Nanopharmaceuticals for Eye Administration: Sterilization, Depyrogenation and Clinical Applications
Simple Summary
Abstract
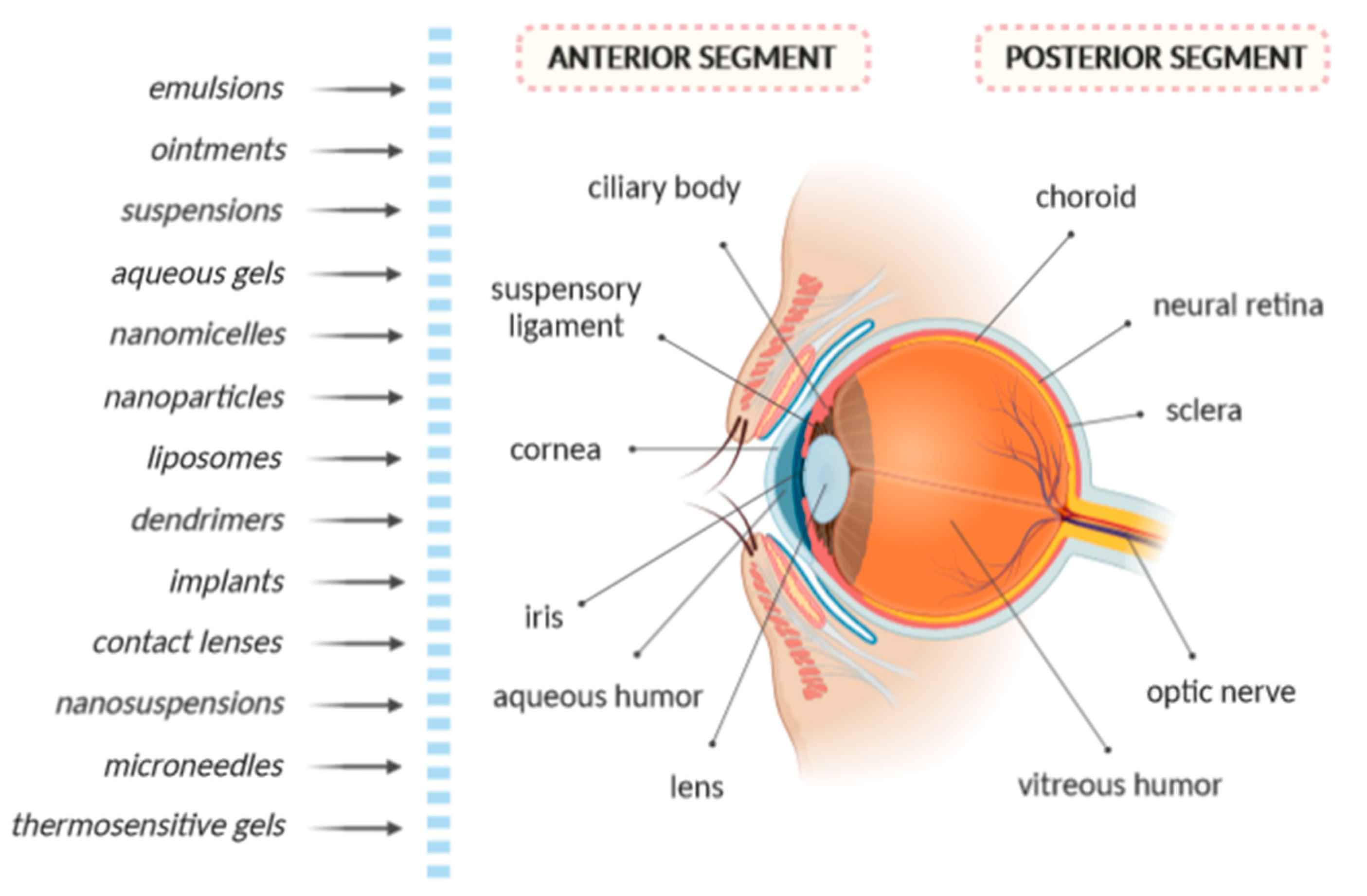
1. Introduction
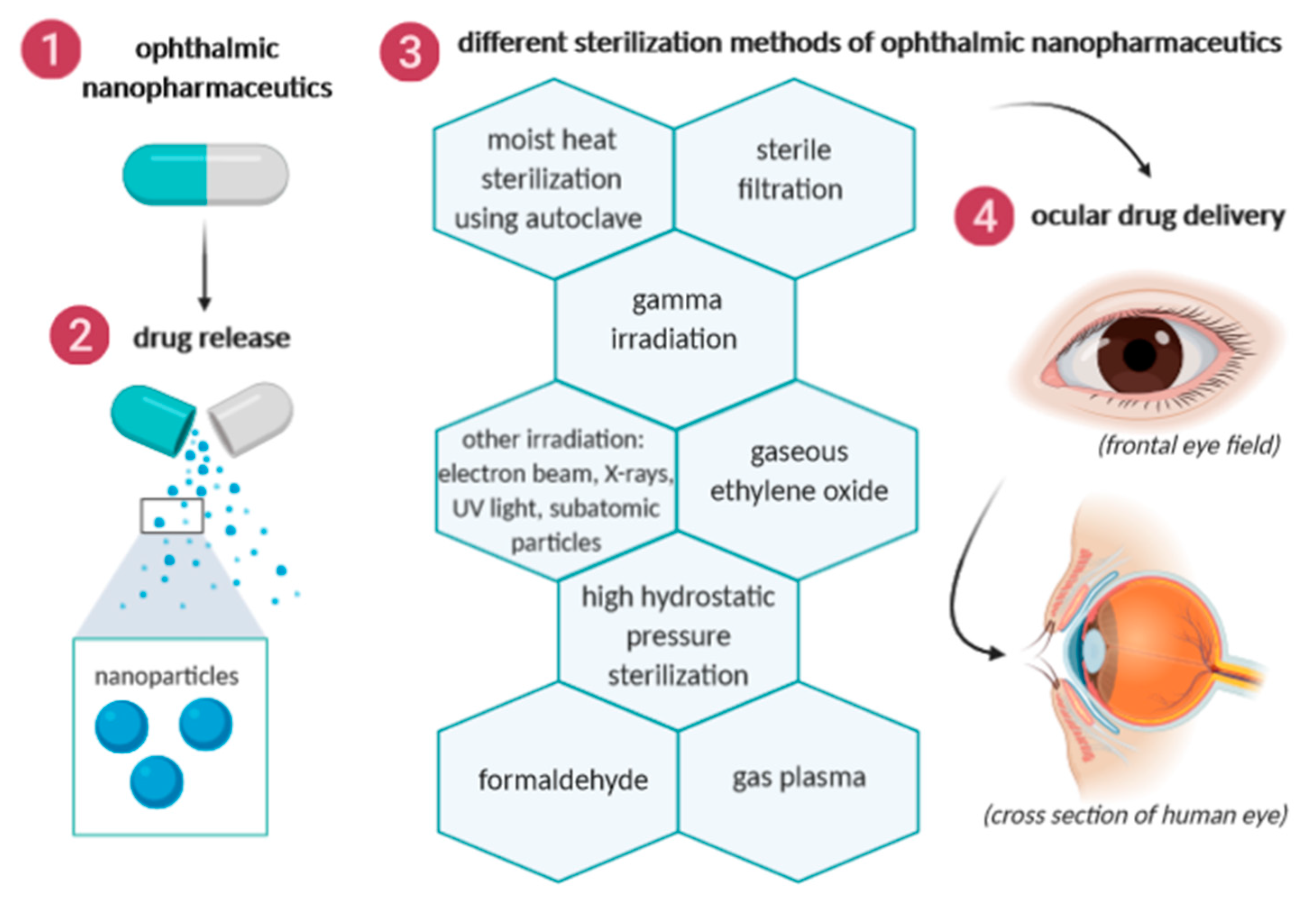
2. Sterilization Methods of Ophthalmic Nanopharmaceuticals
2.1. Moist Heat Sterilization Using Autoclave
| Sterilization Methods | Effect | Advantage | Drawbacks | References |
|---|---|---|---|---|
| Autoclaving: high pressure steam | bactericidal | low cost | chemical degradation, structural modification | [16] |
| Filtration: barrier | physical retention | drug thermally sensitive | viscosity, size | [17,25,26,27] |
| Gamma irradiation: ionizing | damage of genetic material | viscous material, drug and adjuvant thermally sensitive, no residue, effective against bacteria, yeast, fungus | chemical degradation, free radical, rate of drug delivery, gas formation, high cost | [16,28] |
| Gaseous ethylene oxide | bactericidal | low cost, drug and adjuvant thermally sensitive | toxic residue, cascade of oxidation, chemical change | [29] |
| High hydrostatic pressure | affect the cellular structures or functions | bar-resistant nanoparticles (polymeric carriers) | modifies adsorption, physical and chemical stability | [30] |
| Formaldehyde | bactericidal, but highly toxic | low cost | toxicity (truncates proteins) and carcinogens, affect the re-dispersion | [17,31,32] |
| Gas plasma: oxide reduction effect | antimicrobial | low temperature, non-toxic | oxidative, aggregation | [15,17,30] |
2.2. Sterile Filtration
2.3. Gamma Irradiation
2.4. Other Irradiation Methods
2.5. Gaseous Ethylene Oxide
2.6. High Hydrostatic Pressure Sterilization
2.7. Formaldehyde
2.8. Gas Plasma
3. Endotoxin Contamination
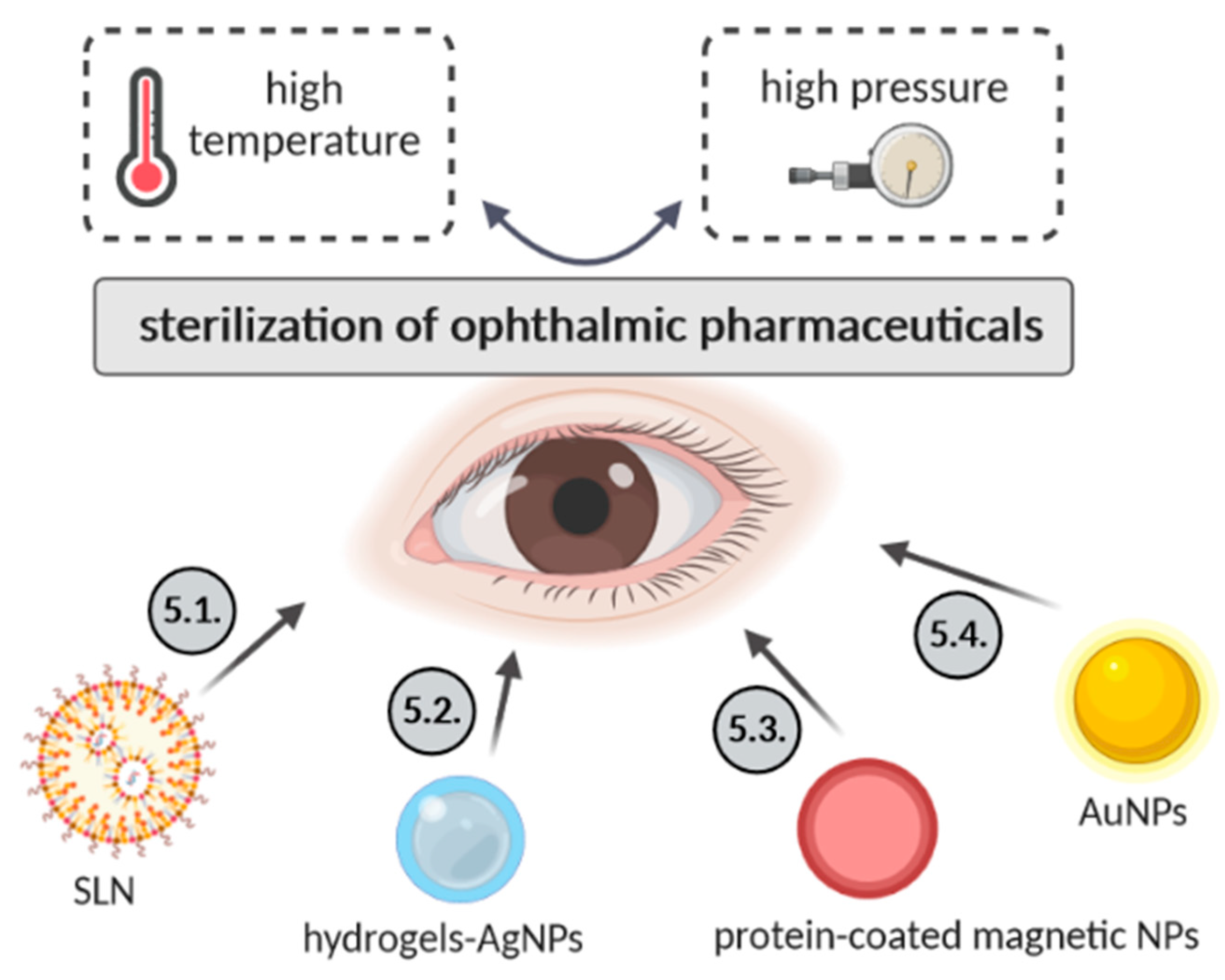
4. Sterilization of Ophthalmic Nanopharmaceuticals
4.1. Solid Lipid Nanoparticles
4.2. Hydrogels Containing Silver Nanoparticles
4.3. Serum Protein-Coated Magnetic Nanoparticles
4.4. Gold Nanoparticles (AuNPs)
5. Guidelines for Cleaning and Sterilization
6. Sterilization of Ophthalmic Formulations
7. Stability of Ophthalmic Formulations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baldim, I.; Oliveira, W.P.; Kadian, V.; Rao, R.; Yadav, N.; Mahant, S.; Lucarini, M.; Durazzo, A.; Da Ana, R.; Capasso, R.; et al. Natural Ergot Alkaloids in Ocular Pharmacotherapy: Known Molecules for Novel Nanoparticle-Based Delivery Systems. Biomolecules 2020, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Veiga, F.; Silva, A.M.; Souto, E.B. Ocular Drug Delivery—New Strategies for Targeting Anterior and Posterior Segments of the Eye. Curr. Pharm. Des. 2016, 22, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; Garcia, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part II—Ocular drug-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 110, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.-L.; Dinu-Pîrvu, C.-E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef]
