Sourcing and Propagation of Pontechium maculatum for Horticulture and Species Restoration
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Ex Vitro Plants from Seeds
2.3. In Vitro Propagation
2.3.1. Shoot Culture
2.3.2. Indirect Organogenesis
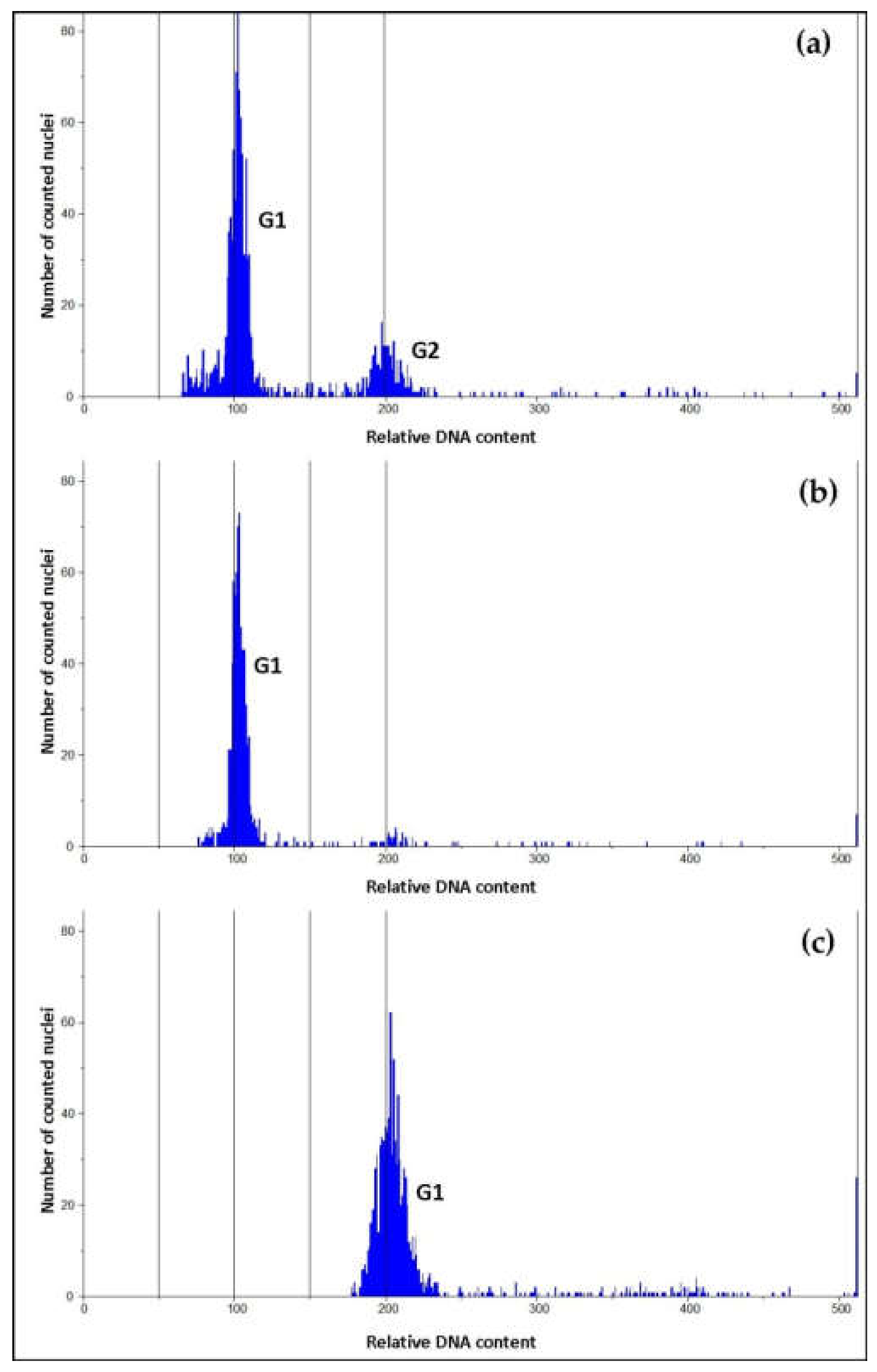
2.3.3. Ploidy Assessment
2.4. Comparison of Generative Potential of Plants in Open Field Study
2.5. Statistical Analysis
3. Results
3.1. In Vitro Propagation
3.1.1. Shoot Culture
3.1.2. Indirect Organogenesis
3.2. Ploidy Assessment
3.3. Comparison of Generative Potential of Plants in Open Field Study
3.3.1. Pollen Viability
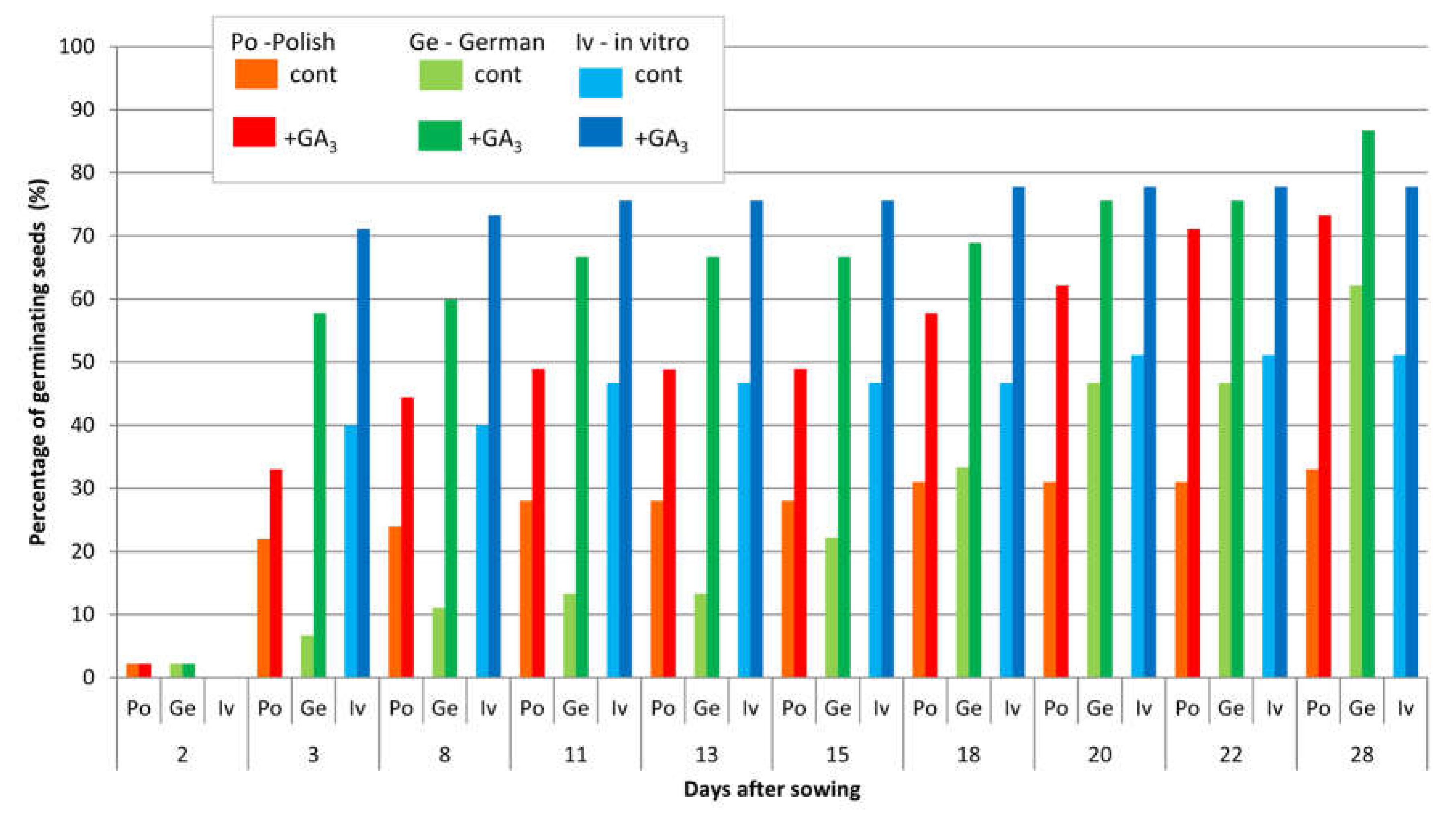
3.3.2. Germination Ability of Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heywood, V.; Casas, A.; Ford-Lloyd, B.; Kell, S.; Maxted, N. Conservation and sustainable use of crop wild relatives. Agric. Ecosyst. Environ. 2007, 121, 245–255. [Google Scholar] [CrossRef]
- Conger, B.V. (Ed.) Cloning Agricultural Plants via in Vitro Techniques, 1st ed; CRC Press: Boca Raton, FL, USA, 1981; pp. 1–280. [Google Scholar] [CrossRef]
- Gajewski, Z.; Boroń, P.; Lenart-Boroń, A.; Nowak, B.; Sitek, E.; Mitka, J. Conservation of Primula farinosa in Poland with respect to the genetic structure of populations. Acta Soc. Bot. Pol. 2018, 87, 3577. [Google Scholar] [CrossRef]
- Jones, T.A. When local isn’t best. Evol. Appl. 2013, 6, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Bucharova, A.; Bossdorf, O.; Hölzel, N.; Kollmann, J.; Prasse, R.; Durka, W. Mix and match: Regional admixture provenancing strikes a balance among different seed-sourcing strategies for ecological restoration. Conserv. Gen. 2019, 20, 7–17. [Google Scholar] [CrossRef]
- Havens, K.; Vitt, P.; Still, S.; Kramer, A.T.; Fant, J.B.; Schatz, K. Seed sourcing for restoration in an era of climate change. Nat. Areas J. 2015, 35, 122–133. [Google Scholar] [CrossRef]
- Letz, R.; Uhríková, A.; Májovský, J. Chromosome number of several interesting taxa of the flora of Slovakia. Biologia 1999, 54, 43–49. [Google Scholar]
- Hilger, H.H.; Böhle, U.R. Pontechium: A new genus distinct from Echium and Lobostemon (Boraginaceae). Taxon 2000, 49, 737–746. [Google Scholar] [CrossRef]
- Chmielewski, P.; Czarnecka, B.; Kucharczyk., M. Echium russicum J.F.Gmel. Żmijowiec czerwony. In Polska Czerwona Księga Roślin. Paprotniki i rośliny kwiatowe [Polish plant red data book. Pteridophyta and Spermatophyta]; Kaźmierczakowa, R., Zarzycki, K., Mirek, Z., Eds.; Akademia Nauk: Kraków, Poland, 2014; pp. 417–418. [Google Scholar]
- Chwil, M.; Weryszko-Chmielewska, E. Nectary structure and nectar secretion of Echium russicum J. F. GMEL. flowers. Acta Agrobot. 2007, 60, 25–33. [Google Scholar] [CrossRef]
- Nicćiforović, N.; Mihailović, V.; Mašković, P.; Solujić, S.; Stojković, A.; Pavlović Muratspahić, D. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem. Toxicol. 2010, 48, 3125–3130. [Google Scholar] [CrossRef]
