Vaccination Route Determines the Kinetics and Magnitude of Nasal Innate Immune Responses in Rainbow Trout (Oncorhynchus mykiss)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
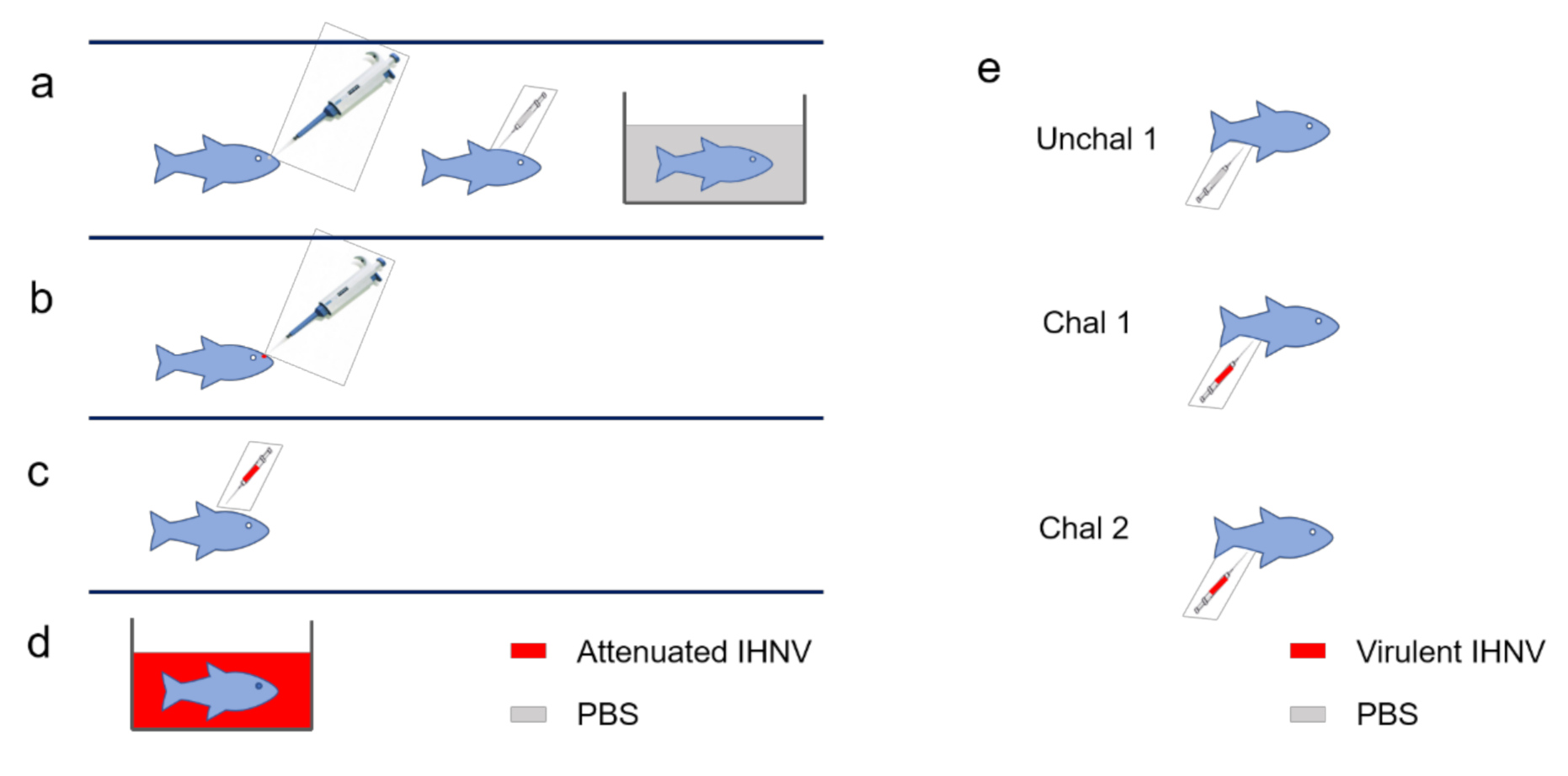
2.2. Vaccination Trials and Challenges
2.3. RNA Isolation and Quantitative Real-Time PCR (qPCR) Analysis
2.4. Statistical Analysis
3. Results
3.1. Local Innate Immune Responses in Trout NALT in Response to Different Vaccination Routes
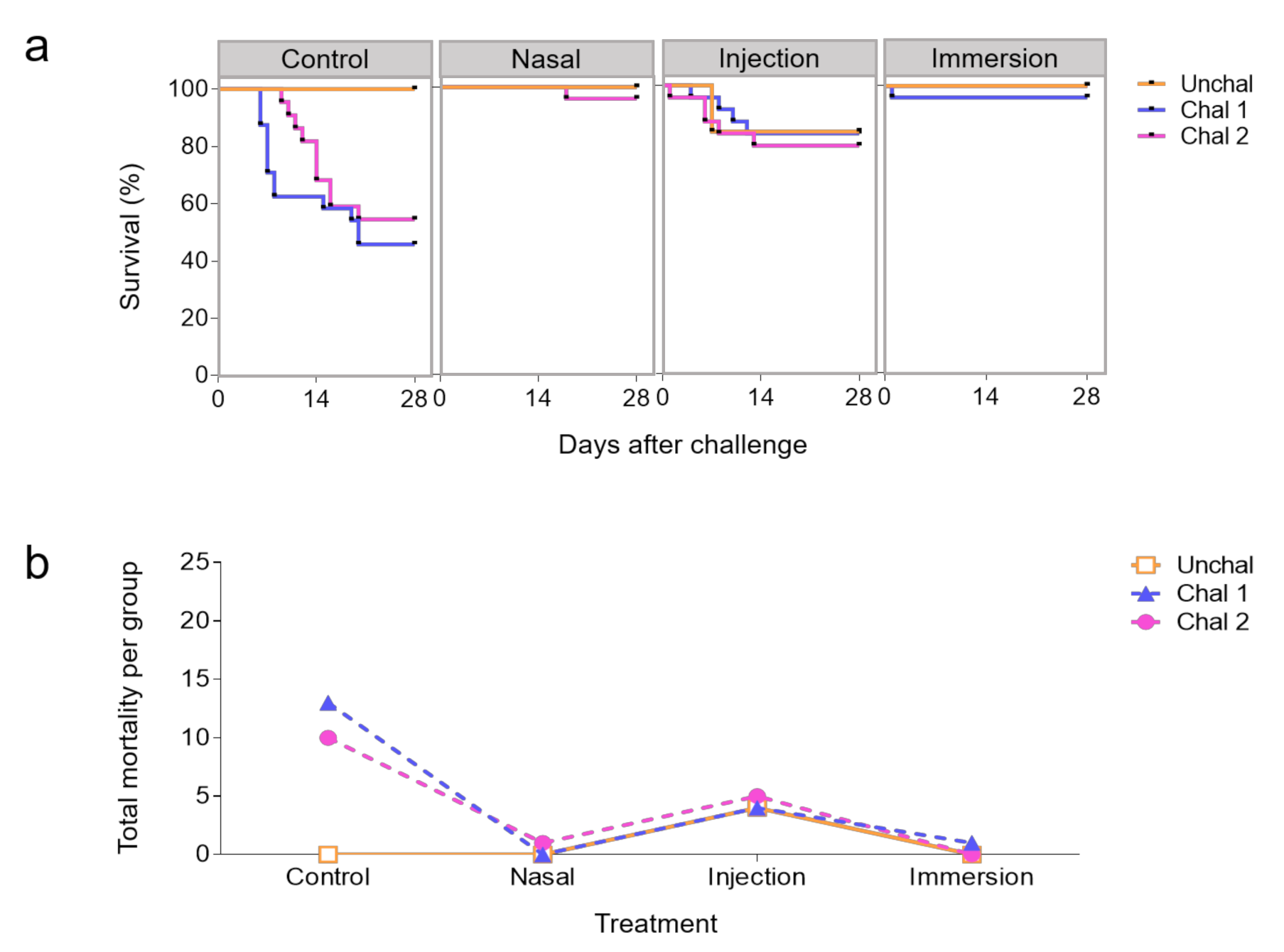
3.2. Protection Against IHNV Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plant, K.P.; Lapatra, S.E. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, B.; Wu, H.; Gao, L.; Liu, Q.; Wang, Q.; Xiao, J.; Zhang, Y. Th17-like immune response in fish mucosal tissues after administration of live attenuated Vibrio anguillarum via different vaccination routes. Fish Shellfish Immunol. 2014, 37, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Tacchi, L.; Musharrafieh, R.; Larragoite, E.T.; Crossey, K.; Erhardt, E.B.; Martin, S.A.M.; LaPatra, S.E.; Salinas, I. Nasal immunity is an ancient arm of the mucosal immune system of vertebrates. Nat. Commun. 2014, 5, 5205. [Google Scholar] [CrossRef]
- LaPatra, S.; Kao, S.; Erhardt, E.B.; Salinas, I. Evaluation of dual nasal delivery of infectious hematopoietic necrosis virus and enteric red mouth vaccines in rainbow trout (Oncorhynchus mykiss). Vaccine 2015, 33, 771–776. [Google Scholar] [CrossRef]
- Salinas, I.; LaPatra, S.E.; Erhardt, E.B. Nasal vaccination of young rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis and enteric red mouth disease. Dev. Comp. Immunol. 2015, 53, 105–111. [Google Scholar] [CrossRef]
- Esteve-Gassent, M.D.; Fouz, B.; Amaro, C. Efficacy of a bivalent vaccine against eel diseases caused by Vibrio vulnificus after its administration by four different routes. Fish Shellfish Immunol. 2004, 16, 93–105. [Google Scholar] [CrossRef]
- Sheng, X.; Chai, B.; Wang, Z.; Tang, X.; Xing, J.; Zhan, W. Polymeric immunoglobulin receptor and mucosal IgM responses elicited by immersion and injection vaccination with inactivated Vibrio anguillarum in flounder (Paralichthys olivaceus). Aquaculture 2019, 505, 1–11. [Google Scholar] [CrossRef]
