The Effect of a 13-Valent Conjugate Pneumococcal Vaccine on Circulating Antibodies Against Oxidized LDL and Phosphorylcholine in Man, A Randomized Placebo-Controlled Clinical Trial
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccination Schedule
2.2. Antibody Measurements
2.3. Power Calculation
2.4. Statistical Analysis
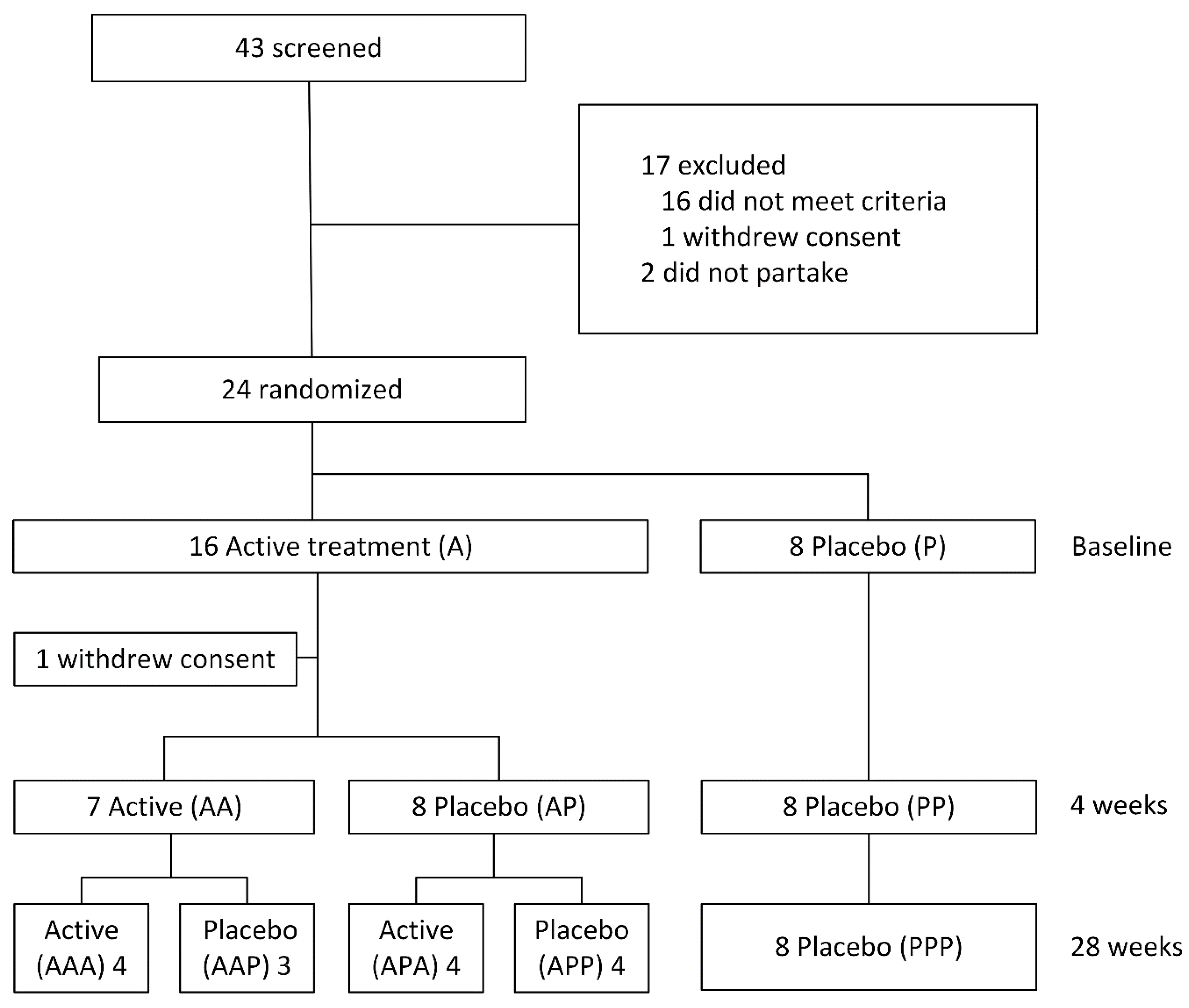
3. Results
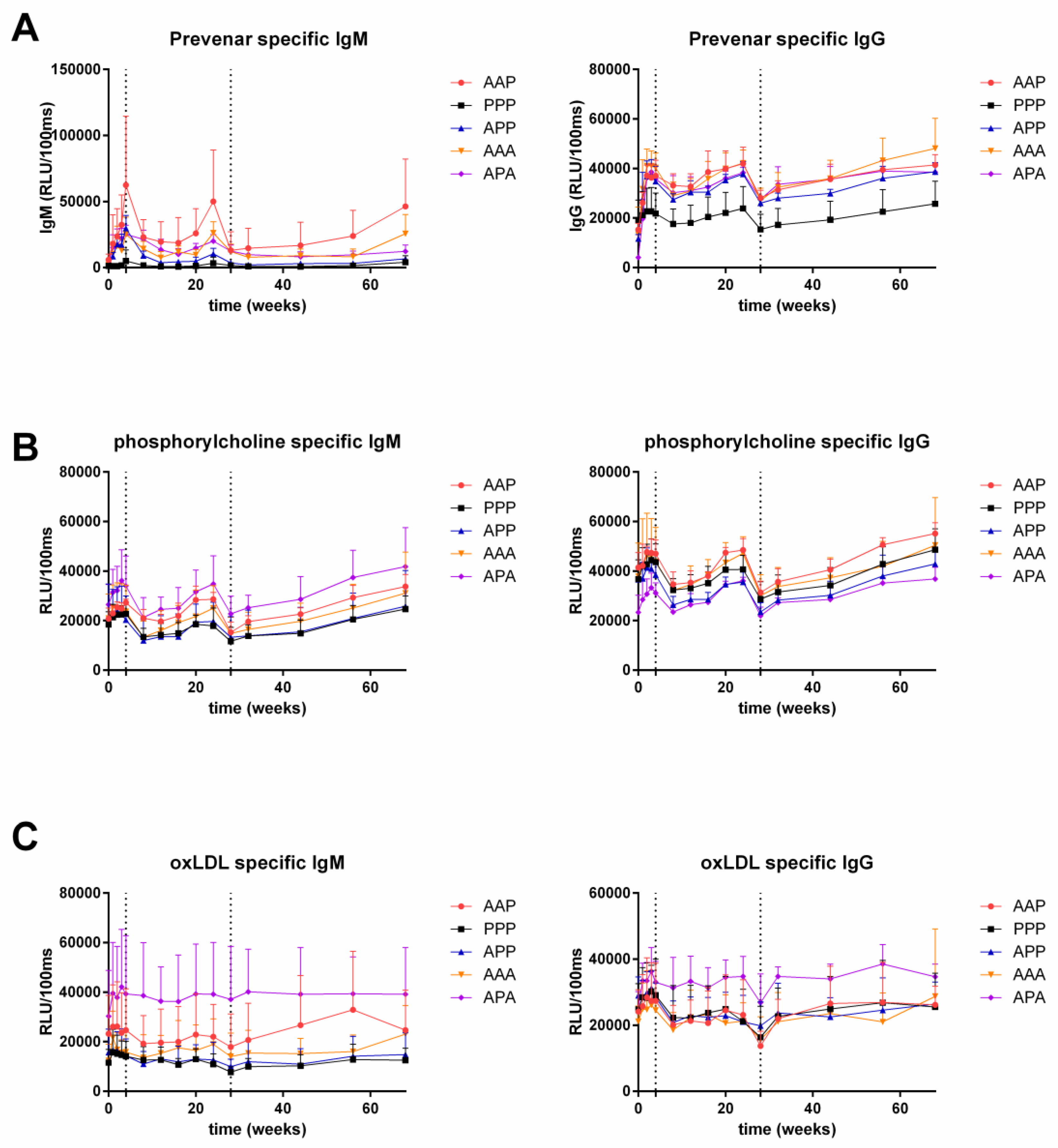
3.1. Anti-Prevenar Antibodies
3.2. Anti-OxLDL and Anti-PC Antibodies
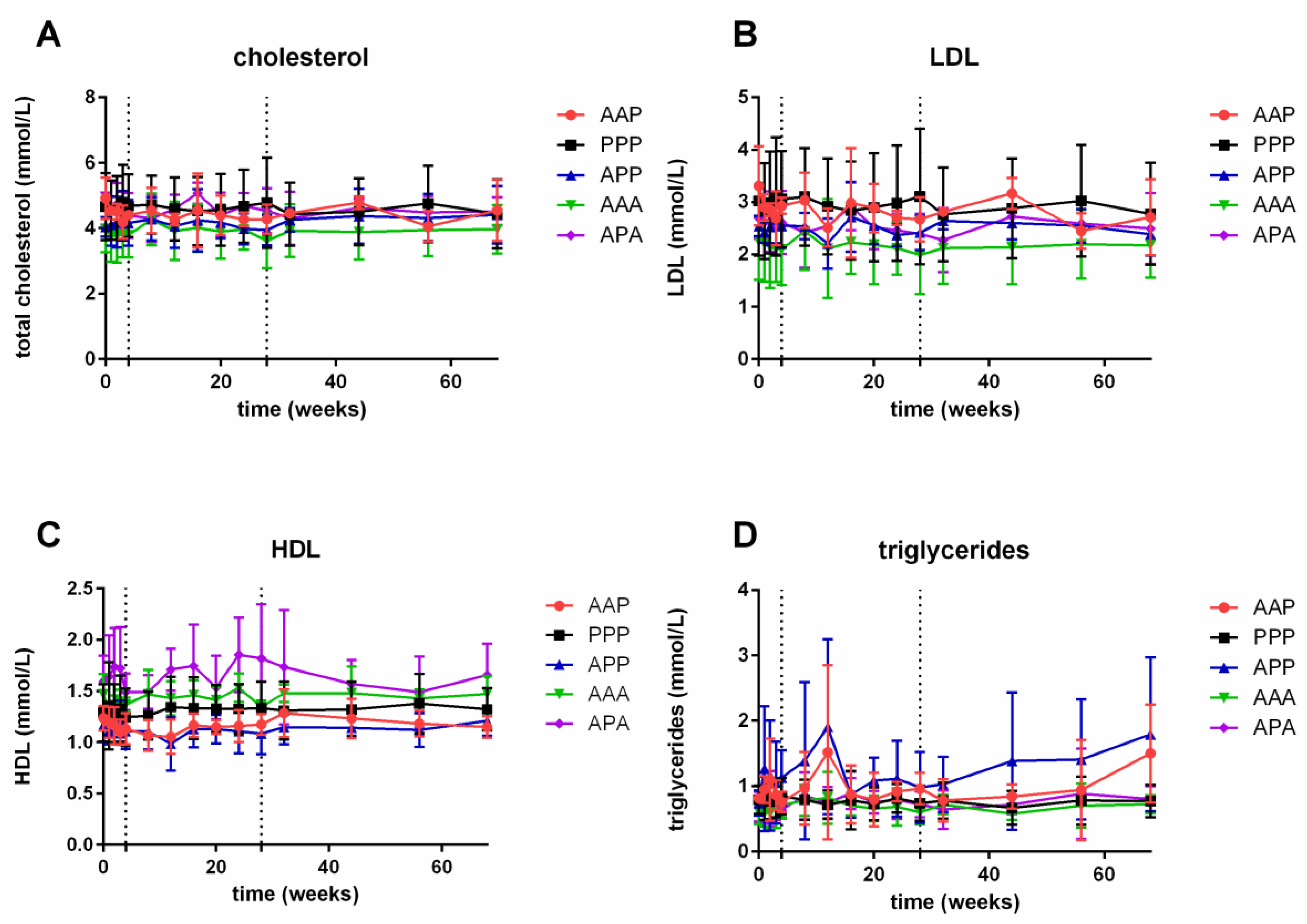
3.3. Lipids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lusis, A.J. Atherosclerosis. Nat. Cell Biol. 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Ketelhuth, D.F.; Hansson, G.K. Cellular immunity, low-density lipoprotein and atherosclerosis: Break of tolerance in the artery wall. Thromb. Haemost. 2011, 106, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.X.; Hörkkö, S.; Chang, M.-K.; Curtiss, L.K.; Palinski, W.; Silverman, G.J.; Witztum, J.L. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Investig. 2000, 105, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, M.; Kemna, M.J.; De Winther, M.P.J.; Boon, L.; Duijvestijn, A.M.; Henatsch, D.; Bos, N.A.; Gijbels, M.J.J.; Tervaert, J.W.C. Passive Immunization with Hypochlorite-oxLDL Specific Antibodies Reduces Plaque Volume in LDL Receptor-Deficient Mice. PLoS ONE 2013, 8, e68039. [Google Scholar] [CrossRef]
- Egrönwall, C.; Evas, J.; Silverman, G.J. Protective Roles of Natural IgM Antibodies. Front. Immunol. 2012, 3, 66. [Google Scholar] [CrossRef]
- Litvack, M.L.; Post, M.; Palaniyar, N. IgM Promotes the Clearance of Small Particles and Apoptotic Microparticles by Macrophages. PLoS ONE 2011, 6, e17223. [Google Scholar] [CrossRef]
- Chang, M.-K.; Bergmark, C.; Laurila, A.; Hörkkö, S.; Han, K.-H.; Friedman, P.; Dennis, E.A.; Witztum, J.L. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: Evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA 1999, 96, 6353–6358. [Google Scholar] [CrossRef]