- Dresler, S.; Kubrak, T.; Bogucka-Kocka, A.; Szymczak, G. Determination of shikonin and rosmarinic acid in Echium vulgare L. and Echium russicum J.F. Gmel. by Capillary Electrophoresis. J. Liq. Chromatogr. Rel. Technol. 2015, 38, 698–701. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Daironas, Z.; Zilfikarov, I.N. Shikonin and rosmarinic-acid derivatives from Echium russicum roots. Chem. Nat. Comd. 2017, 53, 953–955. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Garcia-Maroto, F.; Vilches-Ferron, M.A.; Lopez-Alonso, D. Gamma-linolenic acid from fourteen Boraginaceae species. Ind. Crops Prod. 2003, 18, 85–89. [Google Scholar] [CrossRef]
- Batsatsashvili, K.; Mehdiyeva, N.; Fayvush, G.; Kikvidze, Z.; Khutsishvili, M.; Maisaia, I.; Sikharulidze, S.; Tchelidze, D.; Aleksanyan, A.; Alizade, V.M.; et al. Echium maculatum L. Boraginaceae. In Ethnobotany of the Caucasus. European Ethnobotany; Bussmann, R., Ed.; Springer: Cham, Germany, 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Vladimirov, V.; Dane, F.; Tan, K. New floristic records in the Balkans: 28. Phytol. Balc. 2015, 21, 367–399. [Google Scholar]
- Zając, M.; Zając, A. Elementy Geo—Graficzne Rodzimej Flory Polski [The Geo—Graphical Elements of Native Flora of Poland]; Nakładem Pracowni Chorologii Komputerowej Instytutu Botaniki Uniwersytetu Jagiellońskiego: Kraków, Poland, 2009; p. 93. [Google Scholar]
- Eliáš, P.J.; Dítě, D.; Kliment, J.; Hrivnák, R.; Feráková, V. Red list of ferns and flowering plants of Slovakia. Biologia 2015, 70, 218–228. [Google Scholar] [CrossRef]
- Petrova, A.; Vladimirov, V. Red List of Bulgarian vascular plants. Phytol. Balc. 2009, 15, 63–94. [Google Scholar]
- Cursach, J.; Rita, J. Implications of the reproductive biology of the narrow endemic Naufraga balearica (Apiaceae) for its conservation status. Plant Syst. Evol. 2012, 298, 581–596. [Google Scholar] [CrossRef]
- Massey, J.R.; Whitson, P.D. Species biology, the key to plant preservation. Rhodora 1980, 82, 97–103. [Google Scholar]
- Yankova-Tsvetkova, E.; Ilieva, I.; Stanilova, M.; Stoyanov, S.; Sidjimova, B. Reproductive biology of the endangered Bulgarian endemic Centaurea achtarovii (Asteraceae). Biologia 2018, 73, 1163–1175. [Google Scholar] [CrossRef]
- Pence, V.C. The possibilities and challenges of in vitro methods for plant conservation. Kew. Bull 2010, 65, 539–547. [Google Scholar] [CrossRef]
- Bagheri, F.; Tahvilian, R.; Karimi, N.; Chalabi, M.; Azami, M. Shikonin production by callus culture of Onosma bulbotrichom as active pharmaceutical ingredient. Iran. J. Pharm. Res. 2018, 17, 495–504. [Google Scholar]
- Yaman, C.; Uranbey, S.; Ahmed, H.A.; Özcan, S.; Tugay, O.; Başalma, D. Callus induction and regeneration of Alkanna orientalis var. orientalis and A. sieheana. Bangladesh J. Bot. 2019, 48, 633–640. [Google Scholar] [CrossRef]
