Building Principles for Constructing a Mammalian Blastocyst Embryo
Abstract
1. Introduction
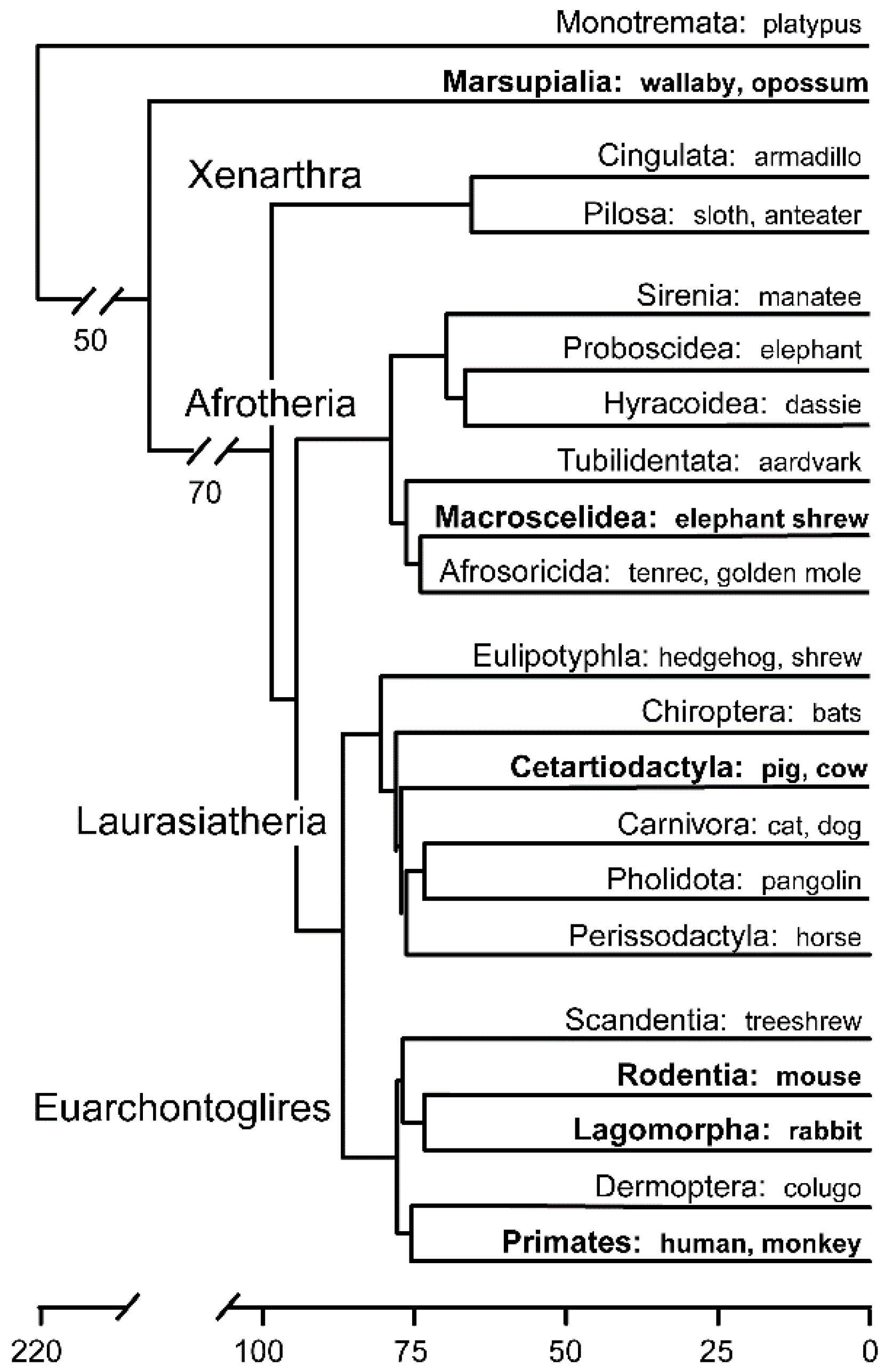
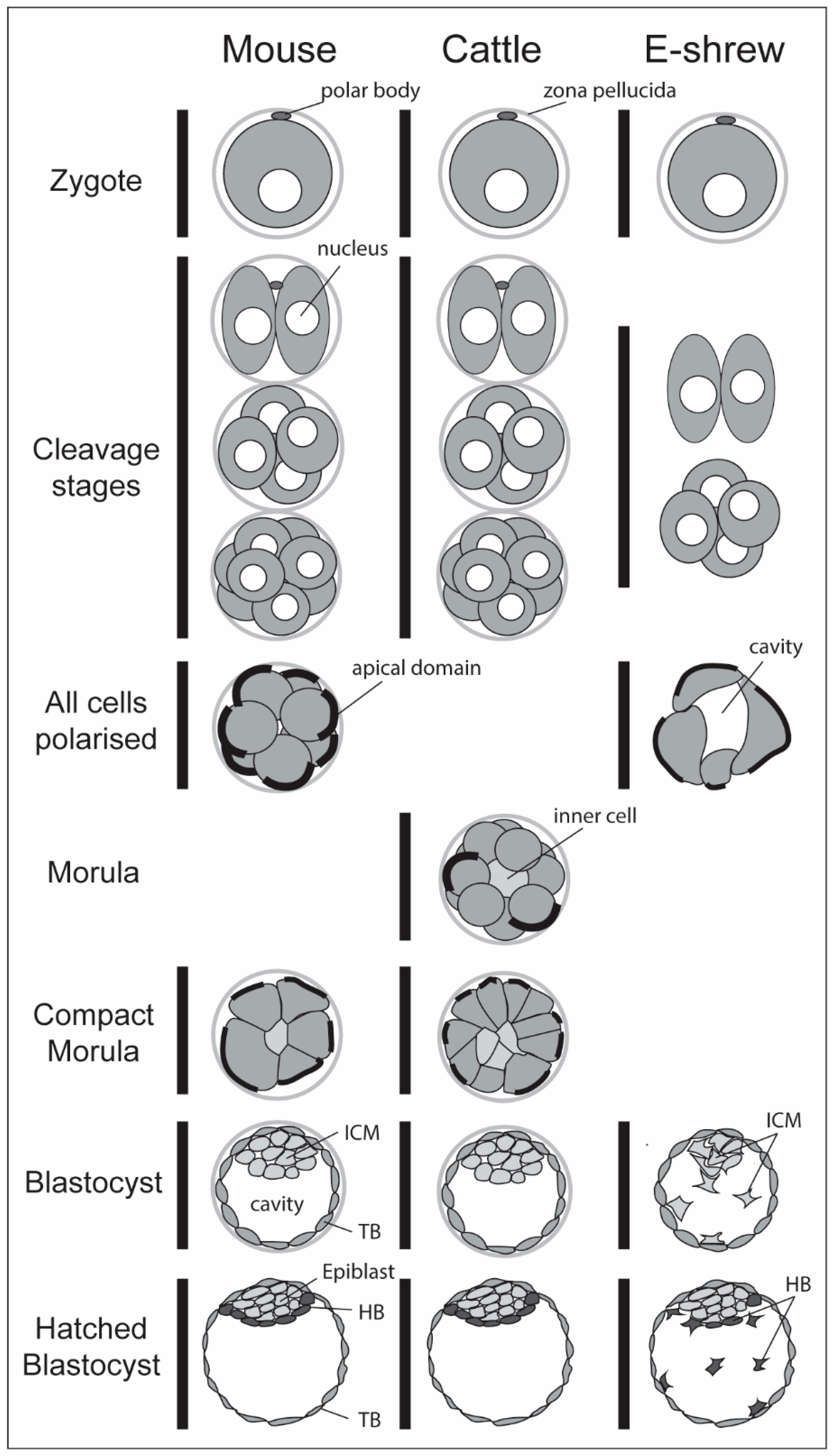
1.1. Mammalian Early Embryological Diversity
1.2. Morphological Events Leading to the Blastocyst
2. Gearing up for Autonomy (Cleavage Stages)
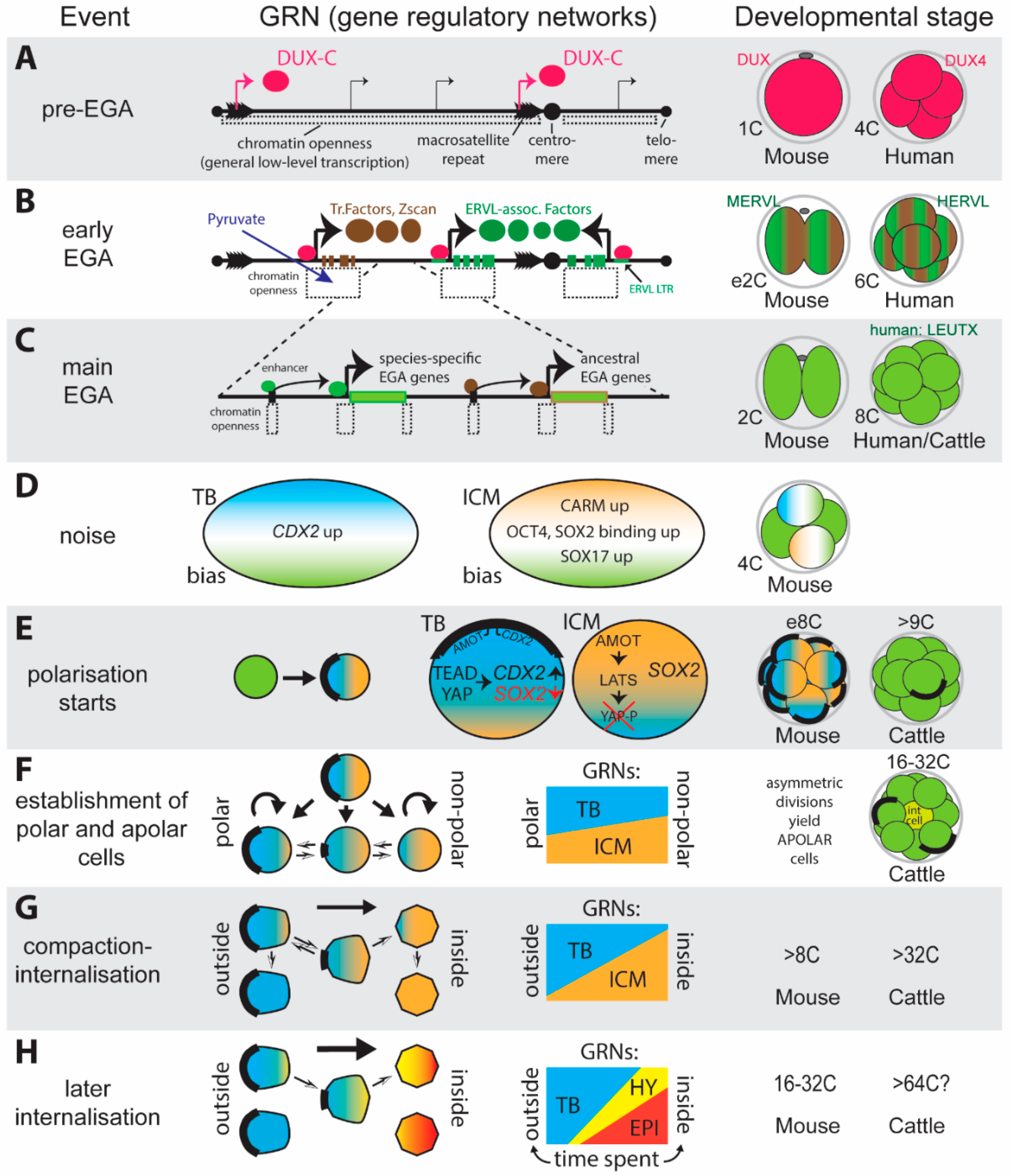
2.1. Triggering EGA Via an External Signal
2.2. Intrinsic Embryonic Genome Activation
2.3. Achieving Totipotency
3. Creating Two Environments in the Morula (Inside and Outside)
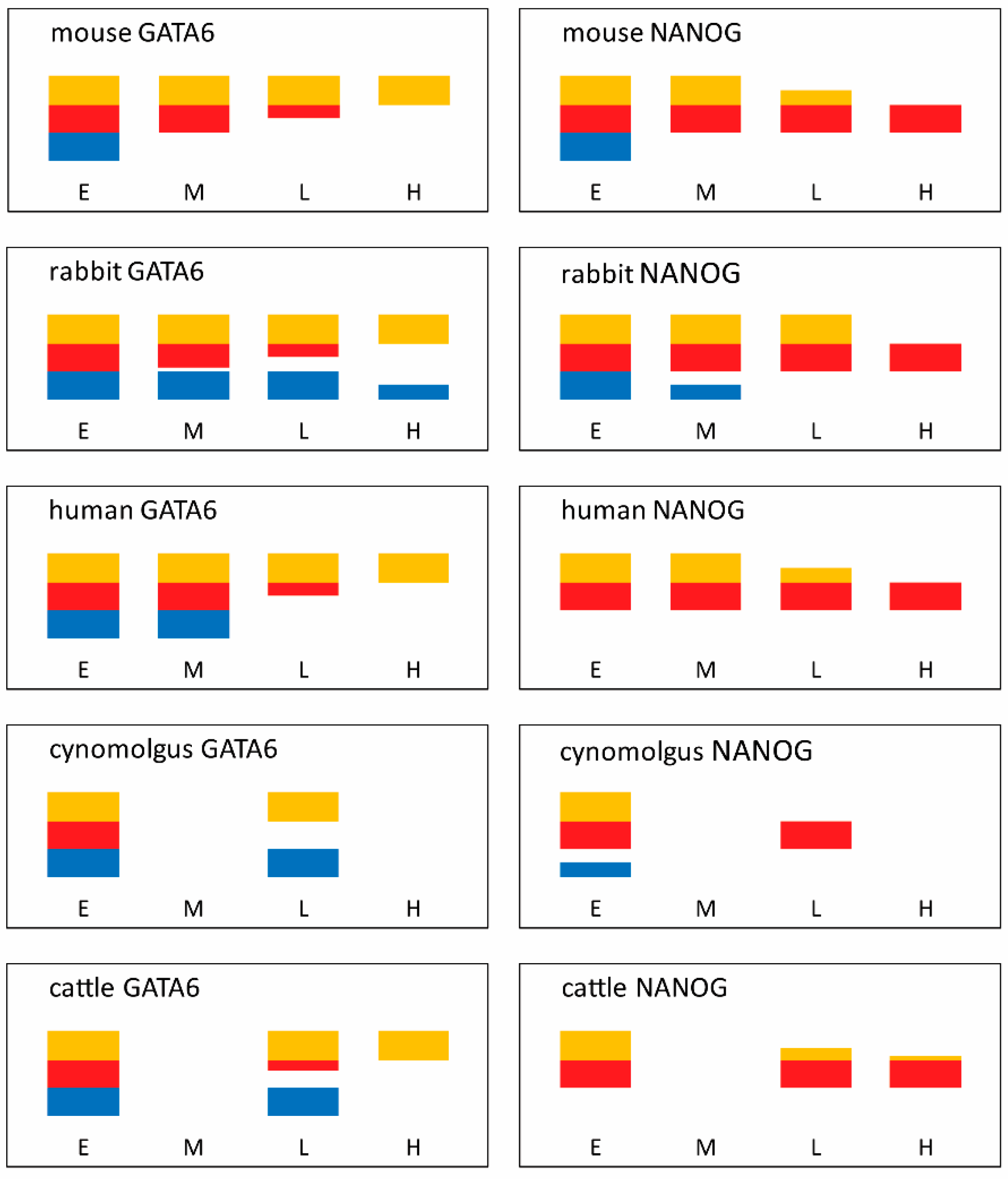
3.1. Establishment of Protein Heterogeneity
3.2. Blastomere Polarisation
3.3. Blastomere Positioning within the Morula
3.4. Polarisation, Compaction and Cell Positioning in Other Mammals
3.5. Epithelialisation of the Outer Cells
4. Establishing the First Lineages (the Blastocyst)
4.1. The Timing of the First Lineage Commitments
4.2. Setting up Stable Lineage Gene Regulatory Networks (GRNs)
4.3. The Trophoblast Lineage
4.3.1. CDX2
4.3.2. GATA2, GATA3
