Specific c-Jun N-Terminal Kinase Inhibitor, JNK-IN-8 Suppresses Mesenchymal Profile of PTX-Resistant MCF-7 Cells through Modulating PI3K/Akt, MAPK and Wnt Signaling Pathways
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Cell Culture
2.3. Trypan Blue Dye Exclusion Assay
2.4. Colony Formation Assay
2.5. Soft Agar Assay
2.6. Fluorescence Microscopy
2.6.1. Propidium Iodide (PI) Staining
2.6.2. 3,3′-Dihexyloxacarbocyanine Iodide (DiOC6) Staining
2.7. Protein Extraction and Immunoblotting
2.8. Wound-Healing Assay
2.9. RNA Isolation, cDNA Synthesis, and RT-PCR
2.10. MTT Cell Viability Assay
2.11. Statistical Analysis
3. Results
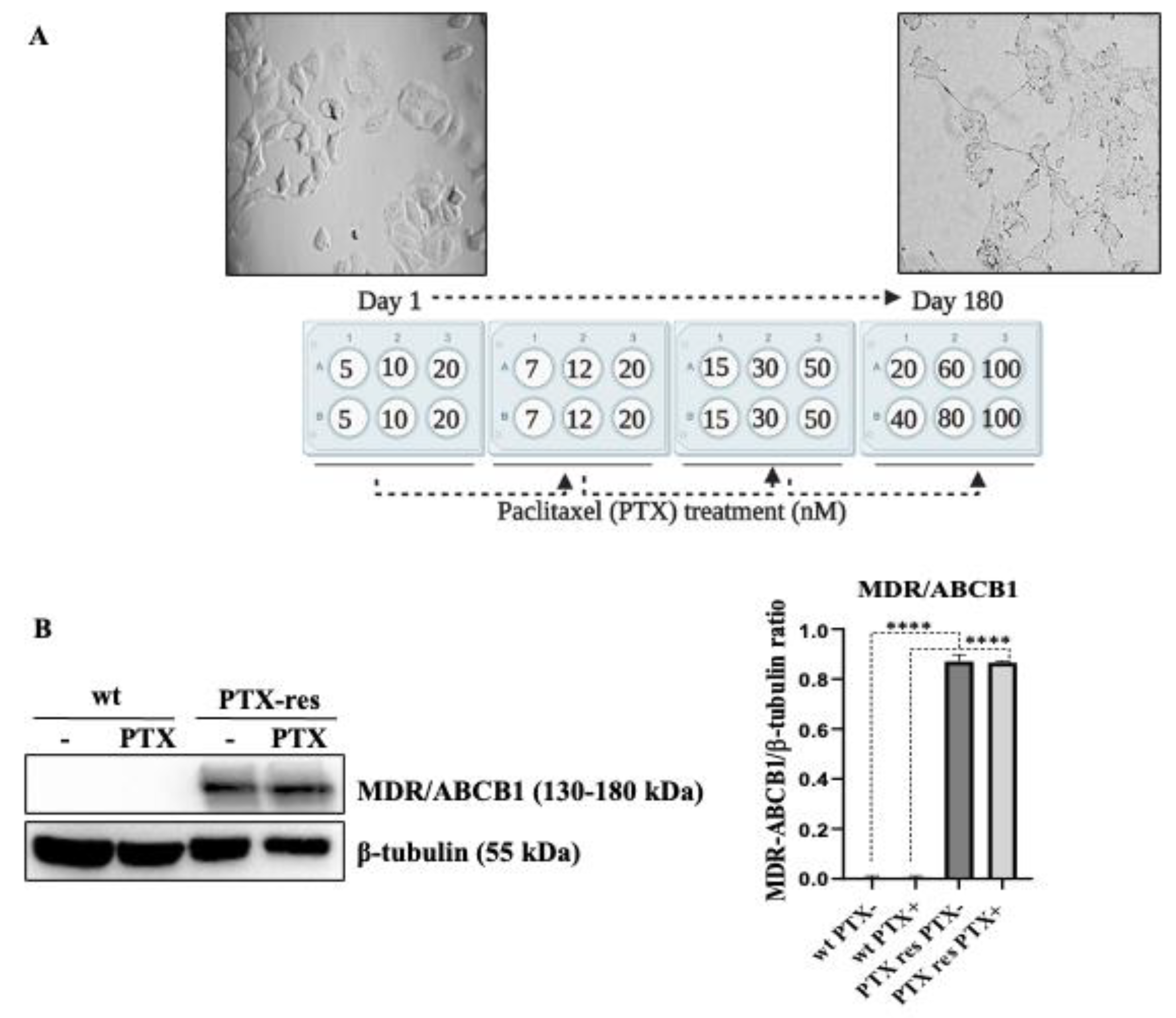
3.1. Establishment and Determination of Drug Resistance of PTX-Res MCF-7 Breast Cancer Cell Line
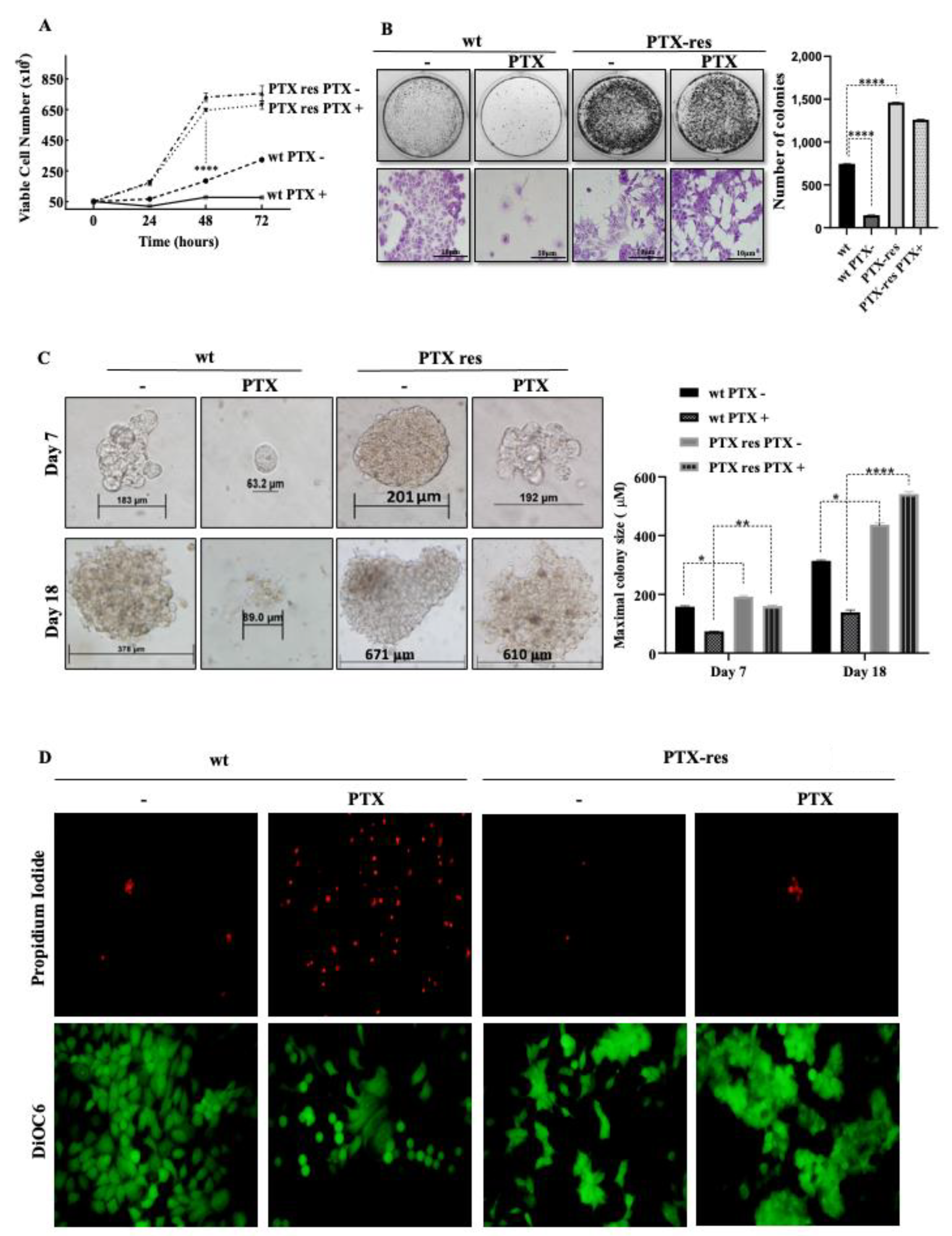
3.2. PTX-Resistance Enhanced the Proliferation and Colony Formation Potential of MCF-7 Cells
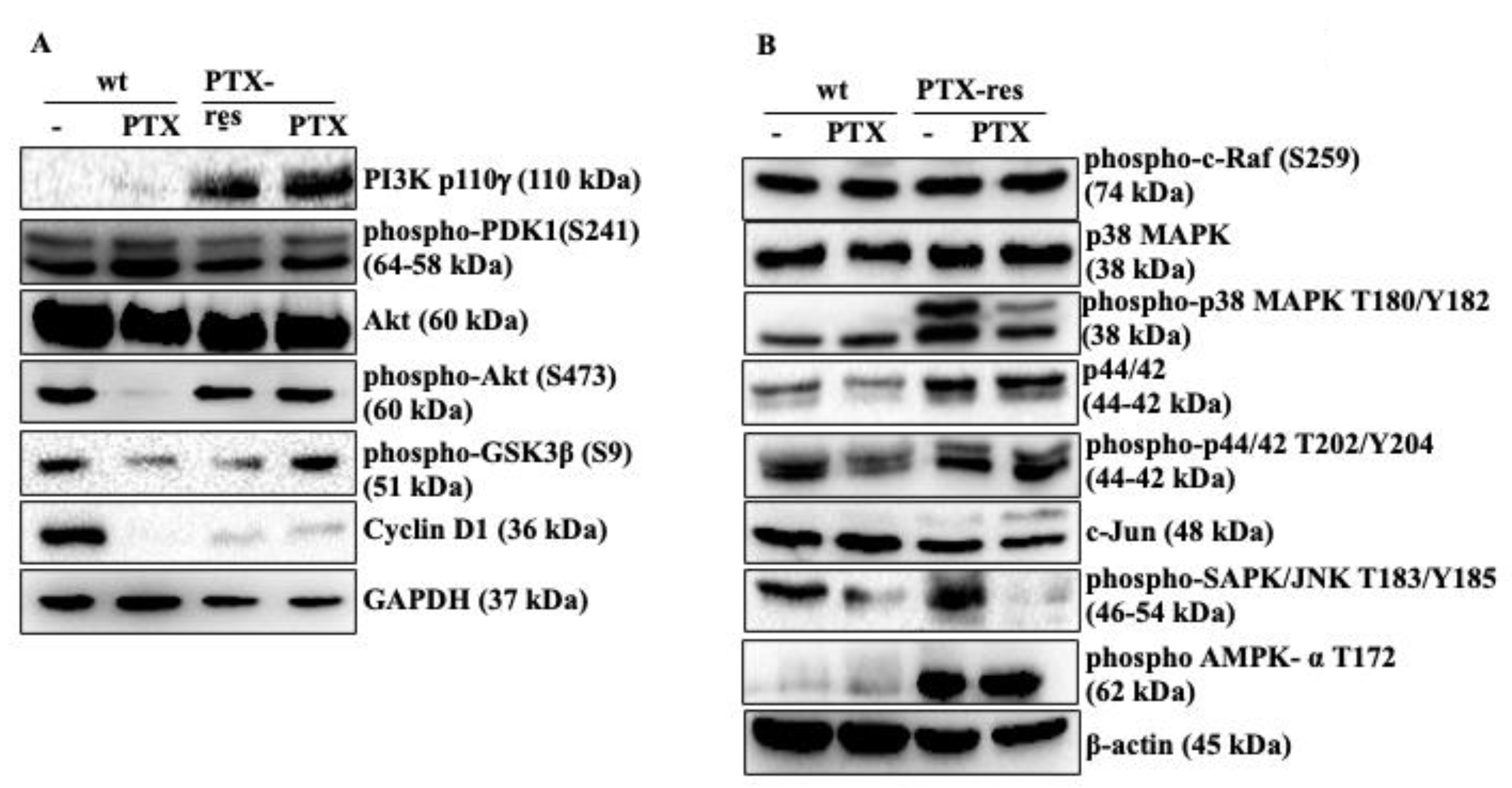
3.3. PTX-Resistance Modulated the PI3K/Akt and MAPK Pathways
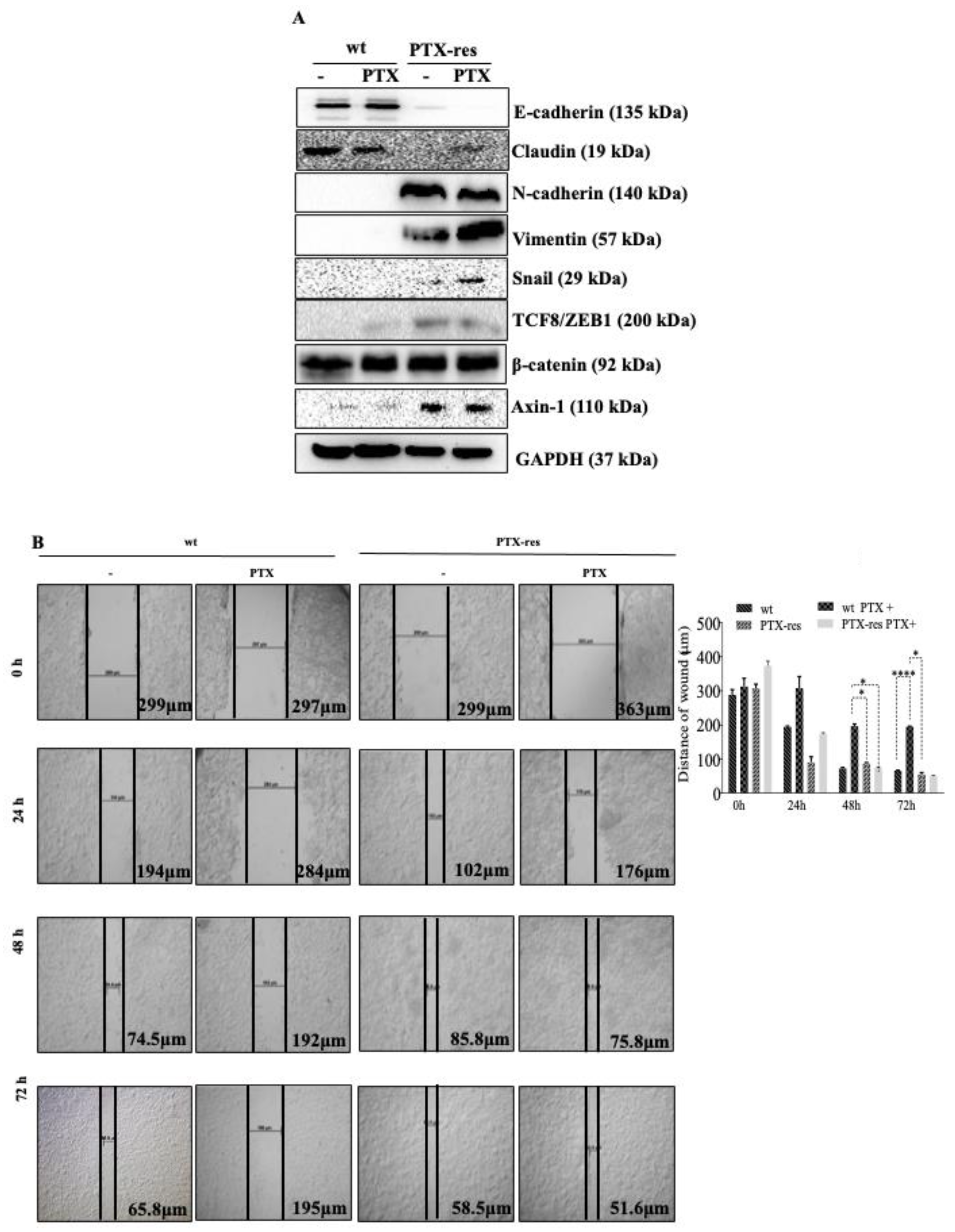
3.4. PTX-Resistance Enhance the Migratory Potential of MCF-7 Cells
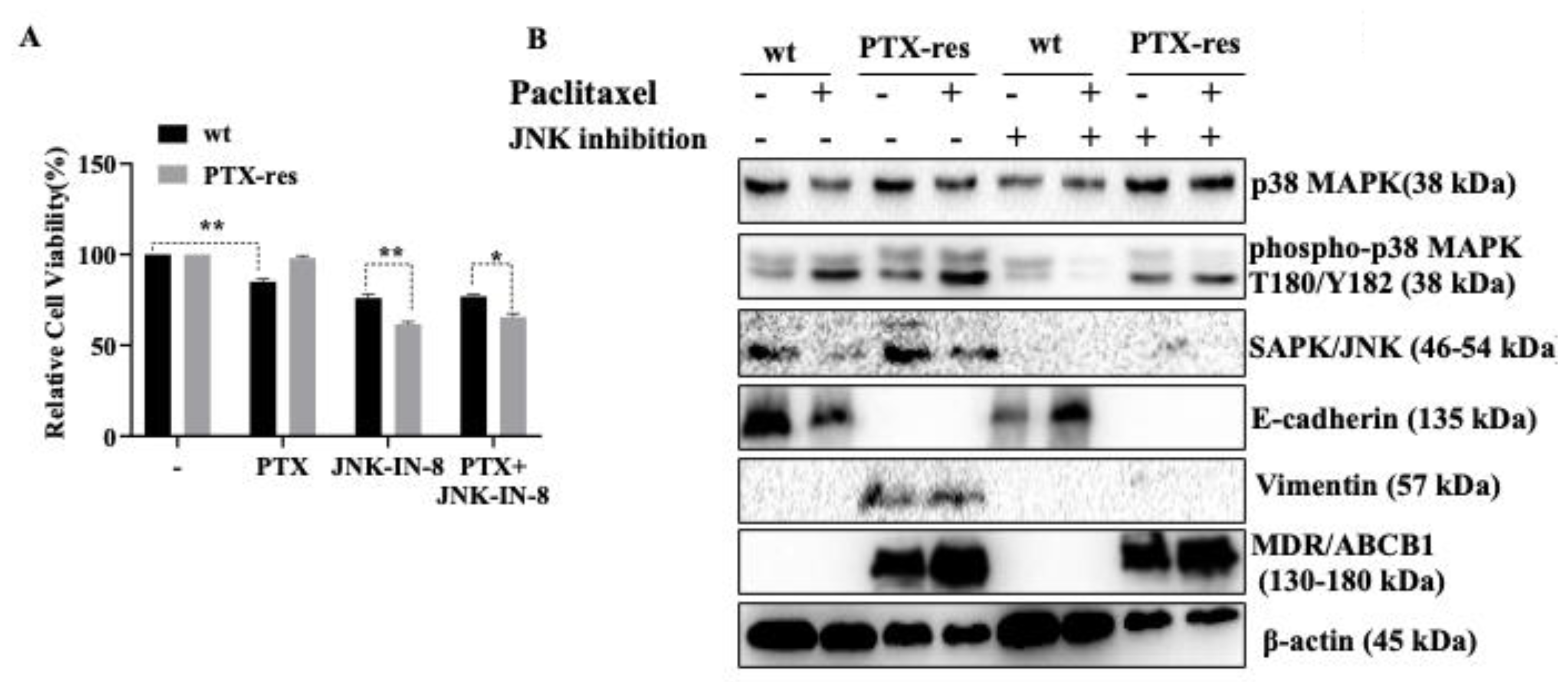
3.5. JNK Inhibitor Suppressed Stress-Activated p38 MAPK and SAPK/JNK, and Mesenchymal Marker Vimentin Expression
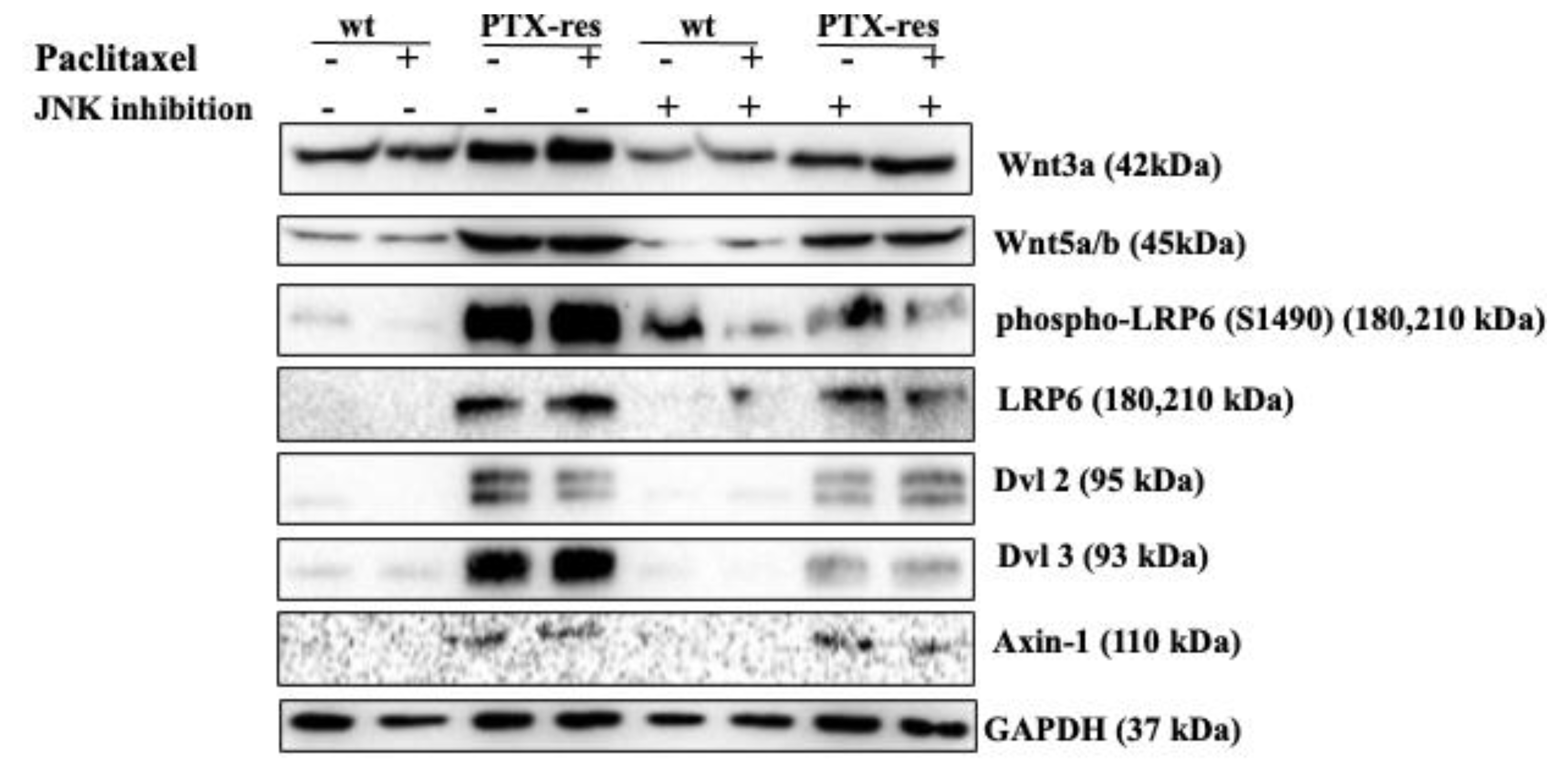
3.6. JNK Inhibitor Suppressed the Expression Profiles of Wnt Signaling Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Breast Cancer; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Ades, F.; Tryfonidis, K.; Zardavas, D. The past and future of breast cancer treatment—From the papyrus to individualised treatment approaches. Ecancermedicalscience 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Y.; Lu, X.; Zhao, Z.; Zhu, L.; Chen, S.; Wu, Q.; Chen, C.; Wang, Z. MiR-125b regulates epithelial-mesenchymal transition via targeting Sema4C in paclitaxel-resistant breast cancer cells. Oncotarget 2014, 6, 3268–3279. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Walsh, V.; Goodman, J. From taxol to taxol®: The changing identities and ownership of an anti-cancer drug. Med. Anthr. 2002, 21, 307–336. [Google Scholar] [CrossRef]
