Low-Light Therapy X; SPIE BiOS: San Francisco, CA, USA, 2015; Volume 9309. [Google Scholar] [CrossRef]
- Ateş, G.B.; Can, A.A.; Gülsoy, M. Investigation of photobiomodulation potentiality by 635 and 809 nm lasers on human osteoblasts. Lasers Med. Sci. 2017, 32, 591–599. [Google Scholar] [CrossRef]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef]
- Mosig, R.A.; Martignetti, J.A. Loss of MMP-2 in murine osteoblasts upregulates osteopontin and bone sialoprotein expression in a circuit regulating bone homeostasis. Dis. Model. Mech. 2012, 6, 397–403. [Google Scholar] [CrossRef]
- Da Silva, A.P.R.B.; Petri, A.D.; Crippa, G.E.; Stuani, A.S.; Stuani, A.S.; Rosa, A.L.; Stuani, M.B. Effect of low-level laser therapy after rapid maxillary expansion on proliferation and differentiation of osteoblastic cells. Lasers Med. Sci. 2011, 27, 777–783. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Xi, S.; Li, X.; Ding, L.; Li, M. The effects of photobiomodulation therapy on mouse pre-osteoblast cell line MC3T3-E1 proliferation and apoptosis via miR-503/Wnt3a pathway. Lasers Med. Sci. 2019, 34, 607–614. [Google Scholar]
- Morsoleto, M.; Sella, V.; Machado, P.; Bomfim, F.; Fernandes, M.H.; Morgado, F.; Filho, G.D.J.L.; Plapler, H.; Lopes, G.D.J. Effect of low power laser in biomodulation of cultured osteoblastic cells of Wistar rats. Acta Cir. Bras. 2019, 34, e201900210. [Google Scholar] [CrossRef]
- Chang, B.; Qiu, H.; Zhao, H.; Yang, X.; Wang, Y.; Ji, T.; Zhang, Y.; Quan, Q.; Li, Y.; Zeng, J.; et al. The Effects of Photobiomodulation on MC3T3-E1 Cells via 630 nm and 810 nm Light-Emitting Diode. Med Sci. Monit. 2019, 25, 8744–8752. [Google Scholar] [CrossRef]
- Coombe, A.R.; Ho, C.-T.G.; Darendeliler, M.A.; Hunter, N.; Philips, J.R.; Chapple, C.C.; Yum, L.W.P. The effects of low level laser irradiation on osteoblastic cells. Clin. Orthod. Res. 2001, 4, 3–14. [Google Scholar] [CrossRef]
- Emes, Y.; Akça, K.; Aybar, B.; Yalçın, S.; Çavuşoğlu, Y.; Baysal, U.; Işsever, H.; Atalay, B.; Vural, P.; Ergüven, M.; et al. Low-level laser therapy vs. pulsed electromagnetic field on neonatal rat calvarial osteoblast-like cells. Lasers Med. Sci. 2012, 28, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Kiyosaki, T.; Mitsui, N.; Mayahara, K.; Omasa, S.; Suzuki, N.; Shimizu, N. Low-intensity laser irradiation stimulates mineralization via increased BMPs in MC3T3-E1 cells. Lasers Surg. Med. 2010, 42, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Kitamura, C.; Fukushima, H.; Nakamichi, I.; Abiko, Y.; Terashita, M.; Jimi, E. Low-level laser irradiation enhances BMP-induced osteoblast differentiation by stimulating the BMP/Smad signaling pathway. J. Cell. Biochem. 2010, 111, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, F.R.C.D.; Sella, V.R.G.; Zanaga, J.Q.; Pereira, N.S.; Nouailhetas, V.L.A.; Plapler, H. RT-PCR standardization and bone mineralization after low-level laser therapy on adult osteoblast cells. Proc. SPIE Int. Soc. Opt. Eng. 2014, 8926, 6. [Google Scholar] [CrossRef]
- Fukuhara, E.; Goto, T.; Matayoshi, T.; Kobayashi, S.; Takahashi, T. Optimal low-energy laser irradiation causes temporal G2/M arrest on rat calvarial osteoblasts. Calcif. Tissue Int. 2006, 79, 443–450. [Google Scholar]