- Mehrabani, M.; Shams-Ardakani, M.; Ghannadi, A.; Dehkordi, N.G.; Sajjadi-Jazi, S.E. Production of rosmarinic acid in Echium amoenum Fisch. and C.A. Mey. cell cultures. Iran. J. Pharm. Res. 2005, 4, 111–115. [Google Scholar] [CrossRef]
- Zare, K.; Khosrowshahli, M.; Nazemiyeh, H.; Movafeghi, A.; Motallebi Azar, A.; Omidi, Y. Callus culture of Echium italicum L. towards production of a shikonin derivative. Nat. Prod. Res. 2011, 25, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Salehian, H.; Kabirnataj, S.; Bagheri, N.; Nematzadeh, G. Evaluation of capability of in vitro micropropagation in Iranian medicinal plant Echium amoenum Fish and C.A. Mey. Int. J. Biosci. 2014, 4, 58–63. [Google Scholar] [CrossRef]
- Turker, A.U.; Yildirim, A.B.; Taş, İ. In vitro adventitious plant regeneration of Echium orientale L., an endemic plant: The evaluation of biological activities and phenolic content. Indian J. Biochem. Biophys. 2018, 55, 264–272. [Google Scholar]
- Us-Camas, R.; Rivera-Solís, G.; Duarte-Aké, F.; de-la-Peña, C. In vitro culture: A epigenetic challenge for plants. Plant Cell. Tissue Organ. Cult. 2014, 118, 187–201. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays of tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Galbraith, W. Analysis of higher plants by flow cytometry and cell sorting. Int. Rev. Cytol. 1989, 116, 165–228. [Google Scholar] [CrossRef]
- Thiem, B.; Śliwińska, E. Flow cytometric analysis of nuclear DNA content in cloudberry (Rubus chamaemorus L.) in vitro cultures. Plant Sci. 2003, 164, 129–134. [Google Scholar] [CrossRef]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Aloni, R.; Langhans, M.; Aloni, E.; Dreieicher, E.; Ullrich, C.I. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. J. Exp. Bot. 2005, 56, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Rahman, M. In vitro micropropagation of Heliotropium indicum Linn.: An important medicinal Herb. J. Pharmacogn. Phytochem. 2018, 8, 1899–1903. [Google Scholar]
- Parray, J.A.; Kamili, A.N.; Jan, S.; Mir, M.Y.; Shameem, N.; Ganai, B.A.; Abd Allah, E.F.; Hashem, A.; Alqarawi, A.A. Manipulation of plant growth regulators on phytochemical constituents and DNA protection potential of the medicinal plant Arnebia benthamii. BioMed. Res. Int. 2018, 2018, 6870139. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, D.H.; Saini, R.K.; Gopal, J.; Keum, Y.S.; Sivanesan, I. Micropropagation and Quantification of Bioactive Compounds in Mertensia maritima (L.) Gray. Int. J. Mol. Sci. 2019, 20, 2141. [Google Scholar] [CrossRef] [PubMed]