- Awtar, K.; Catherine, M.F.; Ilia, A. Drug retention, efflux, and resistance in tumor cells. Cytometry 1997. [Google Scholar] [CrossRef]
- Sparreboom, A.; Danesi, R.; Ando, Y.; Chan, J.; Figg, W.D. Pharmacogenomics of ABC transporters and its role in cancer chemotherapy. Drug Resist. Updat. 2003, 6, 71–84. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Xu, J.-D.; Wang, W.J.; Cao, X.-X.; Liu, X.-P.; Xu, Z.; Chen, Q.; Tang, F.; Chen, Z.-Q. Twist1-Mediated Adriamycin-Induced Epithelial-Mesenchymal Transition Relates to Multidrug Resistance and Invasive Potential in Breast Cancer Cells. Clin. Cancer Res. 2009, 15, 2657–2665. [Google Scholar] [CrossRef]
- Kip, A.W.; Castillo, S.S.; Dennis, P.A. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist. Updat. 2002, 5, 234–248. [Google Scholar] [CrossRef]
- Lunardi, A.; Webster, K.A.; Papa, A.; Padmani, B.; Clohessy, J.G.; Bronson, R.T.; Pandolfi, P.P. Role of aberrant PI3K pathway activation in gallbladder tumorigenesis. Oncotarget 2014, 5, 894–900. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, K.E.; Rojo, F.; She, Q.-B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; Hofmann, F.; Hicklin, D.J.; Ludwig, D.L.; et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006, 66, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Nonghua, L.; Wenting, X.; Zhen, Y. A new role for the PI3K/Akt signaling pathway in the epitelial-mesenchymal trasition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar]