- Jain, D.; Kumar, V.; Singh, S.; Müllertz, A.; Bar Shalom, D. Newer Trends in In Situ Gelling Systems for Controlled Ocular Drug Delivery. J. Anal. Pharm. Res. 2016, 2, 000222. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Pittet, D. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR. Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2002, 51, 1–45. [Google Scholar]
- Aitken, C.; Jeffries, D.J. Nosocomial spread of viral disease. Clin. Microbiol. Rev. 2001, 14, 528–546. [Google Scholar] [CrossRef]
- Cillino, S.; Casuccio, A.; Giammanco, G.M.; Mammina, C.; Morreale, D.; Di Pace, F.; Lodato, G. Tonometers and infectious risk: Myth or reality? Efficacy of different disinfection regimens on tonometer tips. Eye 2007, 21, 541–546. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part II—Production scales and clinically compliant production methods. Nanomaterials 2020, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Sanchez-Lopez, E.; Campos, J.R.; da Ana, R.; Espina, M.; Garcia, M.L.; Severino, P.; Batain, F.; Alves, T.F.R.; Crescencio, K.M.M.; et al. Retinal Drug Delivery: Rethinking Outcomes for the Efficient Replication of Retinal Behavior. Appl. Sci. 2020, 10, 4258. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Fernandes, A.R.; Sanchez-Lopez, E.; Garcia, M.L.; Silva, A.M.; Zakharova, L.Y.; Souto, E.B. Polymer nanogels: Fabrication, structural behavior and biological applications. In Theory and Applications of Nonparenteral Nanomedicines; Kesharwani, P., Taurin, S., Greish, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 5. [Google Scholar] [CrossRef]
- França, Á.; Pelaz, B.; Moros, M.; Sánchez-Espinel, C.; Hernández, A.; Fernández-López, C.; Grazú, V.; de la Fuente, J.M.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; et al. Sterilization Matters: Consequences of Different Sterilization Techniques on Gold Nanoparticles. Small 2010, 6, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, N. Nanoparticle Sterility and Sterilization of Nanomaterials. In Handbook of Immunological Properties of Engineered Nanomaterials, 2nd ed.; Dobrovolskaia, M.A., McNeil, S.E., Eds.; World Scientific: Singapore, 2016; pp. 53–75. [Google Scholar] [CrossRef]
- Vetten, M.A.; Yah, C.S.; Singh, T.; Gulumian, M. Challenges facing sterilization and depyrogenation of nanoparticles: Effects on structural stability and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1391–1399. [Google Scholar] [CrossRef]