- Zdravković-Korać, S.; Tubić, L.; Devrnja, N.; Ćalić, D.; Milojević, J.; Milić, M.; Savić, J. Somatic embryogenesis from stamen filaments of Aesculus flava Sol. and peroxidase activity during the transition from friable to embryogenic callus. Sci. Hortic. 2019, 247, 362–372. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y.D.; Li, Y.; Xia, Y.P. Plantlet regeneration from primary callus cultures of Lilium brownii F.E.Br. ex Miellez var. giganteum Li, G.Y. and Chen, Z.H., a rare bulbous germplasm. In Vitro Cell. Dev. Biol. Plant 2019, 55, 44–59. [Google Scholar] [CrossRef]
- Quambusch, M.; Gruß, S.; Pscherer, T.; Winkelmann, T.; Bartsch, M. Improved in vitro rooting of Prunus avium microshoots using a dark treatment and an auxin pulse. Sci. Hortic. 2017, 220, 52–56. [Google Scholar] [CrossRef]
- Nameth, B.M.; Goron, T.L.; Dinka, S.J.; Morris, A.D.; English, J.; Lewis, D.; Oro, R.; Raizada, M.N. The initial hours of post-excision light are critical for adventitious root regeneration from Arabidopsis thaliana (L.) Heynh. cotyledon explants. In Vitro Cell. Dev. Biol. Plant 2018, 54, 273. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Poudyal, B.K.; Du, G.; Zhang, Y.; Liu, J.; Shi, Q. Studies on browning problem and phenols content on shoots of Yali, Aikansui and Abbe Fetel pears for in vitro culture. Front. Agri. China 2008, 2, 321–330. [Google Scholar] [CrossRef]
- Aliyu, A.B.; Musa, A.M.; Ibrahim, M.A.; Ibrahim, H.; Oyewale, A.O. Preliminary phytochemical screening and antioxidant activity of leave extract of Albizia chevalieri harms (Leguminoseae-Mimosoideae). Bayero J. Pure App. Sci. 2009, 2, 149–153. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; et al. Gibberellins producing endophytic fungus Porostereum spadiceum agh786 rescues growth of salt affected soybean. Front. Microbiol. 2017, 8, 686. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Wang, G.X.; Wang, X.D.; Guo, J.Y. Germination, osmotic adjustment, and antioxidant enzyme activities of gibberellin-pretreated Picea asperata seeds under water stress. New Forests 2010, 39, 231–243. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, M.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech. 2016, 6, 54. [Google Scholar] [CrossRef]
- Prado, M.J.; Herrera, M.T.; Vázquez, R.A.; Romo, S.; González, M.V. Micropropagation of two selected male kiwifruit and analysis of genetic variation with AFLP markers. HortScience 2005, 40, 740–746. [Google Scholar] [CrossRef]
- Farahani, F.; Yari, R.; Masoud, S. Somaclonal variation in Dezful cultivar of olive (Olea europaea subsp. europaea). Gene Conserve 2011, 10, 216–221. [Google Scholar]
- Sivanesan, I.; Jeong, B.R. Identification of somaclonal variants in proliferating shoot cultures of Senecio cruentus cv. Tokyo Daruma. Plant Cell Tissue Organ. Cult. 2012, 111, 247–253. [Google Scholar] [CrossRef]
- Fay, M.F. In what situation is in vitro culture appropriate to plant conservation? Biodivers. Conserv. 1994, 3, 176–183. [Google Scholar] [CrossRef]
