Which Species Should We Focus On? Umbrella Species Assessment in Southwest China
Abstract
1. Introduction
2. Materials and Methods
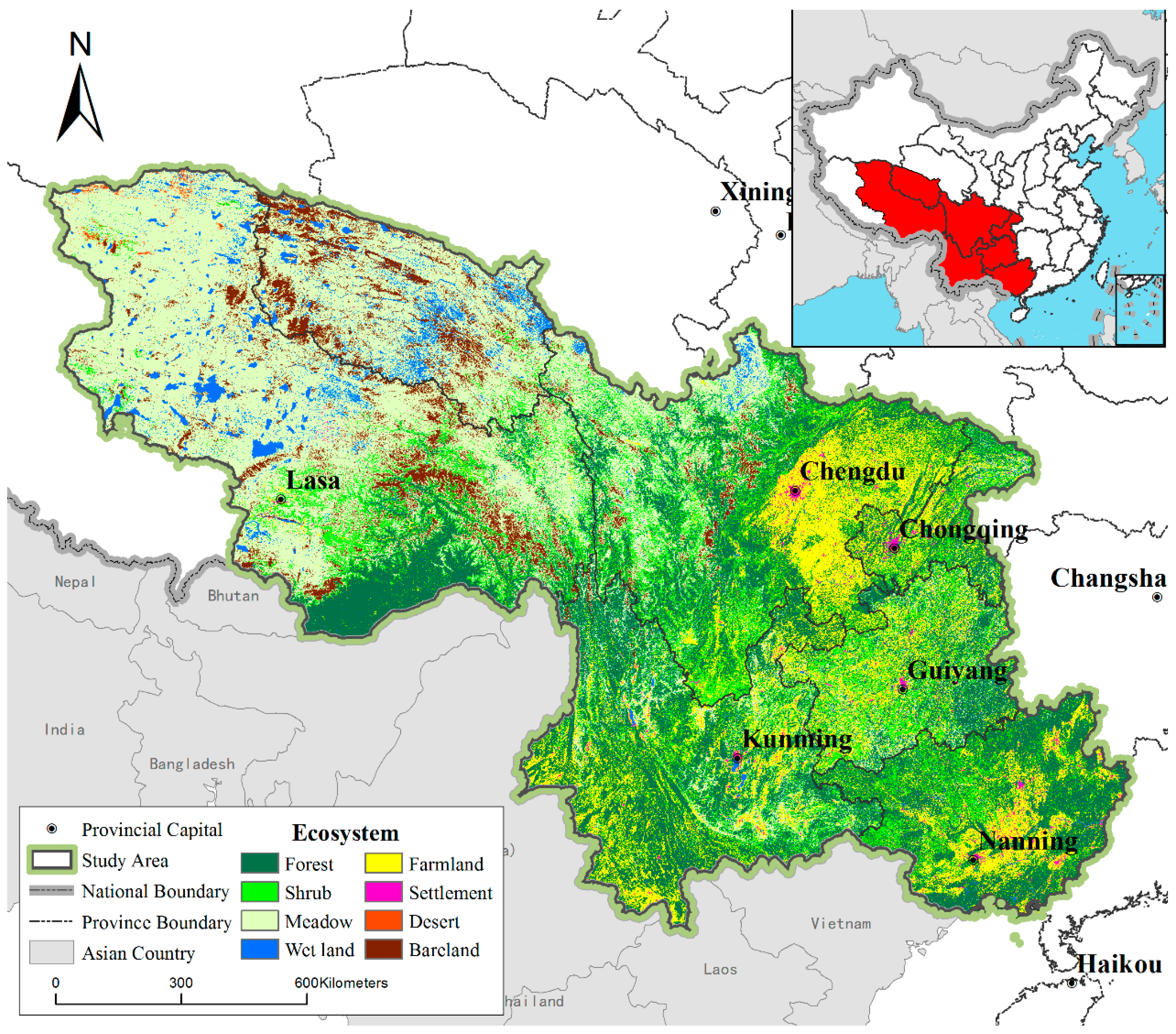
2.1. Study Area
2.2. Species List and Distribution
2.3. Statistical Analysis
2.3.1. Indicator Species Analysis