- Magalhaes, P.O.; Lopes, A.M.; Mazzola, P.G.; Rangel-Yagui, C.; Penna, T.C.; Pessoa, A., Jr. Methods of endotoxin removal from biological preparations: A review. J. Pharm. Pharm. Sci. 2007, 10, 388–404. [Google Scholar] [PubMed]
- Jain, A.; Jain, R.; Jain, S. Autoclave. In Basic Techniques in Biochemistry, Microbiology and Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 9–10. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; Reimers, M.; Ernst, N.; Bova, G.; Nowakowski, E.; Bukowski, J.; Ellis, B.C.; Smith, C.; Sauer, L.; Dionne, K. Validation of autoclave protocols for successful decontamination of category a medical waste generated from care of patients with serious communicable diseases. J. Clin. Microbiol. 2017, 55, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Viveksarathi, K.; Kannan, K. Effect of the moist-heat sterilization on fabricated nanoscale solid lipid particles containing rasagiline mesylate. Int. J. Pharm. Investig. 2015, 5, 87. [Google Scholar]
- Gokce, E.H.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Guneri, T.; Caramella, C. Cyclosporine A loaded SLNs: Evaluation of cellular uptake and corneal cytotoxicity. Int. J. Pharm. 2008, 364, 76–86. [Google Scholar] [CrossRef]
- Oh, S.; Brammer, K.S.; Moon, K.-S.; Bae, J.-M.; Jin, S. Influence of sterilization methods on cell behavior and functionality of osteoblasts cultured on TiO2 nanotubes. Mater. Sci. Eng. C 2011, 31, 873–879. [Google Scholar] [CrossRef]
- Zheng, J.; Clogston, J.D.; Patri, A.K.; Dobrovolskaia, M.A.; McNeil, S.E. Sterilization of Silver Nanoparticles Using Standard Gamma Irradiation Procedure Affects Particle Integrity and Biocompatibility. J. Nanomed. Nanotechnol. 2011, 2011, 001. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Memisoglu-Bilensoy, E.; Hincal, A.A. Sterile, injectable cyclodextrin nanoparticles: Effects of gamma irradiation and autoclaving. Int. J. Pharm. 2006, 311, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y. A new preparation method for ophthalmic drug nanoparticles. Pharm. Anal. Acta 2014, 5, 1000305. [Google Scholar]
- Silindir, M.; Ozer, Y. The effect of radiation on a variety of pharmaceuticals and materials containing polymers. PDA J. Pharm. Sci. Technol. 2012, 66, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Silindir, M.; Özer, A.Y. Sterilization methods and the comparison of e-beam sterilization with gamma radiation sterilization. Fabad J. Pharm. Sci. 2009, 34, 43. [Google Scholar]
- Brigger, I.; Armand-Lefevre, L.; Chaminade, P.; Besnard, M.; Rigaldie, Y.; Largeteau, A.; Andremont, A.; Grislain, L.; Demazeau, G.; Couvreur, P. The stenlying effect of high hydrostatic pressure on thermally and hydrolytically labile nanosized carriers. Pharm. Res. 2003, 20, 674–683. [Google Scholar] [CrossRef]