- Cano, A.; Perez, F.P.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; Del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Eastham, A.M.; Spencer, H.; Soncin, F.; Ritson, S.; Merry, C.; Stern, P.L.; Ward, C.M. Epithelial-Mesenchymal Transition Events during Human Embryonic Stem Cell Differentiation. Cancer Res. 2007, 67, 11254–11262. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Boil. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Martin-Belmonte, F.; Perez-Moreno, M. Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 2011, 12, 23–38. [Google Scholar] [CrossRef]
- Scoazec, J.-Y.; Vouret-Craviari, V. Microbes-induced EMT at the crossroad of inflammation and cancer. Gut Microbes 2012, 3, 176–185. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, F.; Han, L.; Zhao, L.; Chen, J.; Olopade, O.I.; He, M.; Wei, M. HIF-2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways. J. Exp. Clin. Cancer Res. 2018, 37, 256. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt Signaling Pathway in Development and Disease. Annu. Rev. Cell Dev. Boil. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Ling, L.; Nurcombe, V.; Cool, S.M. Wnt signaling controls the fate of mesenchymal stem cells. Gene 2009, 433, 1–7. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, L.; Duan, Y.; Wang, Y.; Xuan, X.; Zhou, L.; Liu, W. Wnt/β-catenin signaling regulates neuronal differentiation of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2013, 439, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ebelt, N.; Kaoud, T.S.; Edupuganti, R.; Van Ravenstein, S.; Dalby, K.N.; Berg, C.L.V.D. A c-Jun N-terminal kinase inhibitor, JNK-IN-8, sensitizes triple negative breast cancer cells to lapatinib. Oncotarget 2017, 8, 104894–104912. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, G.-S.; Lee, J.E.; Kim, J.-H. A leukotriene B4 receptor-2 is associated with paclitaxel resistance in MCF-7/DOX breast cancer cells. Br. J. Cancer 2013, 109, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Kars, M.D.; Işeri, Ö.D.; Gündüz, U. A microarray based expression profiling of paclitaxel and vincristine resistant MCF-7 cells. Eur. J. Pharmacol. 