- Hörkkö, S.; Bird, D.A.; Miller, E.; Itabe, H.; Leitinger, N.; Subbanagounder, G.; Berliner, J.A.; Friedman, P.; Dennis, E.A.; Curtiss, L.K.; et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid–protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Investig. 1999, 103, 117–128. [Google Scholar] [CrossRef]
- Iseme, R.A.; McEvoy, M.; Kelly, B.J.; Agnew, L.L.; Walker, F.R.; Handley, T.; Oldmeadow, C.; Attia, J.; Boyle, M. A role for autoantibodies in atherogenesis. Cardiovasc. Res. 2017, 113, 1102–1112. [Google Scholar] [CrossRef]
- Karvonen, J.; Päivänsalo, M.; Kesäniemi, Y.A.; Hörkkö, S. Immunoglobulin M Type of Autoantibodies to Oxidized Low-Density Lipoprotein Has an Inverse Relation to Carotid Artery Atherosclerosis. Circulation 2003, 108, 2107–2112. [Google Scholar] [CrossRef]
- Su, J.; Georgiades, A.; Wu, R.; Thulin, T.; De Faire, U.; Frostegård, J. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis 2006, 188, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Brilakis, E.S.; Lennon, R.J.; Miller, E.R.; Witztum, J.L.; McConnell, J.P.; Kornman, K.S.; Berger, P.B. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 2006, 48, 425–433. [Google Scholar] [CrossRef]
- Soto, Y.; Condé, H.; Aroche, R.; Brito, V.; Luaces, P.; Nasiff, A.; Obregón, A.; Vazquez, A. Abstract: P821 Autoantibodies to oxidized low density lipoprotein in relation with coronary artery disease. Atherosclerosis Suppl. 2009, 10, e1210. [Google Scholar] [CrossRef]
- Oksjoki, R.; Kovanen, P.T.; Lindstedt, K.A.; Jansson, B.; Pentikäinen, M.O. OxLDL–IgG Immune Complexes Induce Survival of Human Monocytes. Arter. Thromb. Vasc. Biol. 2006, 26, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, J.; Lindstedt, K.A.; Oksjoki, R.; Kovanen, P.T. OxLDL–IgG immune complexes induce