- Lin, J.J.; Hsu, P.Y. Gamma-ray sterilization effects in silica nanoparticles/gamma-APTES nanocomposite-based pH-sensitive polysilicon wire sensors. Sensors 2011, 11, 8769–8781. [Google Scholar] [CrossRef]
- Sommerfeld, P.; Schroeder, U.; Sabel, B.A. Sterilization of unloaded polybutylcyanoacrylate nanoparticles. Int. J. Pharm. 1998, 164, 113–118. [Google Scholar] [CrossRef]
- Wadhwa, A.; Mathura, V.; Lewis, S. Emerging novel nanopharmaceuticals for drug delivery. Asian J. Pharm. Clin. Res. 2018, 11, 35–42. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Fellabaum, C.; Volarevic, V. Risks of using sterilization by gamma radiation: The other side of the coin. Int. J. Med. Sci. 2018, 15, 274. [Google Scholar] [CrossRef] [PubMed]
- Hume, A.J.; Ames, J.; Rennick, L.J.; Duprex, W.P.; Marzi, A.; Tonkiss, J.; Mühlberger, E. Inactivation of RNA viruses by gamma irradiation: A study on mitigating factors. Viruses 2016, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Chapter 2—Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar] [CrossRef]
- Mráz, J.; Hanzlíková, I.; Dušková, Š.; Tvrdíková, M.; Linhart, I. N-(2-Hydroxyethyl)-L-valyl-L-leucine: A novel urinary biomarker of ethylene oxide exposure in humans. Toxicol. Lett. 2020, 326, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Friess, W.; Schlapp, M. Sterilization of gentamicin containing collagen/PLGA microparticle composites. Eur. J. Pharm. Biopharm. 2006, 63, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Moisan, M.; Barbeau, J.; Crevier, M.-C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma sterilization. Methods and mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar] [CrossRef]
- Shintani, H.; Sakudo, A.; Burke, P.; McDonnell, G. Gas plasma sterilization of microorganisms and mechanisms of action. Exp. Ther. Med. 2010, 1, 731–738. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Neun, B.W.; Clogston, J.D.; Ding, H.; Ljubimova, J.; McNeil, S.E. Ambiguities in applying traditional Limulus amebocyte lysate tests to quantify endotoxin in nanoparticle formulations. Nanomedicine 2010, 5, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Smulders, S.; Kaiser, J.P.; Zuin, S.; Van Landuyt, K.L.; Golanski, L.; Vanoirbeek, J.; Wick, P.; Hoet, P.H. Contamination of nanoparticles by endotoxin: Evaluation of different test methods. Part. Fibre Toxicol. 2012, 9, 41. [Google Scholar] [CrossRef]