4.3.3. TFAP2a, TFAP2c
4.4. The Pluripotent Inner Cell Mass Lineage
4.5. The Mouse ICM-Epiblast Gene Regulatory Network
- (1)
- In vivo knock-out experiments: OCT4-deficient embryos develop to the early blastocyst stage but the inner cells stop expressing some epiblast (however NANOG is upregulated) and hypoblast markers, and instead start expressing trophoblast markers. Subsequently, all ICM–derived tissue is lost [143,144]. SOX2, while not required for the initial specification of ICM and epiblast, is critical for maintaining epiblast identity, including continued Oct4 and Nanog expression [49,145]. Loss of NANOG led to normal early E3.5 blastocysts, but subsequent loss of epiblast, with blastocyst ICM-culture outgrowths forming only hypoblast [146,147]. SALL4 is required for both ICM and hypoblast derivation [148]. Some of the factors though did not appear to be involved in lineage decisions in this in vivo functional assay: double knock-outs of the closely related KLF2 and 4 genes [149] or of TFCP2L1 [150], ESRRB [151] or GBX2 [152] led to no impairment of early development in mice.
- (2)
- An early differential expression in inner cells: Sox2, Nanog, Klf2 and Essrb are among the first genes seen to be uniquely expressed in inner cells of 16-24-cell morulas [95].
- (3)
- (4)
- Pluripotent reprogramming ability: Overexpression of a cocktail of genes has been shown to be able to reprogram somatic cells to a naïve pluripotent state (so called “induced pluripotent stem cells” or iPSC). The initial cocktail contained three of the core pluripotency factors—OCT4, SOX2, KLF4—as well as c-MYC [154], Subsequently c-MYC was shown to be dispensable, and NANOG and SALL4 to aid, in the derivation of iPSC [155,156].
4.6. Conservation of the ICM-Epiblast Pluripotency GRN
5. The Third Lineage (Hypoblast)
5.1. The Mouse Hypoblast Gene Regulatory Network (GRN)
5.2. FGF Signalling in the Mouse Hypoblast/Epiblast Lineage Decision
5.3. A Common Hypoblast Gene Regulatory Network (GRN)
5.4. FGF Signalling in Other Mammals
5.5. Alternative Signalling
5.6. The Third Lineage—Conclusion
6. Concluding Remarks
- An intrinsic trigger to switch on the embryonic gene expression program. This trigger (DUX-C) is nearly fail-proof thanks to being present in the genome in high copy numbers.
- The use of inherent random fluctuations (noise) in the gene expression machinery to generate asymmetries between blastomeres, which is likely to play a part in biasing cells during the first and second lineage decisions.
- The adaptation of basic cellular processes (polarisation, compaction—as seen during mesenchymal to epithelial transitions) to asymmetrically segregate lineage specifiers during subsequent cell divisions.
- Amplification of small differences in GRN-biases via reciprocal inhibition between alternative GRN programs. For the first lineage decision such inhibition is achieved predominantly through a small set of master transcriptional regulators, for the second decision additional control is achieved through the use of diffusible signalling molecules.