2011, 657, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.K.; Hyeong, S.C.; Min, K.K.; Hee, J.L.; Seung, H.M.; Hee, J.J. SH003 Enhances Paclitaxel Chemosensitivity in MCF-7/PAX Breast Cancer Cells through Inhibition of MDR1 Activity. Mol. Cell. Biochem. 2016, 426, 1–8. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, J.; Wu, Q.; Cai, Y.; Zhu, L.; Lu, X.; Chen, S.; Chen, C.; Wang, Z. Acquisition of epithelial–mesenchymal transition is associated with Skp2 expression in paclitaxel-resistant breast cancer cells. Br. J. Cancer 2014, 110, 1958–1967. [Google Scholar] [CrossRef]
- Marcucci, F.; Stassi, G.; De Maria, R. Epithelial–mesenchymal transition: A new target in anticancer drug discovery. Nat. Rev. Drug Discov. 2016, 15, 311–325. [Google Scholar] [CrossRef]
- Němcová-Fürstová, V.; Kopperová, D.; Balušíková, K.; Ehrlichová, M.; Brynychová, V.; Václavíková, R.; Daniel, P.; Souček, P.; Kovář, J. Characterization of acquired paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol. Appl. Pharmacol. 2016, 310, 215–228. [Google Scholar] [CrossRef]
- Fu, S.; Chen, X.; Lo, H.-W.; Lin, J. Combined bazedoxifene and paclitaxel treatments inhibit cell viability, cell migration, colony formation, and tumor growth and induce apoptosis in breast cancer. Cancer Lett. 2019, 448, 11–19. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, J.; Chen, S.; Zheng, X.; Hu, S.; Dong, W.; Lu, J.; Xing, J.; Lu, T. Paclitaxel resistance in MCF-7/PTX cells is reversed by paeonol through suppression of the SET/phosphatidylinositol 3-kinase/Akt pathway. Mol. Med. Rep. 2012, 12, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, X.; Meng, T.; You, H.; Lu, T.; Xing, J.; Chen, S. SET protein overexpression contributes to paclitaxel resistance in MCF-7/S cells through PI3K/Akt pathway. J. Drug Target. 2016, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Minamoto, T.; Pyko, I.; Hayashi, Y.; Ham, J.-I. The Pivotal Roles of GSK3β in Glioma Biology. In Molecular Targets of CNS Tumors; IntechOpen: London, UK, 2011. [Google Scholar]