- Li, Y.; Boraschi, D. Endotoxin contamination: A key element in the interpretation of nanosafety studies. Nanomedicine 2016, 11, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fujita, M.; Boraschi, D. Endotoxin contamination in nanomaterials leads to the misinterpretation of immunosafety results. Front. Immunol. 2017, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Ongkudon, C.M.; Chew, J.H.; Liu, B.; Danquah, M.K. Chromatographic removal of endotoxins: A bioprocess engineer’s perspective. ISRN Chromatogr. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Salama, S.E.-M.; Mobarez, E. Depyrogenation methods. Egypt. J. Chem. Environ. Health 2015, 1, 540–551. [Google Scholar]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Souto, E.B.; Muller, R.H. Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. Handb. Exp. Pharmacol. 2010, 197, 115–141. [Google Scholar] [CrossRef]
- Araujo, J.; Garcia, M.L.; Mallandrich, M.; Souto, E.B.; Calpena, A.C. Release profile and transscleral permeation of triamcinolone acetonide loaded nanostructured lipid carriers (TA-NLC): In vitro and ex vivo studies. Nanomedicine 2012, 8, 1034–1041. [Google Scholar] [CrossRef]
- Araujo, J.; Gonzalez, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Nanomedicines for ocular NSAIDs: Safety on drug delivery. Nanomedicine 2009, 5, 394–401. [Google Scholar] [CrossRef]
- Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surf. B Biointerfaces 2010, 81, 412–421. [Google Scholar] [CrossRef]
- Gonzalez-Mira, E.; Egea, M.A.; Souto, E.B.; Calpena, A.C.; Garcia, M.L. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology 2011, 22, 045101. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mira, E.; Nikolic, S.; Calpena, A.C.; Egea, M.A.; Souto, E.B.; Garcia, M.L. Improved and safe transcorneal delivery of flurbiprofen by NLC and NLC-based hydrogels. J. Pharm. Sci. 2012, 101, 707–725. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.; Nikolic, S.; Egea, M.A.; Souto, E.B.; Garcia, M.L. Nanostructured lipid carriers for triamcinolone acetonide delivery to the posterior segment of the eye. Colloids Surf. B Biointerfaces 2011, 88, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Silva, A.M.; Souto, E.B. Design of cationic lipid nanoparticles for ocular delivery: Development, characterization and cytotoxicity. Int. J. Pharm. 2014, 461, 64–73. [Google Scholar] [CrossRef]
- Mahant, S.; Rao, R.; Souto, E.B.; Nanda, S. Analytical tools and evaluation strategies for nanostructured lipid carrier based topical delivery systems. Expert Opin. Drug Deliv. 2020, 17, 963–992. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Cavalli, R.; Caputo, O.; Carlotti, M.E.; Trotta, M.; Scarnecchia, C.; Gasco, M.R. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles. Int. J. Pharm. 1997, 148, 47–54. [Google Scholar] [CrossRef]
