The Use of Myelinating Cultures as a Screen of Glycomolecules for CNS Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Library of Glycomolecules
2.2. Astrocytes Derived from Neurospheres
2.3. Myelinating Spinal Cord Cultures (MC-Dev)
2.4. Myelinating Injured Cultures (MC-Inj)
2.5. Demyelinated Cultures (MC-Demy)
2.6. Immunocytochemistry
2.7. Microscopy and Image Analysis
2.8. Re/myelination Quantification
2.9. Neurite Outgrowth
2.10. Statistical Analysis
3. Results
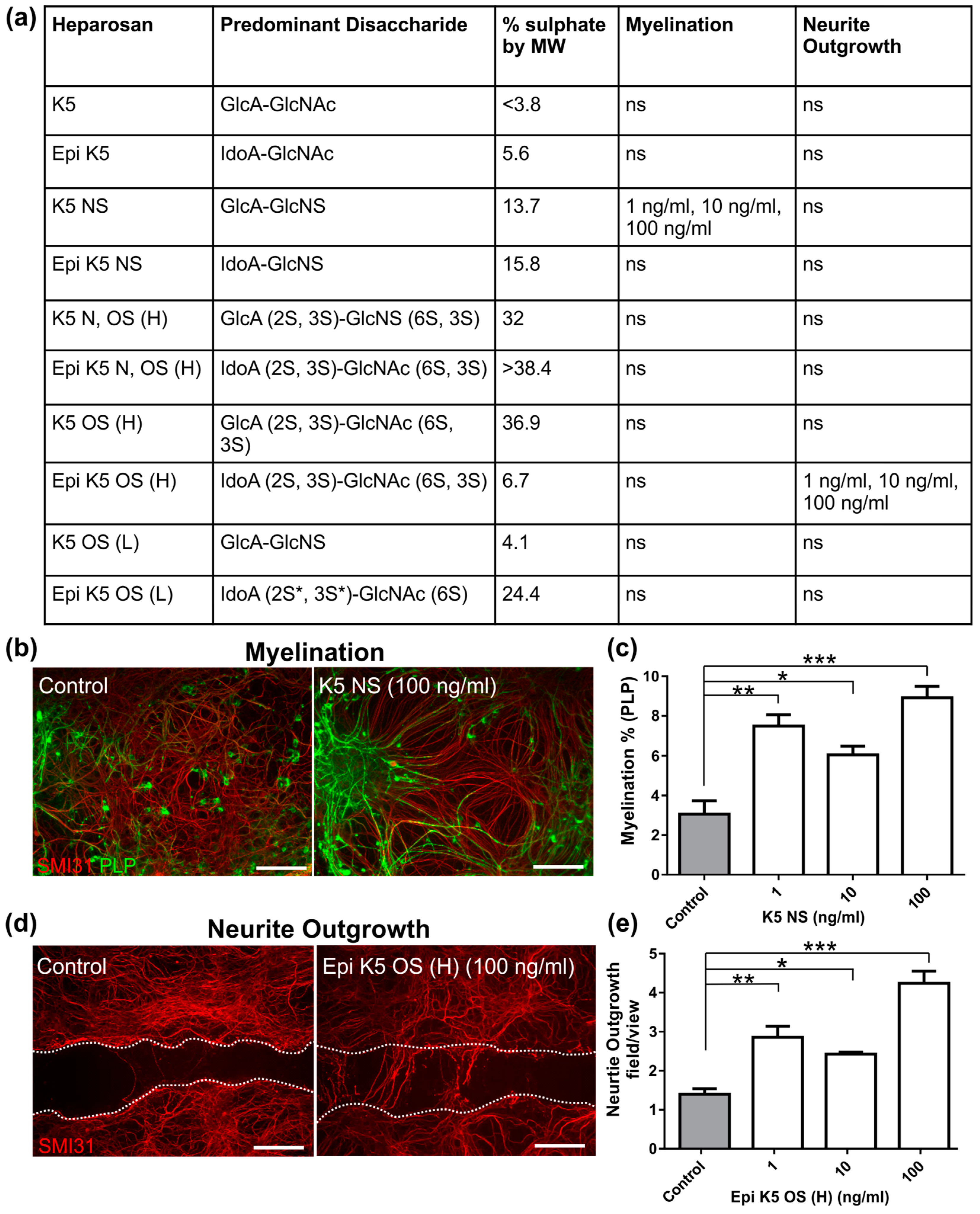
3.1. Heparosans K5 NS and (Epi) K5 OS (H) Promote CNS Repair in MC-Inj
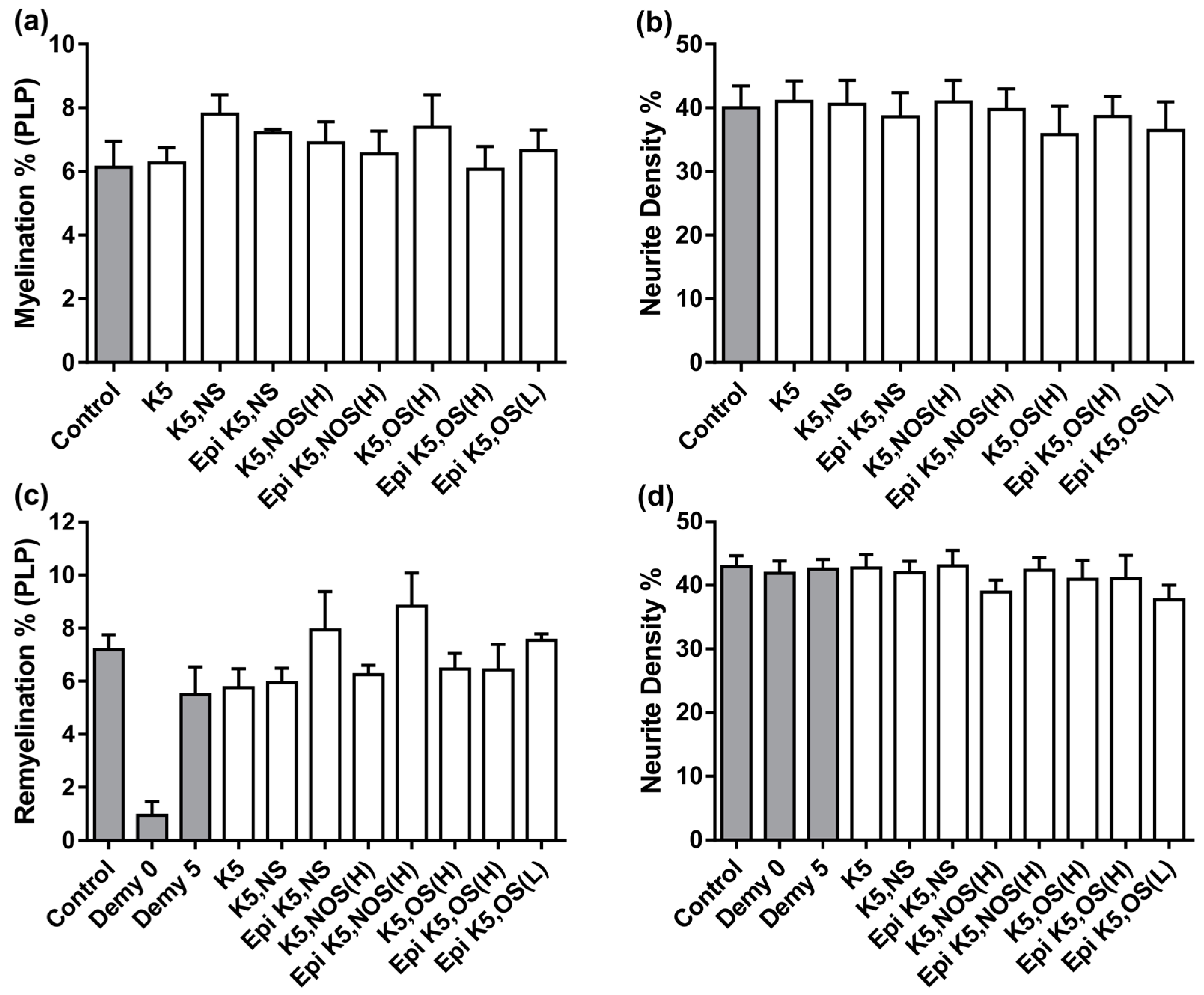
3.2. Heparosans Do Not Affect the Development of Myelination in MC-Dev or Remyelination in MC-Demy
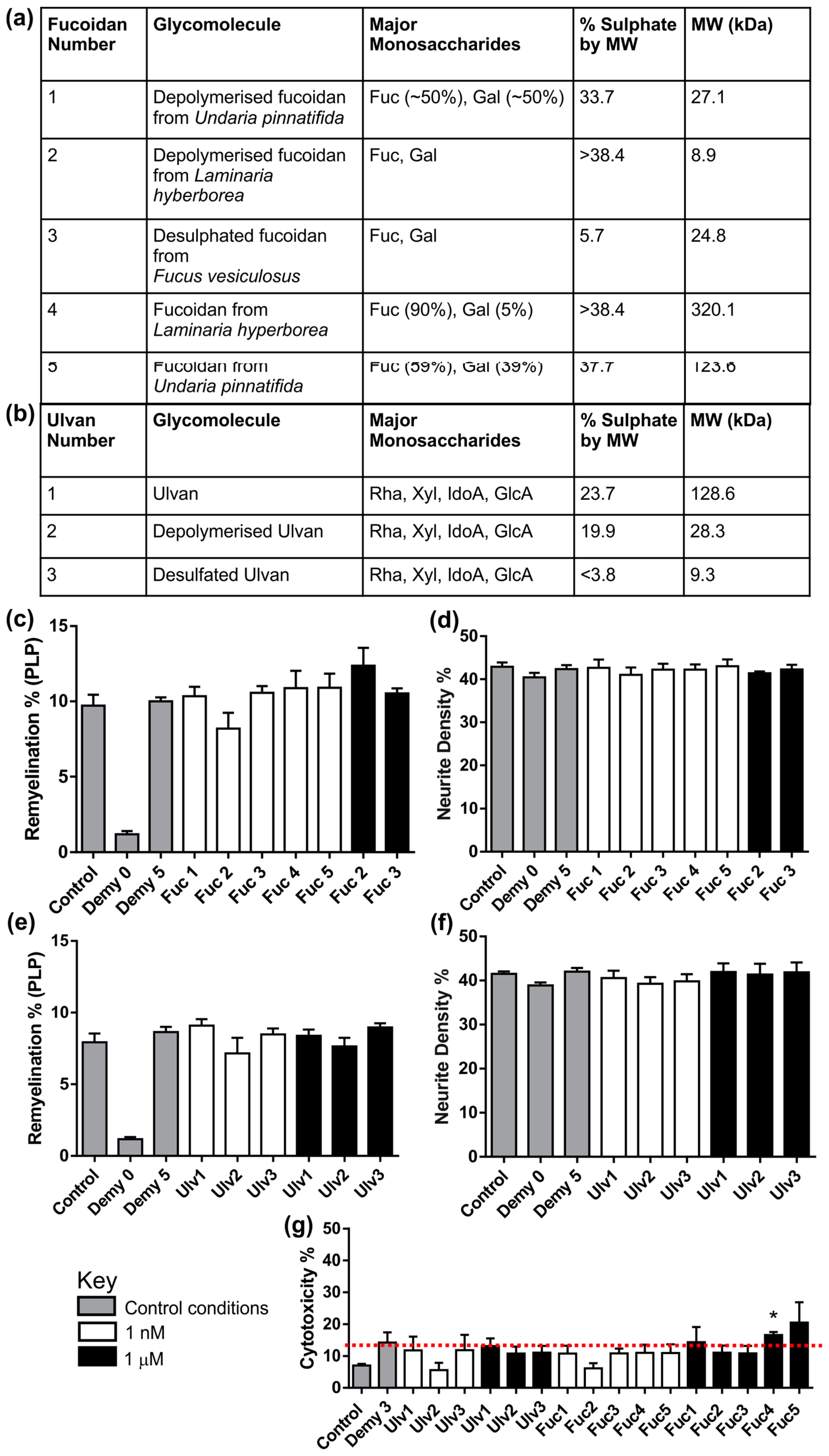
3.3. Fucoidans or Ulvans Did Not Affect the Level of Remyelination in MC-Demy
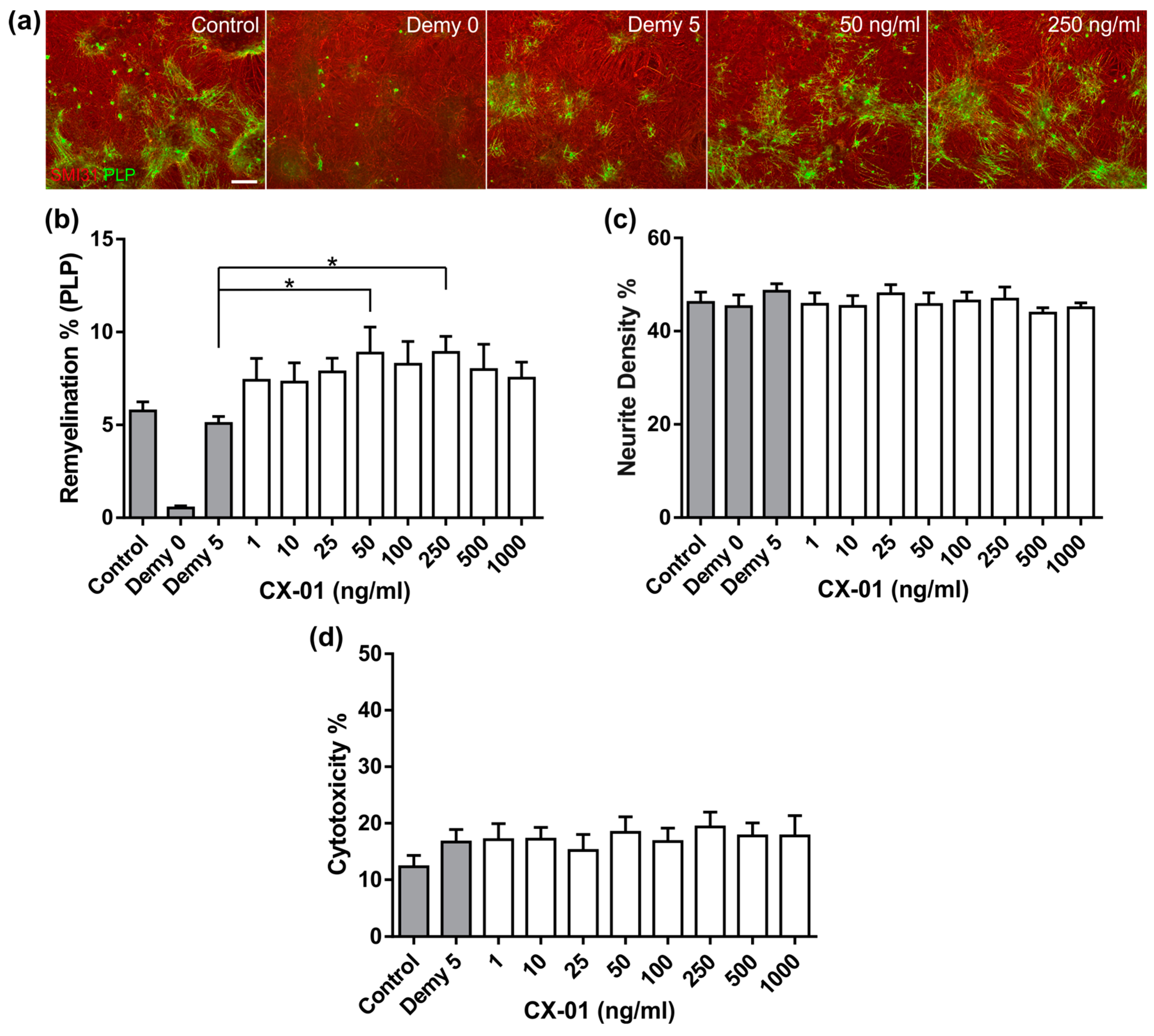
3.4. CX-01 Promotes Remyelination in MC-Demy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorensen, A.; Moffat, K.; Thomson, C.; Barnett, S.C. Astrocytes, but not olfactory ensheathing cells or Schwann cells, promote myelination of CNS axons in vitro. Glia 2008, 56, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, K.; Anderson, K.I.; Strachan, D.; Edgar, J.E.; Barnett, S.C. Time-lapse imaging of the dynamics of CNS glial/axonal interactions in vitro and ex vivo. PLoS ONE 2012, 7, e30775. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nash, B.; Thomson, C.E.; Linington, C.; Arthur, A.T.; McClure, J.D.; McBride, M.W.; Barnett, S.C. Functional duality of astrocytes in myelination. J. Neurosci. 2011, 31, 13028–13038. [Google Scholar] [CrossRef] [PubMed]
- Lamond, R.; Barnett, S.C. Schwann cells but not olfactory ensheathing cells inhibit CNS myelination via the secretion of connective tissue growth factor. J. Neurosci. 2013, 33, 18686–18697. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.L.; Johnstone, S.A.; Mountford, J.C.; Sheikh, S.; Allan, D.B.; Clark, L.; Barnett, S.C. Human mesenchymal stem cells isolated from olfactory biopsies but not bone enhance CNS myelination in vitro. Glia 2013, 61, 368–382. [Google Scholar] [CrossRef]
- Lindsay, S.L.; Johnstone, S.A.; McGrath, M.A.; Mallinson, D.; Barnett, S.C. Comparative miRNA-Based Fingerprinting Reveals Biological Differences in Human Olfactory Mucosa- and Bone-Marrow-Derived Mesenchymal Stromal Cells. Stem Cell Rep. 2016, 6, 729–742. [Google Scholar] [CrossRef]
- Boomkamp, S.D.; Riehle, M.O.; Wood, J.; Olson, M.F.; Barnett, S.C. The development of a rat in vitro model of spinal cord injury demonstrating the additive effects of Rho and ROCK inhibitors on neurite outgrowth and myelination. Glia 2012, 60, 441–446. [Google Scholar] [CrossRef]