2.3.2. Co-Occurring Species Analysis
Correlation Analysis
Factor Analysis
2.4. Umbrella Species Selection
3. Results
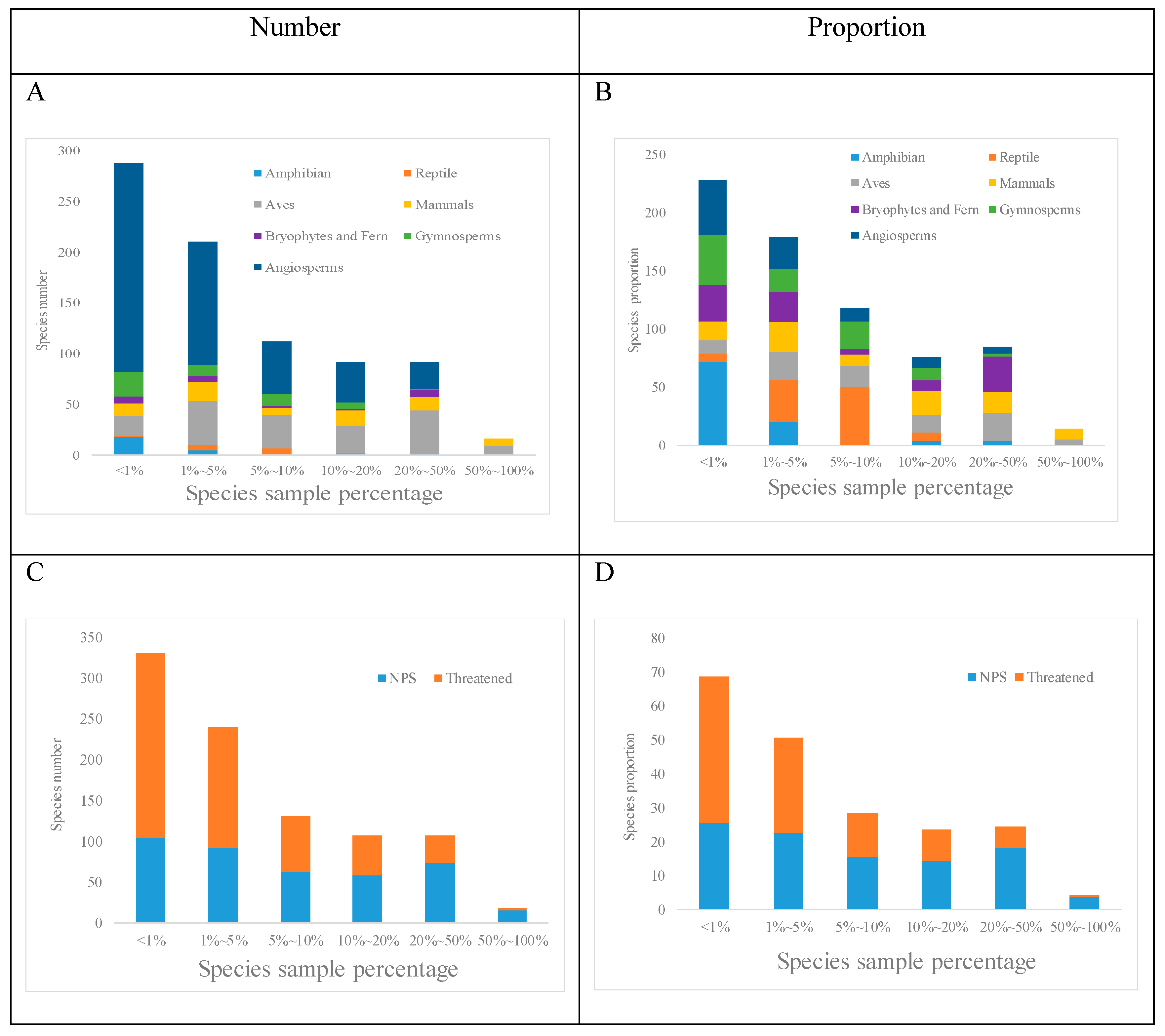
3.1. Biodiversity Spatial Distribution and Plot-by-Species Table
3.2. Statistical Analyses
3.2.1. Indicator Species