expression and secretion of proatherogenic cytokines by cultured human mast cells. Atherosclerosis 2011, 214, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Laczik, R.; Szodoray, P.; Veres, K.; Szomják, E.; Csípő, I.; Sipka, S.; R, Y.S.; Szekanecz, Z.; Soltész, P. Assessment of IgG antibodies to oxidized LDL in patients with acute coronary syndrome. Lupus 2011, 20, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Nishizawa, Y.; Fukumoto, M.; Shimamura, K.; Kimura, J.; Kanda, H.; Emoto, M.; Kawagishi, T.; Morii, H. Inverse relationship between circulating oxidized low density lipoprotein (oxLDL) and anti-oxLDL antibody levels in healthy subjects. Atherosclerosis 2000, 148, 171–177. [Google Scholar] [CrossRef]
- Zhou, X.; Caligiuri, G.; Hamsten, A.; Lefvert, A.K.; Hansson, G.K. LDL Immunization Induces T-Cell–Dependent Antibody Formation and Protection Against Atherosclerosis. Arter. Thromb. Vasc. Biol. 2001, 21, 108–114. [Google Scholar] [CrossRef]
- Binder, C.J.; Hörkkö, S.; Dewan, A.; Chang, M.-K.; Kieu, E.P.; Goodyear, C.S.; Shaw, P.X.; Palinski, W.; Witztum, J.L.; Silverman, G.J. Pneumococcal vaccination decreases atherosclerotic lesion formation: Molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 2003, 9, 736–743. [Google Scholar] [CrossRef]
- Caligiuri, G.; Khallou-Laschet, J.; Vandaele, M.; Gaston, A.-T.; Delignat, S.; Mandet, C.; Kohler, H.V.; Kaveri, S.V.; Nicoletti, A. Phosphorylcholine-Targeting Immunization Reduces Atherosclerosis. J. Am. Coll. Cardiol. 2007, 50, 540–546. [Google Scholar] [CrossRef]
- Faria-Neto, J.R.; Chyu, K.-Y.; Li, X.; Dimayuga, P.C.; Ferreira, C.; Yano, J.; Cercek, B.; Shah, P.K. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis 2006, 189, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, B.G.; Su, J.; Dahlbom, I.; Gronlund, H.; Wikström, M.; Hedblad, B.; Berglund, G.; De Faire, U.; Frostegård, J. Low levels of IgM antibodies against phosphorylcholine—A potential risk marker for ischemic stroke in men. Atherosclerosis 2009, 203, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Gronlund, H.; Hallmans, G.; Jansson, J.H.; Boman, K.K.; Wikström, M.; De Faire, U.; Frostegård, J. Low levels of IgM antibodies against phosphorylcholine predict development of acute myocardial infarction in a population-based cohort from northern Sweden. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 16, 382–386. [Google Scholar] [CrossRef]
- Frostegård, J.; Tao, W.; Georgiades, A.; Råstam, L.; Lindblad, U.; Lindeberg, S. Atheroprotective natural anti-phosphorylcholine antibodies of IgM subclass are decreased in Swedish controls as compared to non-westernized individuals from New Guinea. Nutr. Metab. 2007, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Sing, S.; Golabkesh, Z.; Fiskesund, R.; Gustafsson, T.; Jogestrand, T.; Frostegård, A.G.; Hafström, I.; Liu, A.; Frostegård, J. IgM antibodies against malondialdehyde and phosphorylcholine are together strong protection markers for atherosclerosis in systemic lupus erythematosus: Regulation and underlying mechanisms. Clin. Immunol. 2016, 166, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Suthers, B.; Hansbro, P.; Thambar, S.; McEvoy, M.; Peel, R.; Attia, J. Pneumococcal vaccination may induce anti-oxidized low-density lipoprotein antibodies that have potentially protective effects against cardiovascular disease. Vaccine 2012, 30, 3983–3985. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.; Rijkers, G.; Tervaert, J.W.C. Pneumococcal vaccination does not increase circulating levels of IgM antibodies to oxidized LDL in humans and therefore precludes an anti-atherogenic effect. Atherosclerosis 2007, 190, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S.; Kyrle, P.; Kammer, M.; Eischer, L.; Kozma, M.O.; Binder, C.J. Natural antibodies to oxidation-specific epitopes: Innate immune response and venous thromboembolic disease. J. Thromb. Haemost. 2017, 16, 31–35. [Google Scholar] [CrossRef]