- Saracino, S.; Mozzati, M.; Martinasso, G.; Pol, R.; Canuto, R.A.; Muzio, G. Superpulsed laser irradiation increases osteoblast activity via modulation of bone morphogenetic factors. Lasers Surg. Med. 2009, 41, 298–304. [Google Scholar] [CrossRef]
- Medina-Huertas, R.; Manzano-Moreno, F.J.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; García-Martínez, O.; Ruiz, C. The effects of low-level diode laser irradiation on differentiation, antigenic profile, and phagocytic capacity of osteoblast-like cells (MG-63). Lasers Med. Sci. 2014, 29, 1479–1484. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Fekrazad, R.; Arany, P.; Ye, Q. Molecular impacts of photobiomodulation on bone regeneration: A systematic review. Prog. Biophys. Mol. Biol. 2019, 149, 147–159. [Google Scholar] [CrossRef]
- Mozzati, M.; Martinasso, G.; Cocero, N.; Pol, R.; Maggiora, M.; Muzio, G.; Canuto, R.A. Superpulsed laser therapy on healing process after tooth extraction in patients waiting for liver transplantation. Lasers Med. Sci. 2012, 27, 353–359. [Google Scholar] [CrossRef]
- Mozzati, M.; Martinasso, G.; Cocero, N.; Pol, R.; Maggiora, M.; Muzio, G.; Canuto, R.A. Influence of Superpulsed Laser Therapy on Healing Processes Following Tooth Extraction. Photomed. Laser Surg. 2011, 29, 565–571. [Google Scholar] [CrossRef]
- Park, J.B.; Ahn, S.-J.; Kang, Y.-G.; Kim, E.-C.; Heo, J.S.; Kang, K.L. Effects of increased low-level diode laser irradiation time on extraction socket healing in rats. Lasers Med. Sci. 2013, 30, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Kang, K.L. Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: A pilot study. Lasers Med. Sci. 2012, 27, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Aoki, A.; Mizutani, K.; Lin, T.; Komaki, M.; Shibata, S.; Izumi, Y. High-frequency pulsed low-level diode laser therapy accelerates wound healing of tooth extraction socket: An in vivo study. Lasers Surg. Med. 2016, 48, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Korany, N.S.; Mehanni, S.S.; Hakam, H.M.; El-Maghraby, E.M. Evaluation of socket healing in irradiated rats after diode laser exposure (histological and morphometric studies). Arch. Oral Biol. 2012, 57, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Mergoni, G.; Vescovi, P.; Sala, R.; Merigo, E.; Passerini, P.; Maestri, R.; Corradi, D.; Govoni, P.; Nammour, S.; Bianchi, M.G. The effect of laser therapy on the expression of osteocalcin and osteopontin after tooth extraction in rats treated with zoledronate and dexamethasone. Support. Care Cancer 2015, 24, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, H.; Daigo, Y.; Enoki, N.; Taniguchi, K.; Sato, H. Influence of carbon dioxide laser irradiation on the healing process of extraction sockets. Acta Odontol. Scand. 2010, 69, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.A.; Naif, J.S.; Abdullah, M.A. Effect of Diode Laser on Healing of Tooth Extraction Socket: An Experimental Study in Rabbits. J. Maxillofac. Oral Surg. 2015, 15, 308–314. [Google Scholar] [CrossRef]