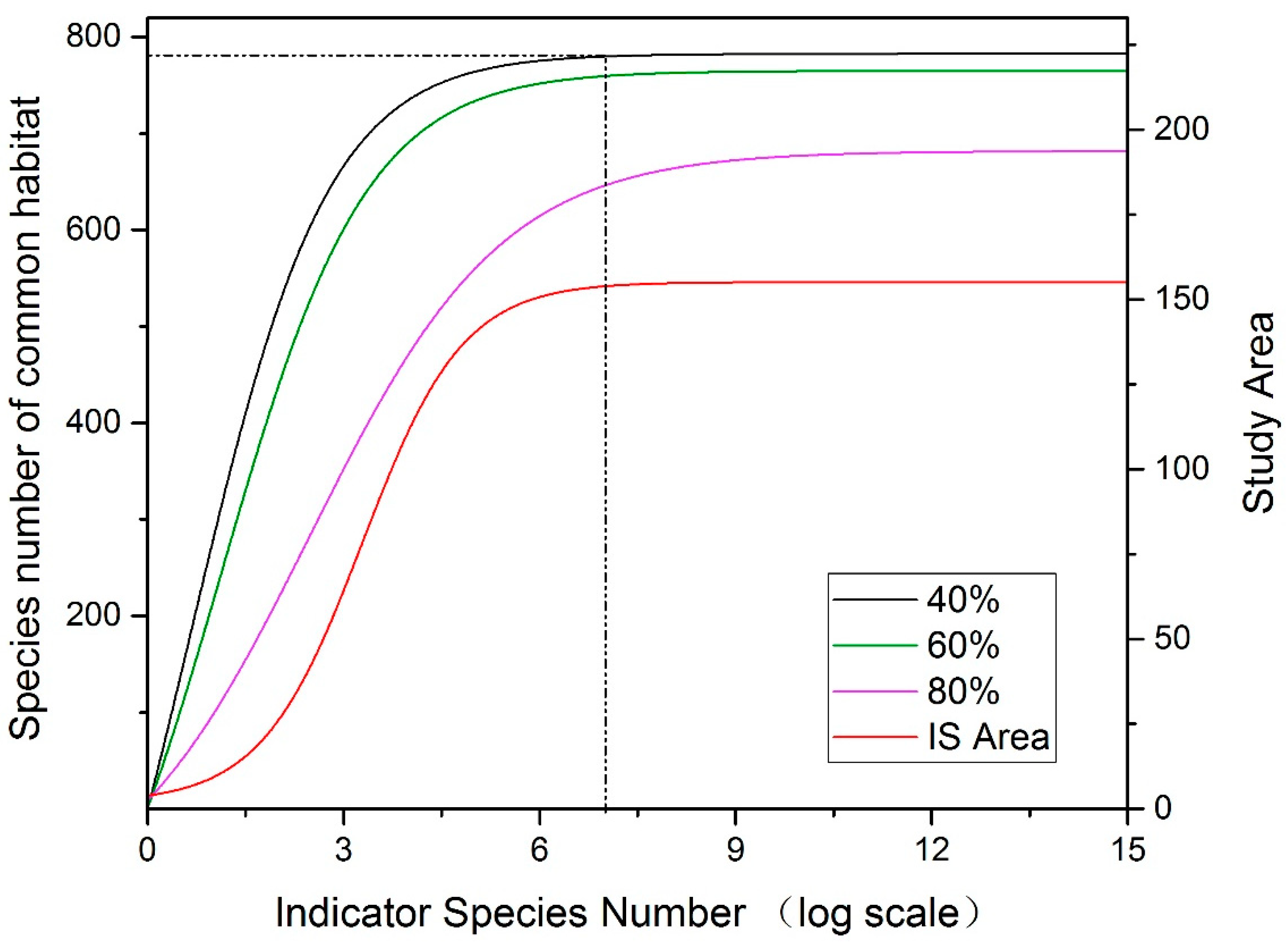
- 1:
- 40% habitat overlap: regarded as two species with a common habitat;
- 2:
- 60% habitat overlap: regarded as two species with a common habitat;
- 3:
- 80% habitat overlap: regarded as two species with a common habitat.