- Nguyen, J.T.; Myers, N.; Palaia, J.; Georgopoulos, A.; Rubins, J.B.; Janoff, E.N. Humoral responses to oxidized low-density lipoprotein and related bacterial antigens after pneumococcal vaccine. Transl. Res. 2007, 150, 172–179. [Google Scholar] [CrossRef]
- Nuorti, J.P.; Whitney, C.G. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR. Morb. Mortal. Wkly. Rep. 2010, 59, 1102–1106. [Google Scholar]
- Musher, D.M.; Sampath, R.; Rodriguez-Barradas, M.C. The Potential Role for Protein-Conjugate Pneumococcal Vaccine in Adults: What Is the Supporting Evidence? Clin. Infect. Dis. 2011, 52, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Burrows, S.R. Human immunology: A case for the ascent of non-furry immunology. Immunol. Cell Biol. 2011, 89, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.S. Translating Mouse Models. Toxicol. Pathol. 2016, 45, 134–145. [Google Scholar] [CrossRef] [PubMed]
| Parameter | n = 24 |
|---|---|
| Age (years) | 28.5 ± 8.5 |
| Gender male (%) | 100 |
| Ethnicity Caucasian (%) | 100 |
| Height (cm) | 180.5 ± 5.3 |
| Weight (kg) | 75.0 ± 11.0 |
| BMI (kg/m2) | 23.0 ± 3.2 |
| Heart rate (min−1) | 58.5 ± 9.0 |
| Systolic blood pressure (mmHg) | 123 ± 9.3 |
| Diastolic blood pressure (mmHg) | 75.1 ± 6.7 |
| IgG | IgM | |||||
|---|---|---|---|---|---|---|
| ED | %95 CI | p Value | ED | %95 CI | p Value | |
| 0–4 weeks (A vs. P) | 19,187.8 | 14,098.7–24,276.9 | <0.0001 | 13,128.5 | 5356.9–20,900.1 | 0.0020 |
| 4–28 weeks (AP vs. PP) | 16,656.9 | 11,626.7–21,687.1 | <0.0001 | 5480.2 | 1143.1–9817.3 | 0.0158 |
| 4–28 weeks (AA vs. PP) | 17,155.5 | 12,449.3–21,861.7 | <0.0001 | 9272.0 | 4568.5–13,975.5 | 0.0005 |
| 28–68 weeks (AAA vs. PPP) | 22,733.6 | 17,512.7–27,954.5 | <0.0001 | 8189.1 | 1407.0–14,971.1 | 0.0209 |