- Boomkamp, S.D.; McGrath, M.A.; Houslay, M.D.; Barnett, S.C. Epac and the high affinity rolipram binding conformer of PDE4 modulate neurite outgrowth and myelination using an in vitro spinal cord injury model. Br. J. Pharmacol. 2014, 171, 2385–2398. [Google Scholar] [CrossRef]
- Elliott, C.; Lindner, M.; Arthur, A.; Brennan, K.; Jarius, S.; Hussey, J.; Chan, A.; Stroet, A.; Olsson, T.; Willison, H.; et al. Functional identification of pathogenic autoantibody responses in patients with multiple sclerosis. Brain 2012, 135, 1819–1833. [Google Scholar] [CrossRef]
- McCanney, G.A.; McGrath, M.A.; Otto, T.D.; Burchmore, R.; Yates, E.A.; Bavington, C.D.; Willison, H.J.; Turnbull, J.E.; Barnett, S.C. Low sulfated heparins target multiple proteins for central nervous system repair. Glia 2019, 67, 668–687. [Google Scholar] [CrossRef]
- Lakatos, A.; Franklin, R.J.; Barnett, S.C. Olfactory ensheathing cells and Schwann cells differ in their in vitro interactions with astrocytes. Glia 2000, 32, 214–225. [Google Scholar] [CrossRef]
- Higginson, J.R.; Thompson, S.M.; Santos-Silva, A.; Guimond, S.E.; Turnbull, J.E.; Barnett, S.C. Differential sulfation remodelling of heparan sulfate by extracellular 6-O-sulfatases regulates fibroblast growth factor-induced boundary formation by glial cells. J. Neurosci. 2012, 32, 15902–15912. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, A.; Fairless, R.; Frame, M.C.; Montague, P.; Smith, G.M.; Toft, A.; Riddell, J.S.; Barnett, S.C. FGF/heparin differentially regulates Schwann cell and olfactory ensheathing cell interactions with astrocytes: A role in astrocytosis. J. Neurosci. 