- Comunian, C.R.; Custódio, A.L.N.; De Oliveira, L.J.; Dutra, C.E.A.; Neto, M.D.F.; Rezende, C.M.F. Photobiomodulation with LED and laser in repair of mandibular socket rabbit: Clinical evaluation, histological, and histomorphometric. Oral Maxillofac. Surg. 2017, 21, 201–206. [Google Scholar] [CrossRef]
- Hamid, M.A.A.; Zaied, A.A.; Zayet, M.K.; Abdelmageed, H.; Hassan, E.A.; Amaroli, A. Efficacy of Flat-Top Hand-Piece Using 980 nm Diode Laser Photobiomodulation on Socket Healing after Extraction: Split Mouth Experimental Model in Dogs. Photochem. Photobiol. 2020. [Google Scholar] [CrossRef]
- Rosero, K.A.V.; Sampaio, R.M.F.; Deboni, M.; Corrêa, L.; Marques, M.M.; Ferraz, E.P.; Naclério-Homem, M.D.G. Photobiomodulation as an adjunctive therapy for alveolar socket preservation: A preliminary study in humans. Lasers Med. Sci. 2020, 35, 1711–1720. [Google Scholar] [CrossRef]
- Romao, M.M.A.; Marques, M.M.; Cortes, A.; Horliana, A.; Moreira, M.S.; LaScala, C.A. Micro-computed tomography and histomorphometric analysis of human alveolar bone repair induced by laser phototherapy: A pilot study. Int. J. Oral Maxillofac. Surg. 2015, 44, 1521–1528. [Google Scholar] [CrossRef]
- Nica, D.F.; Heredea, E.R.; Todea, D.C.M. Alveolus soft and bone tissue regeneration after laser biomodulation—A histological study. Rom. J. Morphol. Embryol. 2019, 60, 1269–1273. [Google Scholar]
- Monea, A.; Beresescu, G.; Boeriu, S.; Tibor, M.; Popsor, S.; Antonescu, D.M. Bone healing after low-level laser application in extraction sockets grafted with allograft material and covered with a resorbable collagen dressing: A pilot histological evaluation. BMC Oral Health 2015, 15, 134. [Google Scholar] [CrossRef]
- Lancieri, L. A new bone surgical laser technique: Technical aspects and applications in dentistry. Front. Biosci. 2011, 3, 463–468. [Google Scholar] [CrossRef][Green Version]
- Kučcerová, H.; Dostálová, T.; Himmlová, L.; Bártová, J.; Mazánek, J. Low-Level Laser Therapy after Molar Extraction. J. Clin. Laser Med. Surg. 2000, 18, 309–315. [Google Scholar] [CrossRef]
- Mester, E.; Szende, B.; Gärtner, P. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother. 1968, 9, 621–626. [Google Scholar]
- Deana, A.M.; De Souza, A.M.; Teixeira, V.P.; Mesquita-Ferrari, R.A.; Bussadori, S.K.; Fernandes, K.P.S. The impact of photobiomodulation on osteoblast-like cell: A review. Lasers Med. Sci. 2018, 33, 1147–1158. [Google Scholar] [CrossRef]
- Escudero, J.S.B.; Perez, M.G.B.; Rosso, M.P.D.O.; Buchaim, D.V.; Pomini, K.T.; Campos, L.M.G.; Audi, M.; Buchaim, R.L. Photobiomodulation therapy (PBMT) in bone repair: A systematic review. Int. J. Care Inj. 2019, 50, 1853–1867. [Google Scholar] [CrossRef]
- Babuccu, C.; Keklikoglu, N.; Baydoğan, M.; Kaynar, A. Cumulative effect of low-level laser therapy and low-intensity pulsed ultrasound on bone repair in rats. Int. J. Oral Maxillofac. Surg. 2014, 43, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Tim, C.R.; Bossini, P.S.; Kido, H.W.; Malavazi, I.; Kress, M.R.V.Z.; Carazzolle, M.F.; Parizotto, N.A.; Rennó, A.C. Effects of low level laser therapy on inflammatory and angiogenic gene expression during the process of bone healing: A microarray analysis. J. Photochem. Photobiol. B Biol. 2016, 154, 8–15. [Google Scholar] [CrossRef]