Funding
Conflicts of Interest
References
- Woodger, J. On biological transformations. In Essays on Growth and Form Presented to D’Arcy Wentworth Thompson; Le Gross Clark, E., Medawar, P.B., Eds.; Clarendon Press: Oxford, UK, 1945; pp. 95–120. [Google Scholar]
- Foley, N.M.; Springer, M.S.; Teeling, E.C. Mammal madness: Is the mammal tree of life not yet resolved? Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150140. [Google Scholar] [CrossRef] [PubMed]
- Wimsatt, W.A. Some comparative aspects of implantation. Biol. Reprod. 1975, 12, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, C.J. Early stages in the embryonic development of Elephantulus. S. Afr. J. Med. Sci. 1942, 7, 55–67. [Google Scholar]
- Goetz, R.H. Early development of the Tenrecoidea. BioMorphosis 1938, 1, 67–79. [Google Scholar]
- Selwood, L.; Johnson, M.H. Trophoblast and hypoblast in the monotreme, marsupial and eutherian mammal: Evolution and origins. Bioessays 2006, 28, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.S.; Viotti, M.; Hadjantonakis, A.K. The Endoderm of the Mouse Embryo Arises by Dynamic Widespread Intercalation of Embryonic and Extraembryonic Lineages. Dev. Cell 2008, 15, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; Krebs, S.; Heininen-Brown, M.; Zakhartchenko, V.; Blum, H.; Wolf, E. Genome activation in bovine embryos: Review of the literature and new insights from RNA sequencing experiments. Anim. Reprod. Sci. 2014, 149, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, R.; Sharpley, M.S.; Chi, F.; Braas, D.; Zhou, Y.; Kim, R.; Clark, A.T.; Banerjee, U. Nuclear Localization of Mitochondrial TCA Cycle Enzymes as a Critical Step in Mammalian Zygotic Genome Activation. Cell 2017, 168, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Khurana, N.K.; Niemann, H. Energy metabolism in preimplantation bovine embryos derived in vitro or in vivo. Biol. Reprod. 2000, 62, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Harvey, A.J. Blastocyst metabolism. Reprod. Fertil. Dev. 2015, 27, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.L.; Nien, C.Y.; Liu, H.Y.; Metzstein, M.M.; Kirov, N.; Rushlow, C. The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature 2008, 456, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Whiddon, J.L.; Langford, A.T.; Wong, C.J.; Zhong, J.W.; Tapscott, S.J. Conservation and innovation in the DUX4-family gene network. Nat. Genet. 2017, 49, 935–940. [Google Scholar] [CrossRef] [PubMed]
- De Iaco, A.; Planet, E.; Coluccio, A.; Verp, S.; Duc, J.; Trono, D. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat. Genet. 2017, 49, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, P.G.; Dorais, J.A.; Grow, E.J.; Whiddon, J.L.; Lim, J.W.; Wike, C.L.; Weaver, B.D.; Pflueger, C.; Emery, B.R.; Wilcox, A.L.; et al. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat. Genet. 2017, 49, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Gearhart, M.D.; Cui, Z.; Bosnakovski, D.; Kim, M.; Schennum, N.; Kyba, M. DUX4 recruits p300/CBP through its C-terminus and induces global H3K27 acetylation changes. Nucleic Acids Res. 2016, 44, 5161–5173. [Google Scholar] [CrossRef] [PubMed]
- Leidenroth, A.; Clapp, J.; Mitchell, L.M.; Coneyworth, D.; Dearden, F.L.; Iannuzzi, L.; Hewitt, J.E. Evolution of DUX gene macrosatellites in placental mammals. Chromosoma 2012, 121, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Perrod, S.; Gasser, S.M. Long-range silencing and position effects at telomeres and centromeres: Parallels and differences. Cell Mol. Life Sci. 2003, 60, 2303–2318. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Yamamoto, R.; Franke, V.; Cao, M.; Suzuki, Y.; Suzuki, M.G.; Vlahovicek, K.; Svoboda, P.; Schultz, R.M.; Aoki, F. The first murine zygotic transcription is promiscuous and uncoupled from splicing and 3′ processing. EMBO J. 2015, 34, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, B.; Chen, H.; Yin, Q.; Liu, Y.; Xiang, Y.; Zhang, B.; Liu, B.; Wang, Q.; Xia, W.; et al. The landscape of accessible chromatin in mammalian preimplantation embryos. Nature 2016, 534, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Ooga, M.; Fulka, H.; Hashimoto, S.; Suzuki, M.G.; Aoki, F. Analysis of chromatin structure in mouse preimplantation embryos by fluorescent recovery after photobleaching. Epigenetics 2016, 11, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Peaston, A.E.; Evsikov, A.V.; Graber, J.H.; de Vries, W.N.; Holbrook, A.E.; Solter, D.; Knowles, B.B. Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev. Cell 2004, 7, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Jouhilahti, E.-M.; Madissoon, E.; Vesterlund, L.; Töhönen, V.; Krjutškov, K.; Plaza Reyes, A.; Petropoulos, S.; Månsson, R.; Linnarsson, S.; Bürglin, T.; et al. The human PRD-like homeobox gene LEUTX has a central role in embryo genome activation. Development 2016, 143, 3459–3469. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Huang, K.; Cai, C.; Cai, L.; Jiang, C.Y.; Feng, Y.; Liu, Z.; Zeng, Q.; Cheng, L.; Sun, Y.E.; et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 2013, 500, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bailey, S.M.; Okuka, M.; Munoz, P.; Li, C.; Zhou, L.; Wu, C.; Czerwiec, E.; Sandler, L.; Seyfang, A.; et al. Telomere lengthening early in development. Nat. Cell Biol. 2007, 9, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Zalzman, M.; Falco, G.; Sharova, L.V.; Nishiyama, A.; Thomas, M.; Lee, S.L.; Stagg, C.A.; Hoang, H.G.; Yang, H.T.; Indig, F.E.; et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 2010, 464, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Seidel, F. Die Entwicklungspotenzen einer isolierten Blastomere des Zweizellenstadiums im Säugetierei. Naturwissenschaften 1952, 39, 355–356. [Google Scholar] [CrossRef]
- Tarkowski, A.K. Experiments on the development of isolated blastomers of mouse eggs. Nature 1959, 184, 1286–1287. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, S.M. The development capacity of blastomeres from 4- and 8-cell sheep embryos. J. Embryol. Exp. Morphol. 1981, 65, 165–172. [Google Scholar] [PubMed]
- Fehilly, C.B.; Willadsen, S.M. Embryo manipulation in farm animals. In Oxford Reviews of Reproductive Biology; Clarke, J.R., Ed.; Clarendon Press: Oxford, UK, 1986; pp. 379–413. [Google Scholar]
- Van de Velde, H.; Cauffman, G.; Tournaye, H.; Devroey, P.; Liebaers, I. The four blastomeres of a 4-cell stage human embryo are able to develop individually into blastocysts with inner cell mass and trophectoderm. Hum. Reprod. 2008, 23, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, P.L. Lineage commitment in the mammalian preimplantation embryo. In Reproduction in Domestic Ruminants VIII; Juengel, J., Miyamoto, A., Webb, R., Eds.; Context: Obihiro, Japan, 2014; Volume 8, pp. 89–103. [Google Scholar]
- Huh, D.; Paulsson, J. Non-genetic heterogeneity from stochastic partitioning at cell division. Nat. Genet. 2011, 43, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.; Muller, J.; Tu, S.; Padilla-Longoria, P.; Guccione, E.; Torres-Padilla, M.E. Single-cell profiling of epigenetic modifiers identifies PRDM14 as an inducer of cell fate in the mammalian embryo. Cell Rep. 2013, 5, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Torres-Padilla, M.-E.; Parfitt, D.-E.; Kouzarides, T.; Zernicka-Goetz, M. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 2007, 445, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Plachta, N.; Bollenbach, T.; Pease, S.; Fraser, S.E.; Pantazis, P. Oct4 kinetics predict cell lineage patterning in the early mammalian embryo. Nat. Cell Biol. 2011, 13, 117–123. [Google Scholar] [CrossRef] [PubMed]
- White, M.D.; Angiolini, J.F.; Alvarez, Y.D.; Kaur, G.; Zhao, Z.W.; Mocskos, E.; Bruno, L.; Bissiere, S.; Levi, V.; Plachta, N. Long-Lived Binding of Sox2 to DNA Predicts Cell Fate in the Four-Cell Mouse Embryo. Cell 2016, 165, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, Q.; Li, X.; Zheng, X.; Zhang, Y.; Qiao, J.; Tang, F.; Tao, Y.; Zhou, Q.; Duan, E. Dynamic transcriptional symmetry-breaking in pre-implantation mammalian embryo development revealed by single-cell RNA-seq. Development 2015, 142, 3468–3477. [Google Scholar] [CrossRef] [PubMed]
- Goolam, M.; Scialdone, A.; Graham, S.J.L.; Macaulay, I.C.; Jedrusik, A.; Hupalowska, A.; Voet, T.; Marioni, J.C.; Zernicka-Goetz, M. Heterogeneity in Oct4 and Sox2 Targets Biases Cell Fate in 4-Cell Mouse Embryos. Cell 2017, 165, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Biase, F.H.; Cao, X.; Zhong, S. Cell fate inclination within 2-cell and 4-cell mouse embryos revealed by single-cell RNA sequencing. Genome Res. 2014, 24, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Ralston, A.; Stephenson, R.O.; Rossant, J. Cell and molecular regulation of the mouse blastocyst. Dev. Dyn. 2006, 235, 2301–2314. [Google Scholar] [CrossRef] [PubMed]