3.2.2. Co-Occurring Species
3.3. Umbrella Species Selection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| No. of Important Species | Category Information | Total | |||
|---|---|---|---|---|---|
| Endangered | Protected in Law | Both Endangered and Protected in Law | |||
| Animals | Amphibian | 21 | 6 | 2 | 25 |
| Reptile | 12 | 7 | 5 | 14 | |
| Aves | 57 | 138 | 19 | 176 | |
| Mammals | 42 | 69 | 39 | 72 | |
| Summary of animals | 132 | 220 | 65 | 287 | |
| Plants | Bryophytes and Fern | 17 | 7 | 1 | 23 |
| Gymnosperms | 44 | 37 | 26 | 55 | |
| Angiosperm | 331 | 143 | 29 | 445 | |
| Summary of plants | 392 | 187 | 56 | 523 | |
| Total | 524 | 407 | 121 | 810 | |
| Scientific Name | Category | Taxa | Region | Mentioned before | Endemic to China | IUCN Red List Category | NPS Category | Importance Level | Indicator Value Index | Non-Repetition Correlation |
|---|---|---|---|---|---|---|---|---|---|---|
| Nyssa yunnanensis | co-occurring species | Angiosperms | F | YES | √ | CR | I | 1 | 0.36 | 164 |
| Neocheiropteris palmatopedata | co-occurring species | Bryophytes and Fern | D | NO | √ | EN | II | 1 | 0.48 | 98 |
| Carya sinensis | co-occurring species | Angiosperms | F | NO | × | EN | II | 1 | 0.55 | 60 |
| Magnolia grandis | co-occurring species | Angiosperms | F | NO | √ | CR | II | 1 | 0.15 | 29 |
| Cycas bifida | co-occurring species | Gymnosperms | D | NO | × | VU | I | 1 | 0.22 | 24 |
| Moschus berezovskii | co-occurring species | Mammals | D | YES | × | EN | I | 1 | 0.58 | 20 |
| Nycticebus bengalensis | co-occurring species/indicator species | Mammals | F | YES | × | VU | I | 1 | 0.60 | 15 |
| Naemorhedus baileyi | co-occurring species/indicator species | Mammals | B | NO | × | VU | I | 1 | 0.63 | 20 |
| Lophophorus lhuysii | co-occurring species | Aves | A | YES | √ | VU | I | 1 | 0.55 | 21 |
| Rhinopithecus roxellana | co-occurring species | Mammals | E | YES | √ | EN | I | 1 | 0.54 | 18 |
| Bretschneidera sinensis | co-occurring species | Angiosperms | E | YES | × | EN | I | 1 | 0.58 | 16 |
| Cuora trifasciata | co-occurring species | Reptile | E | YES | × | CR | II | 1 | 0.42 | 12 |
| Nycticebus pygmaeus | co-occurring species | Mammals | F | YES | × | N/A | I | 1 | 0.15 | 11 |
| Ailuropoda melanoleuca | co-occurring species/indicator species | Mammals | C | YES | √ | VU | I | 1 | 0.80 | 11 |
| Tragopan caboti | co-occurring species | Aves | E | YES | √ | VU | I | 1 | 0.21 | 10 |
| Cycas debaoensis | co-occurring species | Gymnosperms | F | YES | √ | CR | I | 1 | 0.18 | 11 |
| Cycas balansae | co-occurring species | Gymnosperms | F | NO | × | Not Threatened | I | 1 | 0.26 | 8 |
| Neofelis nebulosa | co-occurring species | Mammals | D | YES | × | VU | I | 1 | 0.45 | 5 |
| Kingdonia uniflora | co-occurring species | Angiosperms | D | NO | √ | Not Threatened | I | 1 | 0.53 | 5 |
| Gypaetus barbatus | co-occurring species | Aves | A | NO | × | Not Threatened | I | 1 | 0.52 | 5 |
| Panthera tigris | indicator species | Mammals | B | YES | × | EN | I | 1 | 0.87 | 0 |
| Aquila chrysaetos | indicator species | Aves | B\C\D | YES | × | Not Threatened | I | 1 | 0.73 | 0 |
| Panthera uncia | indicator species | Mammals | A\C\D | YES | × | EN | I | 1 | 0.68 | 0 |