- Li, W.; Cai, J.-H.; Zhang, J.; Tang, Y.-X.; Wan, L. Effects of Cyclooxygenase Inhibitors in Combination with Taxol on Expression of Cyclin D1 and Ki-67 in a Xenograft Model of Ovarian Carcinoma. Int. J. Mol. Sci. 2012, 13, 9741–9753. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Koh, W.S.; Han, S.S. Down-regulation of Raf-1 kinase is associated with paclitaxel resistance in human breast cancer MCF-7/Adr cells. Cancer Lett. 2003, 193, 57–64. [Google Scholar] [CrossRef]
- Park, S.-H.; Seong, M.-A.; Lee, H.-Y. p38 MAPK-induced MDM2 degradation confers paclitaxel resistance through p53-mediated regulation of EGFR in human lung cancer cells. Oncotarget 2016, 7, 8184–8199. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Luo, D.; Liao, D.J.; Sun, Y. CyclinD1 protein plays different roles in modulating chemoresponses in MCF7 and MDA-MB231 cells. J. Carcinog. 2012, 11, 12. [Google Scholar] [CrossRef]
- Lehn, S.; Tobin, N.P.; Berglund, P.; Nilsson, K.; Sims, A.H.; Jirström, K.; Härkönen, P.; Lamb, R.; Landberg, G. Down-Regulation of the Oncogene Cyclin D1 Increases Migratory Capacity in Breast Cancer and Is Linked to Unfavorable Prognostic Features. Am. J. Pathol. 2010, 177, 2886–2897. [Google Scholar] [CrossRef]
- Du, F.; Wu, X.; Liu, Y.; Wang, T.; Qi, X.; Mao, Y.; Jiang, L.; Zhu, Y.; Chen, Y.; Zhu, R.; et al. Acquisition of paclitaxel resistance via PI3K-dependent epithelial-mesenchymal transition in A2780 human ovarian cancer cells. Oncol. Rep. 2013, 30, 1113–1118. [Google Scholar] [CrossRef]
- Işeri, Ö.D.; Kars, M.D.; Arpaci, F.; Atalay, C.; Pak, I.; Gündüz, U. Drug resistant MCF-7 cells exhibit epithelial-mesenchymal transition gene expression pattern. Biomed. Pharmacother. 2011, 65, 40–45. [Google Scholar] [CrossRef]