- Hoare, R.; Ngo, T.P.H.; Bartie, K.L.; Adams, A. Efficacy of a polyvalent immersion vaccine against Flavobacterium psychrophilum and evaluation of immune response to vaccination in rainbow trout fry (Onchorynchus mykiss L.). Vet. Res. 2017, 48, 43. [Google Scholar] [CrossRef]
- Sepahi, A.; Casadei, E.; Tacchi, L.; Muñoz, P.; LaPatra, S.E.; Salinas, I. Tissue microenvironments in the nasal epithelium of rainbow trout (Oncorhynchus mykiss) define two distinct CD8α+ cell populations and establish regional immunity. J. Immunol. 2016, 197, 4453–4463. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Kong, W.; Yin, Y.X.; Dong, F.; Huang, Z.Y.; Yin, G.M.; Dong, S.; Salinas, I.; Zhang, Y.A.; Xu, Z. Mucosal immunoglobulins protect the olfactory organ of teleost fish against parasitic infection. PLoS Pathog. 2018, 14, e1007251. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Paley, R.; Alegria-Moran, R.; Oidtmann, B. Epidemiological characteristics of infectious hematopoietic necrosis virus (IHNV): A review. Vet. Res. 2016, 47, 63. [Google Scholar] [CrossRef] [PubMed]
- Ammayappan, A.; LaPatra, S.E.; Vakharia, V.N. Molecular characterization of the virulent infectious hematopoietic necrosis virus (IHNV) strain 220-90. Virol. J. 2010, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Bootland, L.M.; Leong, J.C. Infectious Hematopoietic Necrosis Virus; Woo, P.T.K., Bruno, D.W., Eds.; Fish Diseases and Disorders, CAB International: New York, NY, USA, 1999; Volume 3, pp. 57–121. [Google Scholar]
- Noonan, B.; Enzmann, P.J.; Trust, T.J. Recombinant infectious hematopoietic necrosis virus and viral hemorrhagic septicemia virus glycoprotein epitopes expressed in Aeromonas salmonicida induce protective immunity in rainbow trout (Oncorhynchus mykiss). Appl. Environ. Microbiol. 1995, 61, 3586–3591. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Y.; Ong, H.K.; Tang, H.C.; Yeap, S.K.; Omar, A.R.; Ho, K.L.; Tan, W.S. Infectious hematopoietic necrosis virus: Advances in diagnosis and vaccine development. PeerJ 2019, 7, e7151. [Google Scholar] [CrossRef]
- LaPatra, S.; Clouthier, S.; Anderson, E. Current Trends in Immunotherapy and Vaccine Development for Viral Diseases of Fish. In Current Trends in the Study of Bacterial and Viral Fish and Shrimp Diseases; World Scientific: Singapore, 2004; pp. 363–389. [Google Scholar]