2007, 27, 7154–7167. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.L.; Fu, H.; Deng, C.; Chen, J.; Chen, J. Modulating the CXCL12-induced cancer cell growth and adhesion by sulfated K5 polysaccharides in vitro. Biomed. Pharmacother. 2015, 73, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.J.; Oh, Y.I.; Chang, S.K.; Hsieh-Wilson, L.C. Tunable heparan sulfate mimetics for modulating chemokine activity. J. Am. Chem. Soc. 2013, 135, 30. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; El Hadri, A.; Richard, S.; Denis, F.; Holte, K.; Duffner, J.; Yu, F.; Galcheva-Gargova, Z.; Capila, I.; Schultes, B.; et al. Synthesis and biological evaluation of a unique heparin mimetic hexasaccharide for structure-activity relationship studies. J. Med. Chem. 2014, 57, 4511–4520. [Google Scholar] [CrossRef]
- Xu, D.; Arnold, K.; Liu, J. Using structurally defined oligosaccharides to understand the interactions between proteins and heparan sulfate. Curr. Opin. Struct. Biol. 2018, 50, 155–161. [Google Scholar] [CrossRef]
- Vann, W.F.; Schmidtm, M.A.; Jannm, B.; Jannm, K. The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli 010:K5:H4. A polymer similar to desulfo-heparin. Eur. J. Biochem. 1981, 166, 359–364. [Google Scholar] [CrossRef]