- Żabicki, P.; Śliwińska, E.; Mitka, J.; Sutkowska, A.; Tuleja, M.; Migdałek, G.; Żabicka, Ż.; Słomka, A.; Kwiatkowska, M.; Kuta, E.; et al. Does somaclonal variation play advantageous role in conservation practice of endangered species? Comprehensive genetic studies of in vitro propagated plantlets of Viola stagnina Kit. (Violaceae). Plant Cell Tissue Organ. Cult. 2019, 136, 339–352. [Google Scholar] [CrossRef]
- Melser, C.; Bijleveld, A.; Klinkhamer, P.G. Late-acting inbreeding depression in both male and female function of Echium vulgare (Boraginaceae). Heredity 1999, 83, 162–170. [Google Scholar] [CrossRef]
- Klemow, K.M.; Clements, D.R.; Threadgill, P.F.; Cavers, P.B. The biology of Canadian weeds. 116. Echium vulgare L. Can. J. Plant Sci. 2002, 82, 235–248. [Google Scholar] [CrossRef]
- Bischoff, A.; Vonlanthen, B.; Steinger, T.; Müller-Schärer, H. Seed provenance matters-Effects on germination of four plant species used for ecological restoration. Basic Appl. Ecol. 2006, 7, 347–359. [Google Scholar] [CrossRef]
- Bischoff, A.; Müller-Schärer, H. Testing population differentiation in plant species–how important are environmental maternal effects. Oikos 2010, 119, 445–454. [Google Scholar] [CrossRef]
- Sharaf, A.R.N.; Hamidoghli, Y.; Zakizadeh, H. In vitro seed germination and micropropagation of primrose (Primula heterochroma Stapf.) an endemic endangered Iranian species via shoot tip explants. Hortic. Environ. Biotechnol. 2011, 52, 298–302. [Google Scholar] [CrossRef]
- Gutterman, Y. Maternal effects on seeds during development. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; Oxford University Press: Oxford, UK, 2000; pp. 59–84. [Google Scholar]
- Helenurm, K.; Schaal, B.A. Genetic and maternal effects on offspring fitness in Lupinus texensis (Fabaceae). Am. J. Bot. 1996, 83, 1596–1608. [Google Scholar] [CrossRef]
- Wulff, R.D.; Causin, H.F.; Benitez, O.; Bacalini, P.A. Intraspecific variability and maternal effects in the response to nutrient addition in Chenopodium album. Can. J. Bot. 1999, 77, 1150–1158. [Google Scholar] [CrossRef]
- Kuzemko, A.A. (Ed.) Finds of Plants and Fungi of the Red Book and the Berne Convention (Resolution 6); Issue Kyiv-Chernivtsi: Kiev, Ukraine, 2019; pp. 1–496. [Google Scholar]
- Linhart, Y.B.; Grant, M.C. Evolutionary significance of local genetic differentiation in plants. Annu. Rev. Ecol. Syst. 1996, 27, 237–277. [Google Scholar] [CrossRef]
- Jacquemyn, H.; de Meester, L.; Jongejans, E.; Honnay, O. Evolutionary changes in plant reproductive traits following habitat fragmentation and their consequences for population fitness. J. Ecol. 2012, 100, 76–87. [Google Scholar] [CrossRef]
- Kar, P.; Chakraborty, A.K.; Bhattacharya, M.; Mishra, T.; Sen, A. Micropropagation, genetic fidelity assessment and phytochemical studies of Clerodendrum thomsoniae Balf. f. with special reference to its anti-stress properties. Res. Plant Biol. 2019, 9, 9–15. [Google Scholar] [CrossRef]