- LaPatra, S.E.; Lauda, K.A.; Jones, G.R.; Walker, S.C.; Shewmaker, W.D. Development of passive immunotherapy for control of infectious hematopoietic necrosis. Dis. Aquat. Org. 1994, 20, 1–6. [Google Scholar] [CrossRef]
- LaPatra, S.E.; Roberti, K.A.; Rohovec, J.S.; Fryer, J.L. Fluorescent Antibody Test for the Rapid Diagnosis of Infectious Hematopoietic Necrosis. J. Aquat. Anim. Health 1989, 1, 29–36. [Google Scholar] [CrossRef]
- Tacchi, L.; Larragoite, E.; Salinas, I. Discovery of J chain in African lungfish (Protopterus dolloi, Sarcopterygii) using high throughput transcriptome sequencing: Implications in mucosal immunity. PLoS ONE 2013, 8, e70650. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Sepahi, A.; Tacchi, L.; Casadei, E.; Takizawa, F.; LaPatra, S.E.; Salinas, I. CK12a, a CCL19-like chemokine that orchestrates both nasal and systemic antiviral immune responses in rainbow trout. J. Immunol. 2017, 199, 3900–3913. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Maehr, T.; Diaz-Rosales, P.; Secombes, C.J.; Wang, T. Bioactivity studies of rainbow trout (Oncorhynchus mykiss) interleukin-6: Effects on macrophage growth and antimicrobial peptide gene expression. Mol. Immunol. 2011, 48, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Maehr, T.; Costa, M.M.; González Vecino, J.L.; Wadsworth, S.; Martin, S.A.M.; Wang, T.; Secombes, C.J. Transforming growth factor-β1b: A second TGF-β1 paralogue in the rainbow trout (Oncorhynchus mykiss) that has a lower constitutive expression but is more responsive to immune stimulation. Fish Shellfish Immunol. 2013, 34, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.R.; Raida, M.K.; Kania, P.W.; Buchmann, K. Response of rainbow trout (Oncorhynchus mykiss) in skin and fin tissue during infection with a variant of Gyrodactylus salaris (Monogenea: Gyrodactylidae). Folia Parasitol. 2009, 56, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Casadei, E.; Wang, T.; Zou, J.; González Vecino, J.L.; Wadsworth, S.; Secombes, C.J. Characterization of three novel β-defensin antimicrobial peptides in rainbow trout (Oncorhynchus mykiss). Mol. Immunol. 2009, 46, 3358–3366. [Google Scholar] [CrossRef]
- Raida, M.K.; Buchmann, K. Bath vaccination of rainbow trout (Oncorhynchus mykiss Walbaum) against Yersinia ruckeri: Effects of temperature on protection and gene expression. Vaccine 2008, 26, 1050–1062. [Google Scholar] [CrossRef]