- Gonçalves, J.B.D.O.; Buchaim, D.V.; Bueno, C.R.D.S.; Pomini, K.T.; Barraviera, B.; Júnior, R.S.F.; Andreo, J.C.; Rodrigues, A.D.C.; Cestari, T.M.; Buchaim, R.L. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J. Photochem. Photobiol. B Biol. 2016, 162, 663–668. [Google Scholar] [CrossRef]
- De Vasconcellos, L.M.R.; Barbara, M.A.M.; Rovai, E.S.; França, M.D.O.; Ebrahim, Z.F.; De Vasconcellos, L.G.O.; Porto, C.D.; Cairo, C.A.A. Titanium scaffold osteogenesis in healthy and osteoporotic rats is improved by the use of low-level laser therapy (GaAlAs). Lasers Med. Sci. 2016, 31, 899–905. [Google Scholar] [CrossRef]
- Pinto, K.N.; Tim, C.R.; Crovace, M.C.; Matsumoto, M.A.; Parizotto, N.A.; Zanotto, E.D.; Peitl, O.; Rennó, A.C. Effects of biosilicate® scaffolds and low-level laser therapy on the process of bone healing. Photomed. Laser Surg. 2013, 31, 252–260. [Google Scholar] [PubMed]
- Batista, J.D.; Zanetta-Barbosa, D.; Cardoso, S.-V.; Dechichi, P.; Rocha, F.S.; Pagnoncelli, R.M. Effect of low-level laser therapy on repair of the bone compromised by radiotherapy. Lasers Med. Sci. 2014, 29, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Acar, A.H.; Yolcu, Ü.; Altındiş, S.; Gül, M.; Alan, H.; Malkoç, S. Bone regeneration by low-level laser therapy and low-intensity pulsed ultrasound therapy in the rabbit calvarium. Arch. Oral Biol. 2016, 61, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Meer, M.; George, R. Efficacy of photobiomodulation on accelerating bone healing after tooth extraction: A systematic review. Lasers Med. Sci. 2018, 34, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Hillier, M.L.; Bell, L.S. Differentiating Human Bone from Animal Bone: A Review of Histological Methods. J. Forensic Sci. 2007, 52, 249–263. [Google Scholar] [CrossRef]
- Viger, M.L.; Sheng, W.; Doré, K.; Alhasan, A.H.; Carling, C.-J.; Lux, J.; Lux, C.D.G.; Grossman, M.; Malinow, R.; Almutairi, A. Near-Infrared-Induced Heating of Confined Water in Polymeric Particles for Efficient Payload Release. ACS Nano. 2014, 8, 4815–4826. [Google Scholar] [CrossRef]
- Fantarella, D.; Kotlow, L. The 9.3-μm CO2 dental laser: Technical development and early clinical experiences. J. Laser Dent. 2014, 22, 10–27. [Google Scholar]
- Pandeshwar, P.; Roa, M.D.; Das, R.; Shastry, S.P.; Kaul, R.; Srinivasreddy, M.B. Photobiomodulation in oral medicine: A review. J. Investig. Clin. Dent. 2015, 7, 114–126. [Google Scholar] [CrossRef]
- Tunér, J.; Jenkins, P.A. Parameter Reproducibility in Photobiomodulation. Photomed. Laser Surg. 2016, 34, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. 808-nm laser therapy with a flat-top handpiece photobiomodulates mitochondria activities of Paramecium primaurelia (Protozoa). Lasers Med. Sci. 2016, 31, 741–747. [Google Scholar] [CrossRef] [PubMed]
| Model Employed | Teeth Extracted | Wavelength and Device | Parameters Irradiated | Therapy Administered | Methods of Detection | Effect of PBM |
|---|---|---|---|---|---|---|