- Rafael, D.; Andrade, F.; Martinez-Trucharte, F.; Basas, J.; Seras-Franzoso, J.; Palau, M.; Gomis, X.; Pérez-Burgos, M.; Blanco, A.; López-Fernández, A.; et al. Sterilization Procedure for Temperature-Sensitive Hydrogels Loaded with Silver Nanoparticles for Clinical Applications. Nanomaterials 2019, 9, 380. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Das, M.; Jain, S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin. Drug Deliv. 2012, 9, 383–402. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Barbosa, G.P.; Debone, H.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Design and characterization of chitosan/zeolite composite films—Effect of zeolite type and zeolite dose on the film properties. Mater. Sci. Eng. C 2016, 60, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Diniz, F.R.; Maia, R.C.A.P.; Rannier, L.; Andrade, L.N.; Chaud, M.V.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; da Costa, L.P.; Souto, E.B.; et al. Silver nanoparticles-composing alginate/gelatin hydrogel improves wound healing in vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef]
- Hissae Yassue-Cordeiro, P.; Zandonai, C.H.; Pereira Genesi, B.; Santos Lopes, P.; Sanchez-Lopez, E.; Garcia, M.L.; Camargo Fernandes-Machado, N.R.; Severino, P.; Souto, E.B.; Ferreira da Silva, C. Development of Chitosan/Silver Sulfadiazine/Zeolite Composite Films for Wound Dressing. Pharmaceutics 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Souto, E.B.; Durazzo, A.; Lucarini, M.; Novellino, E.; Tewari, D.; Wang, D.; Atanasov, A.G.; Santini, A. Big impact of nanoparticles: Analysis of the most cited nanopharmaceuticals and nanonutraceuticals research. Curr. Res. Biotechnol. 2020, 2, 53–63. [Google Scholar] [CrossRef]
- Galante, R.; Pinto, T.J.A.; Colaco, R.; Serro, A.P. Sterilization of hydrogels for biomedical applications: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2472–2492. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Wojahn, S.; Gräfe, C.; Weidner, A.; Clement, J.H. Influence of Sterilization and Preservation Procedures on the Integrity of Serum Protein-Coated Magnetic Nanoparticles. Nanomaterials 2017, 7, 453. [Google Scholar] [CrossRef]
- Zhao, L.; Mei, S.; Wang, W.; Chu, P.K.; Wu, Z.; Zhang, Y. The role of sterilization in the cytocompatibility of titania nanotubes. Biomaterials 2010, 31, 2055–2063. [Google Scholar] [CrossRef]
- Durchschlag, H.; Fochler, C.; Feser, B.; Hausmann, S.; Seroneit, T.; Swientek, M.; Swoboda, E.; Winklmair, A.; Wlček, C.; Zipper, P.J.R.P.; et al. Effects of X-and UV-irradiation on proteins. Radiat. Phys. Chem. 1996, 47, 501–505. [Google Scholar] [CrossRef]
- Vaz, C.M.; de Graaf, L.A.; Reis, R.L.; Cunha, A.M. Effect of crosslinking, thermal treatment and UV irradiation on the mechanical properties and in vitro degradation behavior of several natural proteins aimed to be used in the biomedical field. J. Mater. Sci. Mater. Med. 2003, 14, 789–796. [Google Scholar] [CrossRef]