| Cymbidium aloifolium | indicator species | Angiosperms | E\F | NO | × | Not Threatened | I | 1 | 0.68 | 0 |
| Andrias davidianus | indicator species | Amphibian | E | YES | √ | CR | II | 1 | 0.65 | 0 |
| Sorolepidium glaciale | indicator species | Bryophytes and Fern | B\C\D | NO | √ | Not Threatened | I | 1 | 0.64 | 0 |
| Budorcas taxicolor | indicator species | Mammals | B\C | YES | × | VU | I | 1 | 0.64 | 0 |
| Ailurus fulgens | indicator species | Mammals | B\C\D | YES | × | EN | II | 1 | 0.62 | 0 |
| Eleutherococcus cuspidatus | indicator species | Angiosperms | C | NO | × | EN | Not Listed | 2 | 0.73 | 1 |
| Burretiodendron tonkinense | indicator species | Angiosperms | F | NO | × | EN | Not Listed | 2 | 0.70 | 0 |
| Aythya baeri | indicator species | Aves | E\F | NO | × | CR | Not Listed | 2 | 0.62 | 0 |
| Emberiza aureola | indicator species | Aves | C\E\F | YES | × | EN | Not Listed | 2 | 0.60 | 0 |

References
- Duelli, P.; Obrist, M.K. Biodiversity indicators: The choice of values and measures. Agric. Ecosyst. Environ. 2003, 98, 87–98. [Google Scholar] [CrossRef]
- Agapow, P.M.; Bininda-Emonds, O.R.P.; Crandall, K.A.; Gittleman, J.L.; Mace, G.M.; Marshall, J.C.; Purvis, A. The impact of species concept on biodiversity studies. Q. Rev. Biol. 2004, 79, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future threats to biodiversity and pathways to their prevention. Nature 2017, 546, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Pierson, J.; Barton, P.; Beger, M.; Branquinho, C.; Calhoun, A.; Caro, T.; Greig, H.; Gross, J.; Heino, J.; et al. A new framework for selecting environmental surrogates. Sci. Total Environ. 2015, 538, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M., Jr.; Westgate, M.; Barton, P.; Calhoun, A.; Pierson, J.; Tulloch, A.; Beger, M.; Branquinho, C.; Caro, T.; Gross, J.; et al. Two roles for ecological surrogacy: Indicator surrogates and management surrogates. Ecol. Indic. 2016, 63, 121–125. [Google Scholar] [CrossRef]
- Roberge, J.M.; Angelstam, P. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Stuber, E.F.; Fontaine, J.J. Ecological neighborhoods as a framework for umbrella species selection. Biol. Conserv. 2018, 223, 112–119. [Google Scholar] [CrossRef]
- Branton, M.; Richardson, J.S. Assessing the Value of the Umbrella-Species Concept for Conservation Planning with Meta-Analysis. Conserv. Biol. 2011, 25, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Fleishman, E.; Murphy, D.D.; Brussard, P.E. A new method for selection of umbrella species for conservation planning. Ecol. Appl. 2000, 10, 569–579. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zheng, H.; Xiao, Y.; Polasky, S.; Liu, J.; Xu, W.; Wang, Q.; Zhang, L.; Xiao, Y.; Rao, E.; et al. Improvements in ecosystem services from investments in natural capital. Science 2016, 352, 1455–1459. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, Y.; Zhang, J.; Yang, W.; Zhang, L.; Hull, V.; Wang, Z.; Zheng, H.; Liu, J.; Polasky, S.; et al. Strengthening protected areas for biodiversity and ecosystem services in China. Proc. Natl. Acad. Sci. USA 2017, 114, 1601–1606. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2017.10. 2017. Available online: http://www.iucnredlist.org (accessed on 22 October 2017).