- Jędrzejczyk, I.; Morozowska, M.; Nowińska, R.; Jagodziński, A.M. Primula veris plants derived from in vitro cultures and from seeds: Genetic stability, morphology, and seed characteristics. Turk. J. Bot. 2018, 42, 412–422. [Google Scholar] [CrossRef]
- Duarte-Aké, F.; Castillo-Castro, E.; Pool, F.B.; Espadas, F.; Santamaría, J.M.; Manuel, L.; Robert, M.L.; de-la-Peña, C. Physiological differences and changes in global DNA methylation levels in Agave angustifolia Haw. albino variant somaclones during the micropropagation process. Plant Cell Rep. 2016, 35, 2489–2502. [Google Scholar] [CrossRef] [PubMed]
- Trejgell, A.; Dąbrowska, A.; Tretyn, A. Micropropagation and influence of in vitro culture on development of Cirsium pannonicum (L. f.) LINK regenerants. Acta Sci. Pol. Hortorum Cultus 2012, 11, 81–90. [Google Scholar]
- Falk, D.A.; Holsinger, K.E. Genetics and Conservation of Rare Plants; Oxford University Press: New York, NY, USA, 1991; pp. 1–283. [Google Scholar]
- Vergeer, P.; Sonderen, E.; Ouborg, J.N. Introduction strategies put to the test: Local adaptation versus heterosis. Conserv. Biol. 2004, 18, 812–821. [Google Scholar] [CrossRef]
- Kramer, A.; Havens, K. Plant conservation genetics in a changing world. Trends Plant Sci. 2009, 14, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Kollman, J.; Edwards, P.J. Genetic introgression from distant provenances reduces fitness in local weed populations. J. Appl. Ecol. 2000, 37, 647–659. [Google Scholar] [CrossRef]
- Montalvo, A.; Ellstrand, N. Nonlocal transplantation and outbreeding depression in the subshrub Lotus scoparius (Fabaceae). Am. J. Bot. 2001, 88, 258–269. [Google Scholar] [CrossRef]
- Maschinski, J.; Wright, S.J.; Koptur, S.; Pinto-Torres, E.C. When is local the best paradigm? Breeding history influences conservation reintroduction survival and population trajectories in times of extreme climate events. Biol. Conserv. 2013, 159, 277–284. [Google Scholar] [CrossRef]

| BAP (mg L−1) | Auxins (mg L−1) | Mean Multiplication Rate | Mean Height (mm) | Mean Number of Leaves Per Shoot | Vitrification (%) | |
|---|---|---|---|---|---|---|
| IBA | NAA | |||||
| - | - | - | 8.9 b * | 12.3 a | 3.4 a | 9.1 a |
| 0.1 | 0.05 | - | 5.2 ab | 18.7 a | 6.2 a | 22.8 a |
| 0.25 | - | 0.05 | 5.6 ab | 15.4 a | 4.8 a | 19.9 a |
| 1.5 | 0.1 | - | 4.9 a | 14.6 a | 4.9 a | 6.8 a |
| 0.5 | - | 0.1 | 4.4 a | 21.7 a | 4.8 a | 18.6 a |
| Auxin (mg L−1) | Rooting (%) | Mean Length of Root (mm) | Number of Root | Mean Height (mm) | Mean Number of Leaves Per Shoot | Vitrificatio n (%) | Mean Multiplication Rate |
|---|---|---|---|---|---|---|---|
| 0.1 IAA | 63.8 a * | 29.6 a | 1.9 a | 29.6 a | 4.7 a | 3.5 a | 1.0 a |
| 0.1 IBA | 59.0 a | 42.4 a | 4.5 a | 16.6 a | 5.4 a | 10.7 a | 6.5 a |
| 0.1 NAA | 53.8 a | 22.0 a | 5.7 a | 14.8 a | 4.5 a | 10.1 a | 16.0 a |