- Sun, J.; Ma, D.; Zhang, H.; Liu, X.; Han, X.; Bao, X.; Weinberg, G.; Pfänder, N.; Su, D. Toward monodispersed silver nanoparticles with unusual thermal stability. J. Am. Chem. Soc. 2006, 128, 15756–15764. [Google Scholar] [CrossRef] [PubMed]
- Lleixà Calvet, J.; Grafahrend, D.; Klee, D.; Möller, M. Sterilization effects on starPEG coated polymer surfaces: Characterization and cell viability. J. Mater. Sci. Mater. Med. 2008, 19, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Mamalis, N.; Edelhauser, H.F.; Dawson, D.G.; Chew, J.; LeBoyer, R.M.; Werner, L. Toxic anterior segment syndrome. J. Cataract. Refract. Surg. 2006, 32, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.F.; Mamalis, N. Guidelines for the cleaning and sterilization of intraocular surgical instruments. J. Cataract. Refract. Surg. 2018, 44, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Bogantes, E.; Navas, A.; Naranjo, A.; Amescua, G.; Graue-Hernandez, E.O.; Flynn, H.W., Jr.; Ahmed, I. Toxic anterior segment syndrome: A review. Surv. Ophthalmol. 2019, 64, 463–476. [Google Scholar] [CrossRef]
- Cutler Peck, C.M.; Brubaker, J.; Clouser, S.; Danford, C.; Edelhauser, H.E.; Mamalis, N. Toxic anterior segment syndrome: Common causes. J. Cataract. Refract. Surg. 2010, 36, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.J. The preparation and sterilization of ophthalmic solutions. Calif. Med. 1949, 71, 414–416. [Google Scholar]
- Ahuja, M.; Dhake, A.S.; Sharma, S.K.; Majumdar, D.K. Stability studies on aqueous and oily ophthalmic solutions of diclofenac. Yakugaku Zasshi 2009, 129, 495–502. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Methods | Principle of the Method |
|---|---|
| Ultrafiltration | It eliminates the endotoxin by molecular weight using ultra-fines (10,000 Daltons or greater). It shows relatively good endotoxin clearance. |
| Reverse osmosis | This process uses a filter and highly pressurized conditions. It captures 99.5% of endotoxin and ions or salts but allows water molecules through. It is commonly used to produce highly purified water. |
| Two-phase partitioning | In this system, an aqueous surfactant solution spontaneously separates into two predominantly aqueous, but immiscible, forms in an effective separation. |
| Affinity chromatography | This method acts to bind endotoxin through biding affinity using ligands such as DEAE Sepharose, poly-L-lysine and polymyxin-B. Moreover, this method can be affected by the pH range, temperature, flow rate and the number of electrolytes in the solution. |