- Kajiyama, H.; Shibata, K.; Terauchi, M.; Yamashita, M.; Ino, K.; Nawa, A.; Kikkawa, F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int. J. Oncol. 2007, 31, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Wu, J.; Zhai, M.; Lu, Y.; Huang, B.; Lu, X.; Jiang, X.; Qiao, Z. Axin1 inhibits proliferation, invasion, migration and EMT of hepatocellular carcinoma by targeting miR-650. Am. J. Transl. Res. 2020, 12, 1114–1122. [Google Scholar] [PubMed]
- Grille, S.J.; Bellacosa, A.; Upson, J.; Klein-Szanto, A.J.; Van Roy, F.; Lee-Kwon, W.; Donowitz, M.; Tsichlis, P.N.; LaRue, L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003, 63, 2172–2178. [Google Scholar]
- Mingo-Sion, A.M.; Marietta, P.M.; Koller, E.; Wolf, D.M.; Berg, C.L.V.D. Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene 2004, 23, 596–604. [Google Scholar] [CrossRef]
- Zhang, T.; Inesta-Vaquera, F.; Niepel, M.; Zhang, J.; Ficarro, S.B.; Machleidt, T.; Xie, T.; Marto, J.A.; Kim, N.; Sim, T.; et al. Discovery of Potent and Selective Covalent Inhibitors of JNK. Chem. Boil. 2012, 19, 140–154. [Google Scholar] [CrossRef]
- Notte, A.; Ninane, N.; Arnould, T.; Michiels, C. Hypoxia counteracts taxol-induced apoptosis in MDA-MB-231 breast cancer cells: Role of autophagy and JNK activation. Cell Death Dis. 2013, 4, e638. [Google Scholar] [CrossRef]
- Khajah, M.A.; Mathew, P.M.; Luqmani, Y. Inhibitors of PI3K/ERK1/2/p38 MAPK Show Preferential Activity Against Endocrine-Resistant Breast Cancer Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 1283–1295. [Google Scholar] [CrossRef]
- Sunters, A.; Madureira, P.A.; Pomeranz, K.M.; Aubert, M.; Brosens, J.; Cook, S.J.; Burgering, B.M.; Coombes, R.C.; Lam, E.W.-F. Paclitaxel-Induced Nuclear Translocation of FOXO3a in Breast Cancer Cells Is Mediated by c-Jun NH2-Terminal Kinase and Akt. Cancer Res. 2006, 66, 212–220. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, T.; Liu, D.; Guan, G.; Huang, J.; Proksch, P.; Chen, X.; Lin, W.-H. Notoamide-type alkaloid induced apoptosis and autophagyviaa P38/JNK signaling pathway in hepatocellular carcinoma cells. RSC Adv. 2019, 9, 19855–19868. [Google Scholar] [CrossRef]