- Zou, J.; Grabowski, P.S.; Cunningham, C.; Secombes, C.J. Molecular cloning of interleukin 1beta from rainbow trout Oncorhynchus mykiss reveals no evidence of an ice cut site. Cytokine 1999, 11, 552–560. [Google Scholar] [CrossRef]
- Harun, N.O.; Wang, T.; Secombes, C.J. Gene expression profiling in naïve and vaccinated rainbow trout after Yersinia ruckeri infection: Insights into the mechanisms of protection seen in vaccinated fish. Vaccine 2011, 29, 4388–4399. [Google Scholar] [CrossRef]
- Díaz-Rosales, P.; Bird, S.; Wang, T.H.; Fujiki, K.; Davidson, W.S.; Zou, J.; Secombes, C.J. Rainbow trout interleukin-2: Cloning, expression and bioactivity analysis. Fish Shellfish Immunol. 2009, 27, 414–422. [Google Scholar] [CrossRef]
- Huster, K.M.; Busch, V.; Schiemann, M.; Linkemann, K.; Kerksiek, K.M.; Wagner, H.; Busch, D.H. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 2004, 101, 5610–5615. [Google Scholar] [CrossRef]
- Kaech, S.M.; Tan, J.T.; Wherry, E.J.; Konieczny, B.T.; Surh, C.D.; Ahmed, R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003, 4, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, Ø. An overview of challenges limiting the design of protective mucosal vaccines for finfish. Front. Immunol. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed]
- Bøgwald, J.; Dalmo, R.A. Review on immersion vaccines for fish: An update 2019. Microorganisms 2019, 7, 627. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479–480, 379–392. [Google Scholar] [CrossRef]
- Lauring, A.S.; Jones, J.O.; Andino, R. Rationalizing the development of live attenuated virus vaccines. Nat. Biotechnol. 2010, 28, 573–579. [Google Scholar] [CrossRef]
- Dhar, A.K.; Manna, S.K.; Thomas Allnutt, F.C. Viral vaccines for farmed finfish. Virus Dis. 2014, 25, 1–17. [Google Scholar] [CrossRef]
- Magadan, S.; Jouneau, L.; Boudinot, P.; Salinas, I. Nasal vaccination drives modifications of nasal and systemic antibody repertoires in rainbow trout. J. Immunol. 2019, 203, 1480. [Google Scholar] [CrossRef]
- Sepahi, A.; Kraus, A.; Casadei, E.; Johnston, C.A.; Galindo-Villegas, J.; Kelly, C.; García-Moreno, D.; Muñoz, P.; Mulero, V.; Huertas, M.; et al. Olfactory sensory neurons mediate ultrarapid antiviral immune responses in a TrkA-dependent manner. Proc. Natl. Acad. Sci. USA 2019, 116, 12428–12436. [Google Scholar] [CrossRef]