- Li, B.V.; Pimm, S.L. China’s endemic vertebrates sheltering under the protective umbrella of the giant panda. Conserv. Biol. 2016, 30, 329–339. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.; Ran, J. Predicting suitable habitat of the Chinese monal (Lophophorus lhuysii) using ecological niche modeling in the Qionglai Mountains, China. PeerJ 2017, 5. [Google Scholar] [CrossRef]
- Gilby, B.L.; Olds, A.D.; Connolly, R.M.; Yabsley, N.A.; Maxwell, P.S.; Tibbetts, I.R.; Schoeman, D.S.; Schlacher, T.A. Umbrellas can work under water: Using threatened species as indicator and management surrogates can improve coastal conservation. Estuar. Coast. Shelf Sci. 2017, 199, 132–140. [Google Scholar] [CrossRef]
- Wang, F.; McShea, W.J.; Li, S.; Wang, D. Does one size fit all? A multispecies approach to regional landscape corridor planning. Divers. Distrib. 2018, 24, 415–425. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Liu, Z.; Lippert, P.C.; Graham, S.A.; Coe, R.S.; Yi, H.; Zhu, L.; Liu, S.; Li, Y. Constraints on the early uplift history of the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2008, 105, 4987–4992. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.R. Animal Geography in China; Science Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Sodhi, N.S.; Posa, M.R.C.; Lee, T.M.; Bickford, D.; Koh, L.P.; Brook, B.W. The state and conservation of Southeast Asian biodiversity. Biodivers. Conserv. 2010, 19, 317–328. [Google Scholar] [CrossRef]
- Roberge, J.-M.; Mikusinski, G.; Svensson, S. The white-backed woodpecker: Umbrella species for forest conservation planning? Biodivers. Conserv. 2008, 17, 2479–2494. [Google Scholar] [CrossRef]
- Xu, W.; Vina, A.; Kong, L.; Pimm, S.L.; Zhang, J.; Yang, W.; Xiao, Y.; Zhang, L.; Chen, X.; Liu, J.; et al. Reassessing the conservation status of the giant panda using remote sensing. Nat. Ecol. Evol. 2017, 1, 1635–1638. [Google Scholar] [CrossRef]
- Zomer, R.J.; Trabucco, A.; Wang, M.; Lang, R.; Chen, H.; Metzger, M.J.; Smajgl, A.; Beckschaefer, P.; Xu, J. Environmental stratification to model climate change impacts on biodiversity and rubber production in Xishuangbanna, Yunnan, China. Biol. Conserv. 2014, 170, 264–273. [Google Scholar] [CrossRef]
- State Forestry Administration of China. Lists of Wildlife under Special State Protection; State Forestry Administration of China: Beijing, China, 2010. (In Chinese)
- Shi, X.; Zhang, L.; Zhang, J.; Ouyang, Z.; Xiao, Y. Priority area of biodiversity conservation in Southwest China. CJE 2018, 37, 3721–3728. (In Chinese) [Google Scholar]
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Foata, J.; Mouillot, D.; Culioli, J.-L.; Marchand, B. Influence of season and host age on wild boar parasites in Corsica using indicator species analysis. J. Helminthol. 2006, 80, 41–45. [Google Scholar] [CrossRef]
- De Caceres, M.; Legendre, P.; Wiser, S.K.; Brotons, L. Using species combinations in indicator value analyses. Methods Ecol. Evol. 2012, 3, 973–982. [Google Scholar] [CrossRef]
- Sattler, T.; Pezzatti, G.B.; Nobis, M.P.; Obrist, M.K.; Roth, T.; Moretti, M. Selection of Multiple Umbrella Species for Functional and Taxonomic Diversity to Represent Urban Biodiversity. Conserv. Biol. 2014, 28, 414–426. [Google Scholar] [CrossRef]