| Distillation | Endotoxin is removed by the rapid evaporation of the water molecules and the persistence of the larger lipopolysaccharide molecules in the original environment. |
| Adsorption | The endotoxin molecule is attracted to the activated carbon bed. This mechanism is less efficient and is affected by several environmental factors. |
| Acid-base hydrolysis | Occurs in the binding of lipid A with the polysaccharide nucleus. The isolated molecule is insoluble in an aqueous medium. The main acids used are HCl and glacial acetic acid diluted. |
| Plasma discharge | The UV radiation used in this process is responsible for the inactivation of spores. The main advantage is the possibility of the operation of the process at moderate temperatures, allowing the treatment of heat-degradable materials. |
| Oxidation | Depyrogenation occurs by peroxidation of the fatty acid in the lipid A region (e.g., using hydrogen peroxide) |
| Ethylene oxide | The process is performed in a heated, pressurized chamber, but at a lower temperature. The depyrogenation process occurs by nucleophilic substitution in the glucosamine of lipid A. |
| Moist heat | Traditional autoclaving does not destroy endotoxins. However, when combined with hydrogen peroxide and pressure, it is effective. |
| Dry heat | Endotoxins are destroyed by exposure to high temperature. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, A.; Soles, B.B.; Lopes, A.R.; Vaz, B.F.; Rodrigues, C.M.; Alves, T.F.R.; Klensporf-Pawlik, D.; Durazzo, A.; Lucarini, M.; Severino, P.; et al. Nanopharmaceuticals for Eye Administration: Sterilization, Depyrogenation and Clinical Applications. Biology 2020, 9, 336. https://doi.org/10.3390/biology9100336
Zielińska A, Soles BB, Lopes AR, Vaz BF, Rodrigues CM, Alves TFR, Klensporf-Pawlik D, Durazzo A, Lucarini M, Severino P, et al. Nanopharmaceuticals for Eye Administration: Sterilization, Depyrogenation and Clinical Applications. Biology. 2020; 9(10):336. https://doi.org/10.3390/biology9100336
Chicago/Turabian StyleZielińska, Aleksandra, Beatriz B. Soles, Ana R. Lopes, Beatriz F. Vaz, Camila M. Rodrigues, Thais F. R. Alves, Dorota Klensporf-Pawlik, Alessandra Durazzo, Massimo Lucarini, Patricia Severino, and et al. 2020. "Nanopharmaceuticals for Eye Administration: Sterilization, Depyrogenation and Clinical Applications" Biology 9, no. 10: 336. https://doi.org/10.3390/biology9100336
APA StyleZielińska, A., Soles, B. B., Lopes, A. R., Vaz, B. F., Rodrigues, C. M., Alves, T. F. R., Klensporf-Pawlik, D., Durazzo, A., Lucarini, M., Severino, P., Santini, A., Chaud, M. V., & Souto, E. B. (2020). Nanopharmaceuticals for Eye Administration: Sterilization, Depyrogenation and Clinical Applications. Biology, 9(10), 336. https://doi.org/10.3390/biology9100336