- Ziomek, C.A.; Johnson, M.H. Cell surface interaction induces polarization of mouse 8-cell blastomeres at compaction. Cell 1980, 21, 935–942. [Google Scholar] [CrossRef]
- Stephenson, R.O.; Yamanaka, Y.; Rossant, J. Disorganized epithelial polarity and excess trophectoderm cell fate in preimplantation embryos lacking E-cadherin. Development 2010, 137, 3383–3391. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; Maro, B.; Takeichi, M. The role of cell adhesion in the synchronization and orientation of polarization in 8-cell mouse blastomeres. J. Embryol. Exp. Morphol. 1986, 93, 239–255. [Google Scholar] [PubMed]
- Korotkevich, E.; Niwayama, R.; Courtois, A.; Friese, S.; Berger, N.; Buchholz, F.; Hiiragi, T. The Apical Domain Is Required and Sufficient for the First Lineage Segregation in the Mouse Embryo. Dev. Cell 2017, 40, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, N.; Inoue, K.; Adachi, K.; Kiyonari, H.; Ota, M.; Ralston, A.; Yabuta, N.; Hirahara, S.; Stephenson, R.O.; Ogonuki, N.; et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 2009, 16, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Hirate, Y.; Hirahara, S.; Inoue, K.; Suzuki, A.; Alarcon, V.B.; Akimoto, K.; Hirai, T.; Hara, T.; Adachi, M.; Chida, K.; et al. Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Curr. Biol. 2013, 23, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.Y.; Zernicka-Goetz, M. Angiomotin prevents pluripotent lineage differentiation in mouse embryos via Hippo pathway-dependent and -independent mechanisms. Nat. Commun. 2013, 4, 2251. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, E.; Blij, S.; Frum, T.; Hirate, Y.; Lang, R.A.; Sasaki, H.; Ralston, A. HIPPO pathway members restrict SOX2 to the inner cell mass where it promotes ICM fates in the mouse blastocyst. PLoS Genet. 2014, 10, e1004618. [Google Scholar] [CrossRef] [PubMed]
- Skamagki, M.; Wicher, K.B.; Jedrusik, A.; Ganguly, S.; Zernicka-Goetz, M. Asymmetric localization of Cdx2 mRNA during the first cell-fate decision in early mouse development. Cell Rep. 2013, 3, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Humięcka, M.; Szpila, M.; Kłoś, P.; Maleszewski, M.; Szczepańska, K. Mouse blastomeres acquire ability to divide asymmetrically before compaction. PLoS ONE 2017, 12, e0175032. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Gunther, S.; Reichmann, J.; Krzic, U.; Balazs, B.; de Medeiros, G.; Norlin, N.; Hiiragi, T.; Hufnagel, L.; Ellenberg, J. Inverted light-sheet microscope for imaging mouse pre-implantation development. Nat. Methods 2016, 13, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; Ziomek, C.A. The foundation of two distinct cell lineages within the mouse morula. Cell 1981, 24, 71–80. [Google Scholar] [CrossRef]
- Anani, S.; Bhat, S.; Honma-Yamanaka, N.; Krawchuk, D.; Yamanaka, Y. Initiation of Hippo signaling is linked to polarity rather than to cell position in the pre-implantation mouse embryo. Development 2014, 141, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Biggins, J.S.; Tannan, N.B.; Srinivas, S. Limited predictive value of blastomere angle of division in trophectoderm and inner cell mass specification. Development 2014, 141, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- De Vries, W.N.; Evsikov, A.V.; Haac, B.E.; Fancher, K.S.; Holbrook, A.E.; Kemler, R.; Solter, D.; Knowles, B.B. Maternal β-catenin and E-cadherin in mouse development. Development 2004, 131, 4435–4445. [Google Scholar] [CrossRef] [PubMed]
- Fierro-González, J.C.; White, M.D.; Silva, J.C.; Plachta, N. Cadherin-dependent filopodia control preimplantation embryo compaction. Nat. Cell Biol. 2013, 15, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Samarage, C.R.; White, M.D.; Álvarez, Y.D.; Fierro-González, J.C.; Henon, Y.; Jesudason, E.C.; Bissiere, S.; Fouras, A.; Plachta, N. Cortical Tension Allocates the First Inner Cells of the Mammalian Embryo. Dev. Cell 2015, 34, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Maître, J.-L.; Turlier, H.; Illukkumbura, R.; Eismann, B.; Niwayama, R.; Nédélec, F.; Hiiragi, T. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature 2016, 536, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.H.; Levine, M.S. Properties of developmental gene regulatory networks. Proc. Natl. Acad. Sci. USA 2008, 105, 20063–20066. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, B.D.; Sevilla, A.; Lenz, M.; Müller, F.