- Zhao, S.Q. A new scheme for comprehensive physical regionalization in China. Acta Geogr. Sin. 1983, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the worlds: A new map of life on Earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- De Caceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Yang, Q.S.; Meng, X.X.; Xia, L.; Feng, Z.J. Conservation status and causes of decline of musk deer (Moschus spp.) in China. Biol. Conserv. 2003, 109, 333–342. [Google Scholar] [CrossRef]
- Deng, W.H.; Zheng, G.M. Landscape and habitat factors affecting cabot’s tragopan Tragopan caboti occurrence in habitat fragments. Biol. Conserv. 2004, 117, 25–32. [Google Scholar] [CrossRef]
- Radhakrishna, S.; Goswami, A.B.; Sinha, A. Distribution and conservation of Nycticebus bengalensis in northeastern India. Int. J. Primatol. 2006, 27, 971–982. [Google Scholar] [CrossRef]
- Guo, S.; Ji, W.; Li, B.; Li, M. Response of a group of Sichuan snub-nosed monkeys to commercial logging in the Qinling Mountains, China. Conserv. Biol. 2008, 22, 1055–1064. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, Z. Characteristics and risk assessment of international trade in tortoises and freshwater turtles in China. Chelonian Conserv. Biol. 2008, 7, 28–36. [Google Scholar] [CrossRef]
- Dorji, S.; Vernes, K.; Rajaratnam, R. Habitat Correlates of the Red Panda in the Temperate Forests of Bhutan. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Starr, C.; Nekaris, K.A.I.; Streicher, U.; Leung, L.K.P. Field surveys of the Vulnerable pygmy slow loris Nycticebus pygmaeus using local knowledge in Mondulkiri Province, Cambodia. Oryx 2011, 45, 135–142. [Google Scholar] [CrossRef]
- Zhan, Q.-Q.; Wang, J.-F.; Gong, X.; Peng, H. Patterns of chloroplast DNA variation in Cycas debaoensis (Cycadaceae): Conservation implications. Conserv. Genet. 2011, 12, 959–970. [Google Scholar] [CrossRef]
- Qiao, Q.; Chen, H.; Xing, F.; Wang, F.; Zhong, W.; Wen, X.; Hou, X. Pollination ecology of Bretschneidera sinensis (Hemsley), a rare and endangered tree in China. Pak. J. Bot. 2012, 44, 1897–1903. [Google Scholar]
- Liu, X.; Wu, P.; Songer, M.; Cai, Q.; He, X.; Zhu, Y.; Shao, X. Monitoring wildlife abundance and diversity with infra-red camera traps in Guanyinshan Nature Reserve of Shaanxi Province, China. Ecol. Indic. 2013, 33, 121–128. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, G.; Grumbine, R.E.; Dao, Z.; Sun, W.; Guo, H. Conserving plant species with extremely small populations (PSESP) in China. Biodivers. Conserv. 2013, 22, 803–809. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Papanikolaou, A.D.; Barbet-Massin, M.; Kallimanis, A.S.; Jiguet, F.; Schmeller, D.S.; Pantis, J.D. Evaluating the Connectivity of a Protected Areas’ Network under the Prism of Global Change: The Efficiency of the European Natura 2000 Network for Four Birds of Prey. PLoS ONE 2013, 8, e59640. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Geng, Y.; Liu, X.X.; Ren, S.Y.; Zhou, Y.; Wang, K.Y.; Huang, X.L.; Chen, D.F.; Peng, X.; Lai, W.M. Characterization of a ranavirus isolated from the Chinese giant salamander (Andrias davidianus, Blanchard, 1871) in China. Aquaculture 2013, 384, 66–73. [Google Scholar] [CrossRef]