- Capila, I.; Linhardt, R.J. Heparin-protein interactions. Angew. Chem. Int. Ed. Engl. 2002, 41, 391–412. [Google Scholar] [CrossRef]
- Naggi, A.; Torri, G.; Casu, B.; Oreste, P.; Zoppetti, G.; Lim, J.P.; Lindahlm, U. Toward a biotechnological heparin through combined chemical and enzymatic modification of the Escherichia coli K5 polysaccharide. Semin. Thromb. Hemost. 2001, 27, 437–443. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Kim, S.K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Biol. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological Activities and Potential Health Benefits of Sulfated Polysaccharides Derived from Marine Algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Meenakshi, S.; Umayaparvathi, S.; Saravanan, R.; Manivasagam, T.; Balasubramanian, T. Neuroprotective effect of fucoidan from Turbinaria decurrens in MPTP intoxicated Parkinsonic mice. Int. J. Biol. Macromol. Viol. 2016, 86, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, D.A.E.; van Scheppingen, J.; van der Poel, M.; Bossers, K.; Schuurman, K.G.; Van Eden, C.G.; Hol, E.M.; Hamann, J.; Huitinga, I. Gene expression profiling of multiple sclerosis pathology identifies early patterns of demyelination surrounding chronic active lesions. Front. Immunol. 2017, 8, 1810. [Google Scholar] [CrossRef] [PubMed]