- Corfe, S.A.; Paige, C.J. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin. Immunol. 2012, 24, 198–208. [Google Scholar] [CrossRef]
- Niu, N.; Qin, X. New insights into IL-7 signaling pathways during early and late T cell development. Cell Mol. Immunol. 2013, 10, 187–189. [Google Scholar] [CrossRef]
- Kieper, W.C.; Tan, J.T.; Bondi-Boyd, B.; Gapin, L.; Sprent, J.; Ceredig, R.; Surh, C.D. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 2002, 195, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Hassane, M.; Jouan, Y.; Creusat, F.; Soulard, D.; Boisseau, C.; Gonzalez, L.; Patin, E.C.; Heuzé-Vourc’h, N.; Sirard, J.C.; Faveeuw, C.; et al. Interleukin-7 protects against bacterial respiratory infection by promoting IL-17A-producing innate T-cell response. Mucosal Immunol. 2020, 13, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ueno, Y.; Yamazaki, M.; Hibi, T. Mucosal IL-7-mediated immune responses in chronic colitis-IL-7 transgenic mouse model. Immunol. Res. 1999, 20, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lei, Y.; Cao, Z.; Chen, X.; Sun, Y.; Xu, Y.; Guo, W.; Wang, S.; Liu, C. A β-defensin gene of Trachinotus ovatus might be involved in the antimicrobial and antiviral immune response. Dev. Comp. Immunol. 2019, 92, 105–115. [Google Scholar] [CrossRef]
- Dong, J.J.; Wu, F.; Ye, X.; Sun, C.F.; Tian, Y.Y.; Lu, M.X.; Zhang, R.; Chen, Z.H. Β-defensin in Nile tilapia (Oreochromis niloticus): Sequence, tissue expression, and anti-bacterial activity of synthetic peptides. Gene 2015, 566, 23–31. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Wang, J.; Wang, X.; Peng, S.; Geng, Y.; Wang, K.; Ouyang, P.; Li, Z.; Huang, X.; et al. Identification and characterization of a β-defensin gene involved in the immune defense response of channel catfish, Ictalurus punctatus. Mol. Immunol. 2017, 85, 256–264. [Google Scholar] [CrossRef]
- Gorgoglione, B.; Taylor, N.G.H.; Holland, J.W.; Feist, S.W.; Secombes, C.J. Immune response modulation upon sequential heterogeneous co-infection with Tetracapsuloides bryosalmonae and VHSV in brown trout (Salmo trutta). Fish Shellfish Immunol. 2019, 88, 375–390. [Google Scholar] [CrossRef]
- Legler, D.F.; Thelen, M. Chemokines: Chemistry, biochemistry and biological function. Chim. Int. J. Chem. 2016, 70, 856–859. [Google Scholar] [CrossRef]