| Light Conditions | Medium | Mean | |||||
|---|---|---|---|---|---|---|---|
| MS | MS +0.1 IBA +0.5 BAP | MS +0.05 IBA +0.2 BAP | MS +0.1 NAA +0.5 BAP | MS +0.1 IAA +0.05 BAP | MS +0.1 IAA +0.05 BAP +0.5 GA3 | ||
| Percent of callusing explants | |||||||
| Photoperiod | 0.0 a * ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 A |
| Darkness | 23.1 c ± 17.0 | 14.6 cd ± 4.9 | 16.7 bc ± 6.9 | 9.0 ab ± 4.8 | 57.5 d ± 10.5 | 0.0 a ±0.0 | 20.1 B |
| Mean | 11.6 B | 7.3 AB | 8.4 AB | 4.5 AB | 28.8 C | 0.0 A | |
| Percent of explants with organogenesis | |||||||
| Photoperiod | 0.0 a ±0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 30.0 b ± 21.5 | 5.0 A |
| Darkness | 29.9 b ± 12.1 | 85.4 d ± 4.9 | 83.3d ± 6.8 | 82.1 d ± 9.6 | 42.5 bc ± 10.5 | 65. 5cd ± 22.7 | 64.7 B |
| Mean | 14.9 A | 42.7 B | 41.7 B | 41.1 B | 21.3 A | 47.8 B | |
| Mean number of buds per explants (pcs) | |||||||
| Photoperiod | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 0.0 a ± 0.0 | 2.4 bc ± 0.9 | 0.4 A |
| Darkness | 1.0 ab ± 0.6 | 6.3 e ± 1.6 | 3.8 cd ± 1.4 | 4.9 de ± 1.7 | 3.3 cd ± 0.7 | 3.1 cd ± 1.3 | 3.7 B |
| Mean | 0.5 A | 3.2 C | 1.9 BC | 2.5 BC | 1.7 AB | 2.8 BC | |
| Plant Origin | Mean Number of Vegetative Rosettes Per Plant | Flowering Specimens ** (%) | Mean Number of Inflorescences Per Flowering Plant IP | Length of Inflorescences (cm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | Mean | 2013 | 2014 | 2015 | Mean | 2013 | 2014 | 2015 | Mean | 2013 | 2014 | 2015 | Mean | |
| Polish—seed origin | 2.2 ab * | 3.7 ab | 3.2 ab | 3.0 A | 77.8 a | 44.4 a | 61.1 a | 61.1 A | 1.6 a | 3.3 ab | 3.9 ab | 2.8 A | 12.7 a | 18.2 a | 18.8 a | 16.1 A |
| German—seed origin | 0.0 a | 5.5 b | 5.0 b | 3.7 A | 100 a | 33.3 a | 92.3 b | 75.2 AB | 2.9 ab | 2.6 ab | 7.8 ab | 4.8 B | 17.7 a | 18.0 a | 21.4 a | 16.6 A |
| German—in vitro | 0.0 a | 5.3 b | 3.9 ab | 3.1 A | 100 a | 77.8 a | 100 b | 92.6 AB | 3.8 ab | 3.6 ab | 6.2 ab | 4.6 AB | 19.7 a | 14.9 a | 15.1 a | 19.2 A |
| Mean | 0.9 A | 4.7 B | 4.2 B | 90.4 B | 47.6 A | 80.9 B | 2.6 A | 3.2 A | 6.1 B | 16.3 A | 17.4 A | 19.8 A | ||||
| Plant Origin | Mean Umber of Cymes Per Inflorescence CI | Mean Number of Cymes Per Plant CP = IP × CI | Mean Number of Flowers in Cymes FlC | Number of Seeds Set per One Fruit SFr | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | Mean | 2013 | 2014 | 2015 | Mean | 2013 | 2014 | 2015 | Mean | 2013 | 2014 | 2015 | Mean | |
| Polish—seed origin | 42.4 a * | 23.3 a | 40.6 a | 37.1 A | 63.4 a | 74.7 a | 186 ab | 107.0A | 9.1 b | 7.0 ab | 7.7 ab | 8.0 B | 0.9 a | 1.3 ab | 0.98 ab | 1.0 AB |
| German—seed origin | 63.5 b | 40.9 a | 47.9 a | 53.9 B | 172.6 ab | 76.4 ab | 411.9 b | 252.3B | 8.2 b | 9.6 b | 6.8 ab | 8.0 B | 0.8 a | 1.6 b | 1.20 ab | 1.1 B |