- Collén, P.N.; Jeudy, A.; Sassi, J.F.; Groisillier, A.; Czjzek, M.; Coutinho, P.M.; Helbert, W. A novel unsaturated β-glucuronyl hydrolase involved in ulvan degradation unveils the versatility of stereochemistry requirements in family GH105. J. Biol. Chem. 2014, 289, 6199–6211. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromoecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef]
- Kaeffer, B.; Bénard, C.; Lahaye, M.; Blottière, H.M.; Cherbut, C. Biological properties of ulvan, a new source of green seaweed sulfated polysaccharides, on cultured normal and cancerous colonic epithelial cells. Planta Med. 1999, 65, 527–531. [Google Scholar] [CrossRef]
- Alves, A.; Pinho, E.D.; Neves, N.M.; Sousa, R.A.; Reis, R.L. Processing ulvan into 2D structures: Cross-linked ulvan membranes as new biomaterials for drug delivery applications. Int. J. Pharm. 2012, 426, 76–81. [Google Scholar] [CrossRef]
- Morelli, A.; Chiellini, F. Ulvan as a New Type of Biomaterial from Renewable Resources: Functionalization and Hydrogel Preparation. Macromol. Chem. Phys. 2010, 211, 821–832. [Google Scholar] [CrossRef]
- Kovacsovics, T.J.; Mims, A.; Salama, M.E.; Pantin, J.; Rao, N.; Kosak, K.M.; Ahorukomeye, P.; Glenn, M.J.; Deininger, M.W.N.; Boucher, K.M.; et al. Combination of the low anticoagulant heparin CX-01 with chemotherapy for the treatment of acute myeloid leukemia. Blood Adv. 2018, 2, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Suto, Y.; Cognetti, J.; Browne, K.D.; Kumasaka, K.; Johnson, V.E.; Kaplan, L.; Marks, J.; Smith, D.H.; Pascual, J.L. Early low-anticoagulant desulfated heparin after traumatic brain injury: Reduced brain edema and leukocyte mobilization is associated with improved watermaze learning ability weeks after injury. J. Trauma Acute Care Surg. 2018, 84, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.A.; Weiss, S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 1996, 175, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bottenstein, J.E.; Sato, G.H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc. Natl. Acad. Sci. USA 1979, 76, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, T.; Konola, J.T.; Wekerle, H.; Lees, M.B. Monoclonal antibodies against myelin proteolipid protein: Identification and characterization of two major determinants. J. Neurochem. 1991, 57, 1671–1680. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef]
- Lindner, M.; Thümmler, K.; Arthur, A.; Brunner, S.; Elliott, C.; McElroy, D.; Mohan, H.; Williams, A.; Edgar, J.M.; Schuh, C.; et al. Fibroblast growth factor signalling in multiple sclerosis: Inhibition of myelination and induction of pro-inflammatory environment by FGF9. Brain 2015, 138, 1875–1893. [Google Scholar] [CrossRef]
- McCanney, G.A. Institute of Infection, Immunity and Inflammation, College of Medical, Veterinary and Life Sciences, University of Glasgow: Glasgow, UK, unpublished data. 2019.