- Basu, S.; Cheriyamundath, S.; Ben-Ze’Ev, A. Cell–cell adhesion: Linking Wnt/β-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 2018, 7, 1488. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lu, Y.; Liu, X.; Huang, X.; Keller, E.T.; Qian, C.-N.; Zhang, J. Wnt3a: Functions and implications in cancer. Chin. J. Cancer 2015, 34, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Van Es, J.H.; Barker, N.; Clevers, H. You Wnt some, you lose some: Oncogenes in the Wnt signaling pathway. Curr. Opin. Genet. Dev. 2003, 13, 28–33. [Google Scholar] [CrossRef]
- Tesshi, Y.; Asako, S.T.; Yasuyoshi, N.; Reiko, H.; Keiji, M.; Chihaya, M.; Atsushi, O.; Setsuo, H. Transactivation of the Multidrug Resistance 1 gene by T-cell factor 4/β-catenin complex in early colorectal carcinogenesis. Cancer Res. 2000, 60, 4761–4766. [Google Scholar]
- Mohammed, M.K.; Shao, C.; Wang, J.; Wei, Q.; Wang, X.; Collier, Z.; Tang, S.; Liu, H.; Zhang, F.; Huang, J.; et al. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016, 3, 11–40. [Google Scholar] [CrossRef]
- Sun, T.; Na Co, N.; Wong, N. PFTK1 interacts with cyclin Y to activate non-canonical Wnt signaling in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2014, 449, 163–168. [Google Scholar] [CrossRef]
- Nishita, M.; Enomoto, M.; Yamagata, K.; Minami, Y. Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Boil. 2010, 20, 346–354. [Google Scholar] [CrossRef]
- Tran, H.; Bustos, D.; Yeh, R.; Rubinfeld, B.; Lam, C.; Shriver, S.; Zilberleyb, I.; Lee, M.W.; Phu, L.; Sarkar, A.A.; et al. HectD1 E3 Ligase Modifies Adenomatous Polyposis Coli (APC) with Polyubiquitin to Promote the APC-Axin Interaction. J. Boil. Chem. 2012, 288, 3753–3767. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012. [Google Scholar] [CrossRef]