| Rat [54] | First molars | 980 nm diode with 300 μm fiber | Power = 0.01 W; Energy 0.6 J; Power density = 0.23 W/cm2; fluence = 13.95 for 60 s of irradiation; irradiation time: 60 s or 120 s or 300 s; continuous wave mode of irradiation (CW); spot size area = 0.043 cm2 | For 3 and 7 days, daily | Observation period = 3 and 7 days Real-time PCR: Runx2, Col-1, osteocalcin, PDGF-B, VEGF | improvement |
| Rat [55] with diabetes | First molars | 980 nm diode with 300 μm fiber | 0.01 W; 0.23 W/cm2; 13.95 J/cm2; 60 s; CW; 0.043 cm2 | from 3 to 14 day, daily | Observation period = 3,5,7,14 days Real-time PCR: Runx2, collagen type 1, osteocalcin, GAPDH/ Histomorphometric analyses | improvement |
| Rat [56] | First molars | 904-910 nm diode with 5.9 mm probe | 0.2 W; 0.73 W/cm2; 43.8 J/cm2; 60 s; pulsed mode of irradiation (PM); 30 kHz; 0.087 cm2 | For 3 and 5 days, daily | Observation period = 3 and 7 days Real-time PCR: Col-1, Alp, Runx2, osteocalcin, and BMP-2; PCNA-positive cells/Micro-CT analysis/ Histomorphometric analyses | improvement |
| Rat [57] treated with γ-ray | First molars | 830 nm diode with 18 mm probe | 75 mW; CW; | Not clearly documented | Observation period = 3,7,10 days Histomorphometric analyses | improvement |
| Rat [58] treated with bisphosphonates | First molar (left) | 1064 nm Nd:YAG with 320 μm fiber | 1.25 W; 268.8 W/cm2; 14.37 J/cm2; 300 s; very short pulsed mode of irradiation, 15 Hz | For 6 day, every other day | Observation period = 8 days Western blot analysis: osteocalcin; osteopontin | improvement |
| Rat [59] | First molar (left) | 10,600 nm CO2 with laser tip | 1 W; 55 W/cm2; 40 J/cm2; 15 s; PM; 0.018 cm2 | The day after surgery | Observation period = 3 and 7 days Histomorphometric analyses | improvement |
| Rabbit [60] | First premolars | 808 nm diode irradiation probe = no specified | 0.9 W; 5 W/cm2; 1459 J/cm2; 300 s; CW; 0.18 cm2 | Immediately and every 72 h for 12 days | Observation period = 7,14,30,45 days Histomorphometric analyses | improvement |
| Rabbit [61] | Low inferior first premolar (right) | 830 nm LED; 780 nm diode irradiation probe = no-specified | 830 nm = 30 J/cm2; 150 s; CW 780 nm = 30 J/cm2; 50 s; CW | 48h after surgery and then 9 irradiations | Observation period = 90 days Evaluation of impacted area (infection, hyperemia, oedema) Micro-CT analysis | 830 nm = improvement 780 nm = no-effect |
| Dog [62] | Third premolar | 980nm diode laser with flat-top hand-piece | 0.60 W; 0.77 W/cm2; 36 J; 46 J/cm2; 60 s; CW | Immediately and every 48h for 14-days | Observation period = 3-, 4- and 5-weeks cone-beam computed tomography | improvement |
| Model Employed | Teeth Extracted | Wavelength and Device | Parameters Irradiated | Therapy Administered | Methods of Detection | Effect of PBM |
|---|---|---|---|---|---|---|
| Human [63] | First and second molars | 808 nm diode irradiation probe = no specified | Power = 0.1 W; Power density = 3.6 W/cm2; fluence = 89 J/cm2; irradiation time: 25 s; continuous wave mode of irradiation (CW); spot size area = 0.028 cm2 | Irradiated at day 0, 1, 2, 3, 4, 7, 15, in 5 points (2 vestibulars, 1 occlusal, 2 linguals) | Observation period = 45 days Micro-computed-tomography (mCT) Histomorphometric analysis | improvement |