- Viola, A.; Luster, A.D. Chemokines and their receptors: Drug targets in immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 171–197. [Google Scholar] [CrossRef]
- Montero, J.; Ordas, M.C.; Alejo, A.; Gonzalez-Torres, L.; Sevilla, N.; Tafalla, C. CK12, a rainbow trout chemokine with lymphocyte chemo-attractant capacity associated to mucosal tissues. Mol. Immunol. 2011, 48, 1102–1113. [Google Scholar] [CrossRef]
- Castro, R.; Abós, B.; Pignatelli, J.; von Gersdorff Jørgensen, L.; González Granja, A.; Buchmann, K.; Tafalla, C. Early immune responses in rainbow trout liver upon viral hemorrhagic septicemia virus (VHSV) infection. PLoS ONE 2014, 9, e111084. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.; Garcia, J.; Ordas, M.C.; Casanova, I.; Gonzalez, A.; Villena, A.; Coll, J.; Tafalla, C. Specific regulation of the chemokine response to viral hemorrhagic septicemia virus at the entry site. J. Virol. 2011, 85, 4046–4056. [Google Scholar] [CrossRef] [PubMed]
- Boudinot, P.; Langevin, C.; Secombes, C.J.; Levraud, J.-P. The peculiar characteristics of fish type I interferons. Viruses 2016, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Langevin, C.; Aleksejeva, E.; Passoni, G.; Palha, N.; Levraud, J.-P.; Boudinot, P. The Antiviral Innate Immune Response in Fish: Evolution and Conservation of the IFN System. J. Mol. Biol. 2013, 425, 4904–4920. [Google Scholar] [CrossRef]
- Rao, Y.; Su, J. Insights into the antiviral immunity against grass carp (Ctenopharyngodon idella) reovirus (GCRV) in grass carp. J. Immunol. Res. 2015, 2015, 670437. [Google Scholar] [CrossRef]
- Ballesteros, N.A.; Alonso, M.; Saint-Jean, S.R.; Perez-Prieto, S.I. An oral DNA vaccine against infectious haematopoietic necrosis virus (IHNV) encapsulated in alginate microspheres induces dose-dependent immune responses and significant protection in rainbow trout (Oncorrhynchus mykiss). Fish Shellfish Immunol. 2015, 45, 877–888. [Google Scholar] [CrossRef]
- Nombela, I.; Carrion, A.; Puente-Marin, S.; Chico, V.; Mercado, L.; Perez, L.; Coll, J.; Ortega-Villaizan, M.D.M. Infectious pancreatic necrosis virus triggers antiviral immune response in rainbow trout red blood cells, despite not being infective. F1000Res 2017, 6, 1968. [Google Scholar] [CrossRef]