- Van Wijk, X.M.; Van Kuppevelt, T.H. Heparan sulfate in angiogenesis: A target for therapy. Angiogenesis 2014, 17, 443–462. [Google Scholar] [CrossRef]
- Khachigian, L.M.; Parish, C.R. Phosphomannopentaose sulfate (PI-88): Heparan sulfate mimetic with clinical potential in multiple vascular pathologies. Cardiovasc. Drug Rev. 2004, 22, 1–6. [Google Scholar] [CrossRef]
- Cibula, D.; Dusek, J.; Jarkovsky, J.; Dundr, P.; Querleu, D.; van der Zee, A.; Kucukmetin, A.; Kocian, R. A prospective multicenter trial on sentinel lymph node biopsy in patients with early-stage cervical cancer (SENTIX). Int. J. Gynecol. Cancer 2019, 29, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Eshed-Eisenbach, Y.; Gordon, A.; Sukhanov, N.; Peles, E. Specific inhibition of secreted NRG1 types I-II by heparin enhances Schwann Cell myelination. Glia 2016, 64, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Borgenström, M.; Jalkanen, M.; Salmivirta, M. Sulfated derivatives of Escherichia coli K5 polysaccharides as modulators of fibroblast growth factor signaling. J. Biol. Chem. 2003, 278, 49882–49889. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Do, A.T.; Lozynska, O.; Kusche-Gullberg, M.; Lindahl, U.; Emerson, C.P. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J. Cell Biol. 2003, 162, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmit, A.; Koyama, T.; Dejima, K.; Hayashi, Y.; Kamimuram, K.; Nakatom, H. Drosophila heparan sulfate 6-O endosulfatase regulates Wingless morphogen gradient formation. Dev. Biol. 2010, 345, 204–214. [Google Scholar] [CrossRef] [PubMed]
- David, M.D.; Cantí, C.; Herreros, J. Wnt-3a and Wnt-3 differently stimulate proliferation and neurogenesis of spinal neural precursors and promote neurite outgrowth by canonical signaling. J. Neurosci. Res. 2010, 88, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Blakely, B.D.; Bye, C.R.; Fernando, C.V.; Horne, M.K.; Macheda, M.L.; Stacker, S.A.; Arenas, E.; Parish, C.L. Wnt5a regulates midbrain dopaminergic axon growth and guidance. PLoS ONE 2011, 6, e18373. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; McLay, N.; Pye, D.A. Structural studies of heparan sulfate hexasaccharides: New insights into iduronate conformational behavior. J. Am. Chem. Soc. 2008, 130, 12435–12444. [Google Scholar] [CrossRef] [PubMed]
- Guglier, S.; Hricovíni, M.; Raman, R.; Polito, L.; Torri, G.; Casu, B.; Sasisekharan, R.; Guerrini, M. Minimum FGF2 binding structural requirements of heparin and heparan sulfate oligosaccharides as determined by NMR spectroscopy. Biochemistry 2008, 47, 13862–13869. [Google Scholar] [CrossRef]
- Zhao, L.; Lai, S.; Huang, R.; Wu, M.; Gao, N.; Xu, L.; Qin, H.; Peng, W.; Zhao, J. Structure and anticoagulant activity of fucosylated glycosaminoglycan degraded by deaminative cleavage. Carbohydr. Polym. 2013, 98, 1514–1523. [Google Scholar] [CrossRef]
- McCanney, G.A.; Whithead, M.J.; McGrath, M.A.; Lindsay, S.L.; Barnett, S.C. Neural cell cultures to study spinal cord injury. Drug Discov. Today Dis. Model. 2018, 25–26, 11–20. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCanney, G.A.; Lindsay, S.L.; McGrath, M.A.; Willison, H.J.; Moss, C.; Bavington, C.; Barnett, S.C. The Use of Myelinating Cultures as a Screen of Glycomolecules for CNS Repair. Biology 2019, 8, 52. https://doi.org/10.3390/biology8030052
McCanney GA, Lindsay SL, McGrath MA, Willison HJ, Moss C, Bavington C, Barnett SC. The Use of Myelinating Cultures as a Screen of Glycomolecules for CNS Repair. Biology. 2019; 8(3):52. https://doi.org/10.3390/biology8030052
Chicago/Turabian StyleMcCanney, George A., Susan L. Lindsay, Michael A. McGrath, Hugh J. Willison, Claire Moss, Charles Bavington, and Susan C. Barnett. 2019. "The Use of Myelinating Cultures as a Screen of Glycomolecules for CNS Repair" Biology 8, no. 3: 52. https://doi.org/10.3390/biology8030052
APA StyleMcCanney, G. A., Lindsay, S. L., McGrath, M. A., Willison, H. J., Moss, C., Bavington, C., & Barnett, S. C. (2019). The Use of Myelinating Cultures as a Screen of Glycomolecules for CNS Repair. Biology, 8(3), 52. https://doi.org/10.3390/biology8030052