| Human [64] | Lower molars | 808 nm diode irradiation probe = no specified | 0.1 W; 2.5 W/cm2; 75 J/cm2; 30 s; CW; 0.04 cm2 | Irradiated at day 0, 1, 2, 3, 4, 5, 7, in 5 points (2 vestibulars, 1 occlusal, 2 linguals) | Observation period = 40 days Micro-computed-tomography (mCT) Histomorphometric analysis | improvement |
| Human [65] | molars | 940 nm diode irradiation probe = no specified | 0.9 W; 36 J; 80 s; CW | Irradiated at day 0, 1, 2, 3, 4, 7, in 2 points (1 buccal, 1 lingual) | Observation period = 56 days Histomorphometric analysis | improvement |
| Human [52] with hepatic disease | Molars | 904-910 nm diode irradiation probe = no specified | 0.2 W; 0.2 W/cm2; 180 J/cm2; 900 s; 1 cm2; super-pulsed (SP) 200 ns; 3 kHz | Irradiated at day 0, 3 and 5 | Observation period = 7 days. Real-time PCR = IL-1B; IL-6; IL-10; Col-1; Col-3; TGF-B2; COX-2; BMP-4; BMP-7; PPAR-B | Inflammation = improved Bone = no-effect |
| Human [53] | molars | 904-910 nm diode irradiation probe = no specified | 0.2 W; 0.2 W/cm2; 180 J/cm2; 900 s; 1 cm2; SP 200 ns; 3 kHz | Irradiated at day 0, 3 and 5 | Observation period = 7 days. Real-time PCR = IL-1B; IL-6; IL-10; Col-1; Col-3; TGF-B2; COX-2; BMP-4; BMP-7; PPAR-B | Inflammation = improved Bone = no-effect |
| Human [66] treated with allograft | Anterior, posterior, with roots fused teeth | Osseo-pulsed phototherapy | 0.02 W/cm2; 1200 s | Irradiated for 21 days, daily | Observation period = 60 days Histomorphometric analysis | Improvement |
| Human [67] treated with biomaterial | Molars and premolars | 810 nm diode | 1 W; 1 W/cm2; 50 J/cm2; 50 s; 1 cm2; CW | Irradiated at day 0, 3, 5 and 7 | Observation period = 4 months. Radiographs Histomorphometric analysis | Improvement |
| Human [68] | Molars | 670 nm diode 632.8 nm He-Ne | 670 nm = 20 mW, 1.5 J/cm2; 292 Hz, 9000 Hz, 20 mW, 1.5 mW; 5 Hz, 20 mW, 1.5 J/cm2 632.8 nm = 5 mW, 5 Hz, 1.5 J/cm2 | Irradiated for 4 days, daily | Observation period = 6 months. Bone density with digital radiovisiography. Protein staining = immunoglobulin A, albumin | No-effect on bone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaroli, A.; Colombo, E.; Zekiy, A.; Aicardi, S.; Benedicenti, S.; De Angelis, N. Interaction between Laser Light and Osteoblasts: Photobiomodulation as a Trend in the Management of Socket Bone Preservation—A Review. Biology 2020, 9, 409. https://doi.org/10.3390/biology9110409
Amaroli A, Colombo E, Zekiy A, Aicardi S, Benedicenti S, De Angelis N. Interaction between Laser Light and Osteoblasts: Photobiomodulation as a Trend in the Management of Socket Bone Preservation—A Review. Biology. 2020; 9(11):409. https://doi.org/10.3390/biology9110409
Chicago/Turabian StyleAmaroli, Andrea, Esteban Colombo, Angelina Zekiy, Stefano Aicardi, Stefano Benedicenti, and Nicola De Angelis. 2020. "Interaction between Laser Light and Osteoblasts: Photobiomodulation as a Trend in the Management of Socket Bone Preservation—A Review" Biology 9, no. 11: 409. https://doi.org/10.3390/biology9110409
APA StyleAmaroli, A., Colombo, E., Zekiy, A., Aicardi, S., Benedicenti, S., & De Angelis, N. (2020). Interaction between Laser Light and Osteoblasts: Photobiomodulation as a Trend in the Management of Socket Bone Preservation—A Review. Biology, 9(11), 409. https://doi.org/10.3390/biology9110409