- Yong, D.L.; Liu, Y.; Low, B.W.; Espanola, C.P.; Choi, C.-Y.; Kawakami, K. Migratory songbirds in the East Asian-Australasian Flyway: A review from a conservation perspective. Bird Conserv. Int. 2015, 25, 1–37. [Google Scholar] [CrossRef]
- Alexander, J.S.; Cusack, J.J.; Pengju, C.; Kun, S.; Riordan, P. Conservation of snow leopards: Spill-over benefits for other carnivores? Oryx 2016, 50, 239–243. [Google Scholar] [CrossRef]
- Velho, N.; Srinivasan, U.; Singh, P.; Laurance, W.F. Large mammal use of protected and community-managed lands in a biodiversity hotspot. Anim. Conserv. 2016, 19, 199–208. [Google Scholar] [CrossRef]
- Dutta, T.; Sharma, S.; DeFries, R. Targeting restoration sites to improve connectivity in a tiger conservation landscape in India. PeerJ 2018, 6. [Google Scholar] [CrossRef]
- Breckheimer, I.; Haddad, N.M.; Morris, W.F.; Trainor, A.M.; Fields, W.R.; Jobe, R.T.; Hudgens, B.R.; Moody, A.; Walters, J.R. Defining and Evaluating the Umbrella Species Concept for Conserving and Restoring Landscape Connectivity. Conserv. Biol. 2014, 28, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Branton, M.A.; Richardson, J.S. A test of the umbrella species approach in restored floodplain ponds. J. Appl. Ecol. 2014, 51, 776–785. [Google Scholar] [CrossRef]
- Crosby, A.D.; Elmore, R.D.; Leslie, D.M., Jr.; Will, R.E. Looking beyond rare species as umbrella species: Northern Bobwhites (Colinus virginianus) and conservation of grassland and shrubland birds. Biol. Conserv. 2015, 186, 233–240. [Google Scholar] [CrossRef]

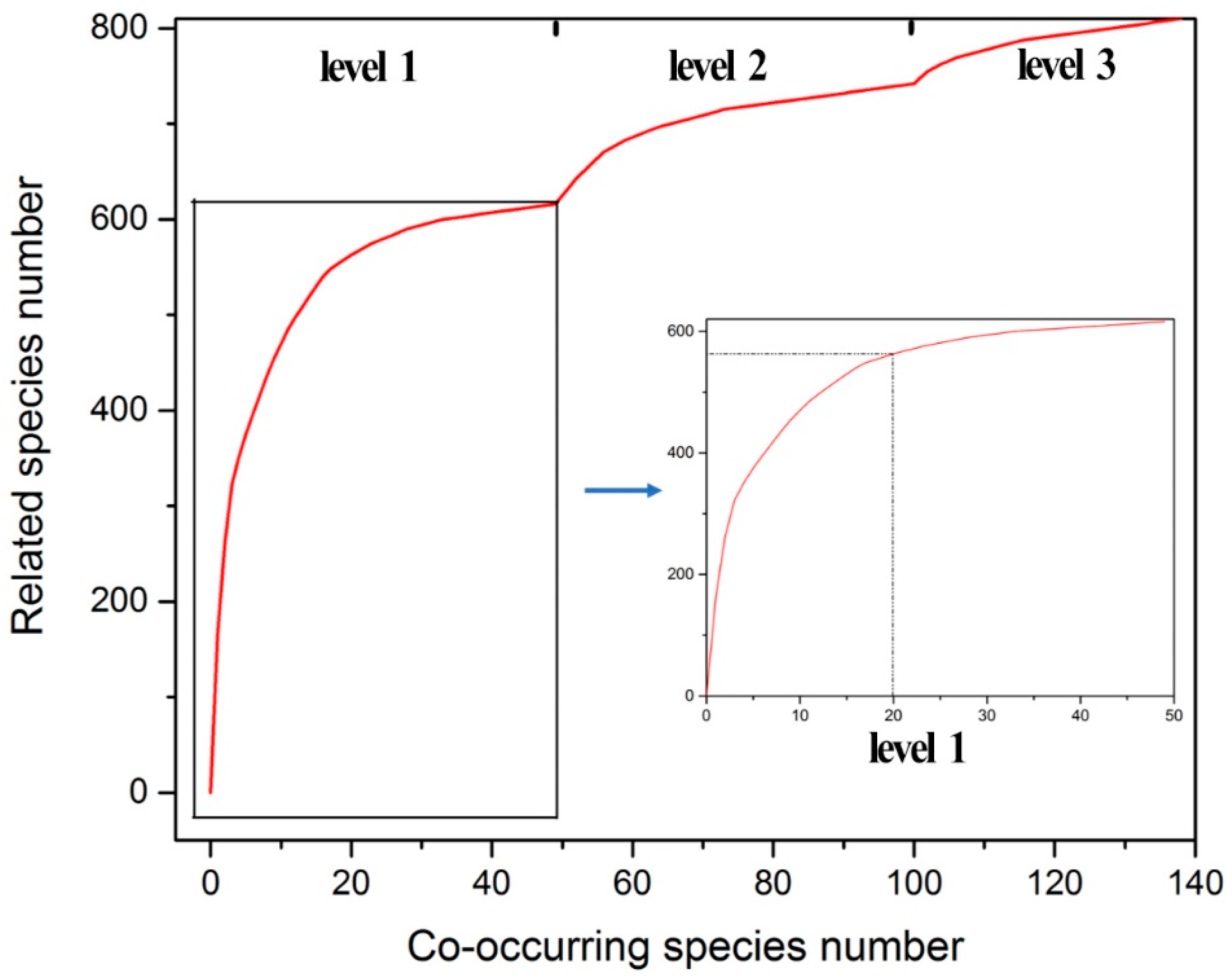
| Evaluation Index | Conservation Status Classification Criteria | ||
|---|---|---|---|
| Level 1 | Level 2 | Level 3 | |
| Threatened | species that are both NPS and CR/EN | Other CR/EN species | VU species |
| Conservation level | Other first-category nationally protected species | --- | Other second-category nationally protected species |
| Endemism | Narrow endemic species | Endemic to province species | Others species |
| Total | 136 | 235 | 439 |
| Category | Amphibian | Reptile | Birds | Mammals | Bryophytes and Ferns | Gymnosperms | Angiosperms | Total |
|---|---|---|---|---|---|---|---|---|
| Indicator species | 1 | 0 | 3 | 7 | 1 | 0 | 3 | 15 |
| Co-occurring species | 0 | 1 | 3 | 7 | 1 | 3 | 5 | 20 |
| Total | 1 | 1 | 6 | 11 | 2 | 3 | 8 | 32 1 |
| Proportion (%) | 3 | 3 | 19 | 34 | 6 | 9 | 25 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Gong, C.; Zhang, L.; Hu, J.; Ouyang, Z.; Xiao, Y. Which Species Should We Focus On? Umbrella Species Assessment in Southwest China. Biology 2019, 8, 42. https://doi.org/10.3390/biology8020042
Shi X, Gong C, Zhang L, Hu J, Ouyang Z, Xiao Y. Which Species Should We Focus On? Umbrella Species Assessment in Southwest China. Biology. 2019; 8(2):42. https://doi.org/10.3390/biology8020042
Chicago/Turabian StyleShi, Xuewei, Cheng Gong, Lu Zhang, Jian Hu, Zhiyun Ouyang, and Yi Xiao. 2019. "Which Species Should We Focus On? Umbrella Species Assessment in Southwest China" Biology 8, no. 2: 42. https://doi.org/10.3390/biology8020042
APA StyleShi, X., Gong, C., Zhang, L., Hu, J., Ouyang, Z., & Xiao, Y. (2019). Which Species Should We Focus On? Umbrella Species Assessment in Southwest China. Biology, 8(2), 42. https://doi.org/10.3390/biology8020042