- Saraiva, L.R.; Ahuja, G.; Ivandic, I.; Syed, A.S.; Marioni, J.C.; Korsching, S.I.; Logan, D.W. Molecular and neuronal homology between the olfactory systems of zebrafish and mouse. Sci. Rep. 2015, 5, 11487. [Google Scholar] [CrossRef]
- Casadei, E.; Salinas, I. Comparative models for human nasal infections and immunity. Dev. Comp. Immunol. 2019, 92, 212–222. [Google Scholar] [CrossRef]
- Richard, M.; van den Brand, J.M.A.; Bestebroer, T.M.; Lexmond, P.; de Meulder, D.; Fouchier, R.A.M.; Lowen, A.C.; Herfst, S. Influenza A viruses are transmitted via the air from the nasal respiratory epithelium of ferrets. Nat. Commun. 2020, 11, 766. [Google Scholar] [CrossRef]
- Singal, C.M.S.; Jaiswal, P.; Seth, P. SARS-CoV-2, More than a Respiratory Virus: Its Potential Role in Neuropathogenesis. ACS Chem. Neurosci. 2020, 11, 1887–1899. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Name | Primer Sequence (5′-3′) | Amplicon Size | Reference |
|---|---|---|---|---|
| ck12 | ck12a F | CTCTGAGGTACCCGTGGATTGC | 277 bp | [22] |
| ck12a R | CCTTAGGGACTATTGTTCTTCAGC | |||
| il10 | il10 F | CTGCTGGACGAAGGGATTCTAC | 277 bp | [23] |
| il10 R | GGCCTTTATCCTGCATCTTCTC | |||
| il7r | il7r F | GTGGAGAAGAATTGGTTGAC | 117 bp | [24] |
| il7r R | CCTCCATTTCATCATCGGTGTC | |||
| tgfb | tgfb F | CATGTCCATCCCCCAGAACT | 361 bp | [25] |
| tgfb R | GGACAACTGTTCCACCTTGTGTT | |||
| tnfa | tnfa F | GGGGACAAACTGTGGACTGA | 66 bp | [26] |
| tnfa R | GAAGTTCTTGCCCTGCTCTG | |||
| omdb-3 | omdb-3 F | GCTTGTGGAATACAAGAGTCATCTGC | 138 bp | [27] |
| omdb-3 R | GCATACATTCGGCCATGTACATCC | |||
| il8 | il8 F | AGAATGTCAGCCAGCCTTGT | 69 bp | [28] |
| il8 R | TCTCAGACTCATCCCCTCAGT | |||
| il1b | il1b F | ACATTGCCAACCTCATCATCG | 91 bp | [29] |
| il1b R | TTGAGCAGGTCCTTGTCCTTG | |||
| il6 | il6 F | ACTCCCCTCTGTCACACACC | 295 bp | [26] |
| il6 R | GGCAGACAGGTCCTCCACTA | |||
| il17a | il17a F | CGTGTCGAAGTACCTGGTTGTGT | 212 bp | [30] |
| il17a R | GGTTCTCCACTGTAGTGCTTTTCCA | |||
| omdb-1 | omdb-1 F | GGTTTTCCTATTGCTTAATGTTGTGG | 302 bp | [27] |
| omdb-1 R | GACACACAGTTAAGTCATGG | |||
| omdb-2 | omdb-2 F | GCTGACAGCAGTGCAAGCTGATGACAC | 143 bp | [27] |
| omdb-2 R | GCAAAGCACAGCATCTTAATCTGC | |||
| omdb-4 | omdb-4 F | GCAACTCTTCTAAAGAACAGT | 238 bp | [27] |
| omdb-4 R | CGTGGGCGACACAGCATACAAATCC | |||
| ef-1a | ef-1a F | CAAGGATATCCGTCGTGGCA | 353 bp | [31] |
| ef-1a R | ACAGCGAAACGACCAAGAGG |
| 7 Dpv | 28 Dpv | |||||
|---|---|---|---|---|---|---|