| 28–68 weeks (AAP vs. PPP) | 18,439.7 | 12,869.1–24,010.2 | <0.0001 | 10,320.1 | −23.1–20,663.3 | 0.0505 |
| 28–68 weeks (APA vs. PPP) | 25,805.3 | 19,200.6–32,409.9 | <0.0001 | 987.0 | −6717.0–8691.0 | 0.7903 |
| 28–68 weeks (APP vs. PPP) | 17,116.7 | 11,720.9–22,512.5 | <0.0001 | 2233.9 | −4392.3–8860.1 | 0.4862 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grievink, H.W.; Gal, P.; Ozsvar Kozma, M.; Klaassen, E.S.; Kuiper, J.; Burggraaf, J.; Binder, C.J.; Moerland, M. The Effect of a 13-Valent Conjugate Pneumococcal Vaccine on Circulating Antibodies Against Oxidized LDL and Phosphorylcholine in Man, A Randomized Placebo-Controlled Clinical Trial. Biology 2020, 9, 345. https://doi.org/10.3390/biology9110345
Grievink HW, Gal P, Ozsvar Kozma M, Klaassen ES, Kuiper J, Burggraaf J, Binder CJ, Moerland M. The Effect of a 13-Valent Conjugate Pneumococcal Vaccine on Circulating Antibodies Against Oxidized LDL and Phosphorylcholine in Man, A Randomized Placebo-Controlled Clinical Trial. Biology. 2020; 9(11):345. https://doi.org/10.3390/biology9110345
Chicago/Turabian StyleGrievink, Hendrika W., Pim Gal, Maria Ozsvar Kozma, Erica S. Klaassen, Johan Kuiper, Jacobus Burggraaf, Christoph J. Binder, and Matthijs Moerland. 2020. "The Effect of a 13-Valent Conjugate Pneumococcal Vaccine on Circulating Antibodies Against Oxidized LDL and Phosphorylcholine in Man, A Randomized Placebo-Controlled Clinical Trial" Biology 9, no. 11: 345. https://doi.org/10.3390/biology9110345
APA StyleGrievink, H. W., Gal, P., Ozsvar Kozma, M., Klaassen, E. S., Kuiper, J., Burggraaf, J., Binder, C. J., & Moerland, M. (2020). The Effect of a 13-Valent Conjugate Pneumococcal Vaccine on Circulating Antibodies Against Oxidized LDL and Phosphorylcholine in Man, A Randomized Placebo-Controlled Clinical Trial. Biology, 9(11), 345. https://doi.org/10.3390/biology9110345