| German—in vitro | 68.3 b | 44.6 a | 38.6 a | 50.2 AB | 229. ab | 130.7 ab | 259.9 ab | 212.1AB | 4.42 a | 7.1 ab | 5.9 ab | 5.8 A | 0.6 a | 0.8 a | 0.88 a | 0.8 A |
| Mean | 56.4 B | 35.1 A | 42.9 A | 143.6 A | 94.7 A | 295.1 B | 7.3 A | 7.9 A | 6.8 A | 0.8 A | 1.1 B | 1.0 AB | ||||
| Plant Origin | Number of Seeds Set Per Flowering Plant AP = CP × FlC× SFr | Potential Seed Production Per Flowering Plant PP = CP × FlC × 4 | Efficacy of Seed Set (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | Mean | 2013 | 2014 | 2015 | Mean | 2013 | 2014 | 2015 | Mean | |
| Polish—seed origin | 490.0a * | 679.3a | 1403.6ab | 840.4A | 2305.9a | 2090.2a | 5208.3a | 3221.1A | 21.3 | 32.5 | 27.0 | 26.8 |
| German—seed origin | 1174.9a | 1196.1ab | 3361.0b | 2093.3B | 5662.0a | 2935.3a | 13180.6b | 8210.7B | 20.8 | 40.8 | 25.5 | 29.0 |
| German—in vitro | 628.9a | 705.3a | 1349.4ab | 921.4A | 4058.4a | 3712.3a | 6133.6ab | 4735.7AB | 15.5 | 19.0 | 22.0 | 18.8 |
| Mean | 797.7A | 817. 6A | 2159.9B | 4045.4A | 2869.2A | 8601.2B | 19.2 | 30.7 | 24.8 | |||
| Plant Origin | Pollen Viability (%) | |||
|---|---|---|---|---|
| 2013 | 2014 | 2015 | Mean | |
| Polish—seed origin | 45.6 ab* | 48.1 ab | 58.5 bcd | 50.7 A |
| German—seed origin | 63.1 cd | 51.3 ab | 70.2 d | 61.5 B |
| German—in vitro | 69.6 d | 44.8 a | 87.8 e | 67.4 C |
| Mean | 59.4 B | 48.0 A | 72.1 C | |
| Plant Origin | Control | +GA3 | Mean |
|---|---|---|---|
| In the year of harvest | |||
| Polish—seed origin | 41.7 a * | 71.6 ab | 56.6 A |
| German—seed origin | 53.3 ab | 83.3 b | 68.3 A |
| German—in vitro | 48.4 a | 73.6 ab | 61.0 A |
| Mean | 47.8 B | 76.2 A | |
| One year after harvest | |||
| Polish—seed origin | 74.0 a | 81.1 a | 77.8 A |
| German—seed origin | 72.2 a | 77.8 a | 75.0 A |
| German—in vitro | 58.9 a | 69.7 a | 64.3 A |
| Mean | 76.2 A | 68.5 A | |
| Two years after harvest | |||
| Polish—seed origin | 88.7 b | 86.7 b | 87.7 B |
| German—seed origin | 73.3 b | 100 b | 86.7 B |
| German—in vitro | 46.6 a | 46.6 a | 46.6 A |
| Mean | 69.5 A | 77.7 A | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, B.; Sitek, E.; Augustynowicz, J. Sourcing and Propagation of Pontechium maculatum for Horticulture and Species Restoration. Biology 2020, 9, 317. https://doi.org/10.3390/biology9100317
Nowak B, Sitek E, Augustynowicz J. Sourcing and Propagation of Pontechium maculatum for Horticulture and Species Restoration. Biology. 2020; 9(10):317. https://doi.org/10.3390/biology9100317
Chicago/Turabian StyleNowak, Barbara, Ewa Sitek, and Joanna Augustynowicz. 2020. "Sourcing and Propagation of Pontechium maculatum for Horticulture and Species Restoration" Biology 9, no. 10: 317. https://doi.org/10.3390/biology9100317
APA StyleNowak, B., Sitek, E., & Augustynowicz, J. (2020). Sourcing and Propagation of Pontechium maculatum for Horticulture and Species Restoration. Biology, 9(10), 317. https://doi.org/10.3390/biology9100317