- Song, X.; Wang, S.; Li, L. New insights into the regulation of Axin function in canonical Wnt signaling pathway. Protein Cell 2014, 5, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Schork, N.J.; Marschke, K.B.; Ng, S.C.; Hermann, T.W.; Zhang, J.; Sanders, J.M.; Tooker, P.; Malo, N.; Zapala, M.A.; et al. Identification of polymorphisms associated with hypertriglyceridemia and prolonged survival induced by bexarotene in treating non-small cell lung cancer. Anticancer. Res. 2011, 31, 2303–2311. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozfiliz Kilbas, P.; Sonmez, O.; Uysal-Onganer, P.; Coker Gurkan, A.; Obakan Yerlikaya, P.; Arisan, E.D. Specific c-Jun N-Terminal Kinase Inhibitor, JNK-IN-8 Suppresses Mesenchymal Profile of PTX-Resistant MCF-7 Cells through Modulating PI3K/Akt, MAPK and Wnt Signaling Pathways. Biology 2020, 9, 320. https://doi.org/10.3390/biology9100320
Ozfiliz Kilbas P, Sonmez O, Uysal-Onganer P, Coker Gurkan A, Obakan Yerlikaya P, Arisan ED. Specific c-Jun N-Terminal Kinase Inhibitor, JNK-IN-8 Suppresses Mesenchymal Profile of PTX-Resistant MCF-7 Cells through Modulating PI3K/Akt, MAPK and Wnt Signaling Pathways. Biology. 2020; 9(10):320. https://doi.org/10.3390/biology9100320
Chicago/Turabian StyleOzfiliz Kilbas, Pelin, Ozlem Sonmez, Pinar Uysal-Onganer, Ajda Coker Gurkan, Pinar Obakan Yerlikaya, and Elif Damla Arisan. 2020. "Specific c-Jun N-Terminal Kinase Inhibitor, JNK-IN-8 Suppresses Mesenchymal Profile of PTX-Resistant MCF-7 Cells through Modulating PI3K/Akt, MAPK and Wnt Signaling Pathways" Biology 9, no. 10: 320. https://doi.org/10.3390/biology9100320
APA StyleOzfiliz Kilbas, P., Sonmez, O., Uysal-Onganer, P., Coker Gurkan, A., Obakan Yerlikaya, P., & Arisan, E. D. (2020). Specific c-Jun N-Terminal Kinase Inhibitor, JNK-IN-8 Suppresses Mesenchymal Profile of PTX-Resistant MCF-7 Cells through Modulating PI3K/Akt, MAPK and Wnt Signaling Pathways. Biology, 9(10), 320. https://doi.org/10.3390/biology9100320