| Unchal | Chal 1 | Chal 2 | Unchal | Chal 1 | Chal 2 | |
| control | 0 | 54.2 | 45.5 | 0 | 84 | 84 |
| IN | 0 | 0 | 4 | 0 | 0 | 0 |
| i.m. | 16 | 16.7 | 20.8 | 0 | 0 | 0 |
| imm | 0 | 4 | 0 | 0 | 24 | 16 |
| Dpv | Pathogen | Vaccinated | P-Value | Significance |
|---|---|---|---|---|
| 7 | IHNV | control vs. IN | <0.0001 | *** |
| 7 | IHNV | control vs. i.m. | 0.0021 | ** |
| 7 | IHNV | control vs. imm | <0.0001 | *** |
| 7 | IHNV | IN vs. i.m. | 0.0072 | ** |
| 7 | IHNV | IN vs. imm | 1 | |
| 7 | IHNV | i.m. vs. imm | 0.0072 | ** |
| 7 | none | control vs. IN | 1 | |
| 7 | none | control vs. i.m. | 0.1099 | |
| 7 | none | control vs. imm | 1 | |
| 7 | none | IN vs. i.m. | 0.1099 | |
| 7 | none | IN vs. imm | 1 | |
| 7 | none | i.m. vs. imm | 0.1099 | |
| 28 | IHNV | control vs. IN | <0.0001 | *** |
| 28 | IHNV | control vs. i.m. | <0.0001 | *** |
| 28 | IHNV | control vs. imm | <0.0001 | *** |
| 28 | IHNV | IN vs. i.m. | 1 | |
| 28 | IHNV | IN vs. imm | 0.0012 | ** |
| 28 | IHNV | i.m. vs. imm | 0.0018 | ** |
| 28 | none | control vs. IN | 1 | |
| 28 | none | control vs. i.m. | 1 | |
| 28 | none | control vs. imm | 1 | |
| 28 | none | IN vs. i.m. | 1 | |
| 28 | none | IN vs. imm | 1 | |
| 28 | none | i.m. vs. imm | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, F.; Tacchi, L.; Xu, Z.; LaPatra, S.E.; Salinas, I. Vaccination Route Determines the Kinetics and Magnitude of Nasal Innate Immune Responses in Rainbow Trout (Oncorhynchus mykiss). Biology 2020, 9, 319. https://doi.org/10.3390/biology9100319
Dong F, Tacchi L, Xu Z, LaPatra SE, Salinas I. Vaccination Route Determines the Kinetics and Magnitude of Nasal Innate Immune Responses in Rainbow Trout (Oncorhynchus mykiss). Biology. 2020; 9(10):319. https://doi.org/10.3390/biology9100319
Chicago/Turabian StyleDong, Fen, Luca Tacchi, Zhen Xu, Scott E. LaPatra, and Irene Salinas. 2020. "Vaccination Route Determines the Kinetics and Magnitude of Nasal Innate Immune Responses in Rainbow Trout (Oncorhynchus mykiss)" Biology 9, no. 10: 319. https://doi.org/10.3390/biology9100319
APA StyleDong, F., Tacchi, L., Xu, Z., LaPatra, S. E., & Salinas, I. (2020). Vaccination Route Determines the Kinetics and Magnitude of Nasal Innate Immune Responses in Rainbow Trout (Oncorhynchus mykiss). Biology, 9(10), 319. https://doi.org/10.3390/biology9100319