-J.; Schuldt, B.M.; Schuppert, A.A.; Ridden, S.J.; Stumpf, P.S.; Fidalgo, M.; Ma’ayan, A.; et al. Nanog-dependent feedback loops regulate murine embryonic stem cell heterogeneity. Nat. Cell Biol. 2012, 14, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Suzuki, H.; Yang, X.; Jiang, S.; Foote, R.H. Analysis of polarity of bovine and rabbit embryos by scanning electron microscopy. Biol. Reprod. 1994, 50, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Van Soom, A.; Boerjan, M.L.; Bols, P.E.; Vanroose, G.; Lein, A.; Coryn, M.; de Kruif, A. Timing of compaction and inner cell allocation in bovine embryos produced in vivo after superovulation. Biol. Reprod. 1997, 57, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, C.J. The placentation of Elephantulus. Trans. R. Soc. S. Afr. 1949, 32, 435–629. [Google Scholar] [CrossRef]
- Barcroft, L.C.; Hay-Schmidt, A.; Caveney, A.; Gilfoyle, E.; Overstrom, E.W.; Hyttel, P.; Watson, A.J. Trophectoderm differentiation in the bovine embryo: Characterization of a polarized epithelium. J. Reprod. Fertil. 1998, 114, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Tsukita, S.; Furuse, M. Tight junctions containing claudin 4 and 6 are essential for blastocyst formation in preimplantation mouse embryos. Dev. Biol. 2007, 312, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Zenker, J.; White, M.D.; Gasnier, M.; Alvarez, Y.D.; Lim, H.Y.G.; Bissiere, S.; Biro, M.; Plachta, N. Expanding Actin Rings Zipper the Mouse Embryo for Blastocyst Formation. Cell 2018, 173, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.; Barcroft, L.C. Regulation of blastocyst formation. Front. Biosci. 2001, 6, D708–D730. [Google Scholar] [CrossRef] [PubMed]
- Benos, D.J.; Balaban, R.S. Energy requirements of the developing mammalian blastocyst for active ion transport. Biol. Reprod. 1980, 23, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J.; Baumann, C.G.; Brison, D.R.; McEvoy, T.G.; Sturmey, R.G. Metabolism of the viable mammalian embryo: Quietness revisited. Mol. Hum. Reprod. 2008, 14, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.F.; Wales, R.G. Glycolysis and glucose oxidation by the sheep conceptus at different oxygen concentrations. Reprod. Fertil. Dev. 1993, 5, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, L.C.; Leese, H.J. Energy metabolism of the trophectoderm and inner cell mass of the mouse blastocyst. J. Exp. Zool. 1993, 267, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Houghton, F.D.; Thompson, J.G.; Kennedy, C.J.; Leese, H.J. Oxygen consumption and energy metabolism of the early mouse embryo. Mol. Reprod. Dev. 1996, 44, 476–485. [Google Scholar] [CrossRef]
- Trimarchi, J.R.; Liu, L.; Porterfield, D.M.; Smith, P.J.S.; Keefe, D.L. Oxidative Phosphorylation-Dependent and -Independent Oxygen Consumption by Individual Preimplantation Mouse Embryos1. Biol. Reprod. 2000, 62, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Dumollard, R.; Carroll, J.; Duchen, M.R.; Campbell, K.; Swann, K. Mitochondrial function and redox state in mammalian embryos. Sem. Cell Dev. Biol. 2009, 20, 346–353. [Google Scholar] [CrossRef]
- Kaneko, K.J.; DePamphilis, M.L. TEAD4 establishes the energy homeostasis essential for blastocoel formation. Development 2013, 140, 3680–3690. [Google Scholar] [CrossRef] [PubMed]
- Home, P.; Saha, B.; Ray, S.; Dutta, D.; Gunewardena, S.; Yoo, B.; Pal, A.; Vivian, J.L.; Larson, M.; Petroff, M.; et al. Altered subcellular localization of transcription factor TEAD4 regulates first mammalian cell lineage commitment. Proc. Natl. Acad. Sci. USA 2012, 109, 7362–7367. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N.; Takahashi, K.; Emura, N.; Hashizume, T.; Sawai, K. Effects of downregulating TEAD4 transcripts by RNA interference on early development of bovine embryos. J. Reprod. Dev. 2017, 63, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.T.; Bantilan, N.S.; Wang, V.N.; Nellett, K.M.; Cruz, Y.P. Expression patterns of Oct4, Cdx2, Tead4, and Yap1 proteins during blastocyst formation in embryos of the marsupial, Monodelphis domestica Wagner. Evol. Dev. 2013, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Dyce, J.; George, M.; Goodall, H.; Fleming, T.P. Do trophectoderm and inner cell mass cells in the mouse blastocyst maintain discrete lineages? Development 1987, 100, 685–698. [Google Scholar] [PubMed]
- Copp, A.J. Interaction between inner cell mass and trophectoderm of the mouse blastocyst. II. The fate of the polar trophectoderm. J. Embryol. Exp. Morphol. 1979, 51, 109–120. [Google Scholar] [PubMed]
- Berg, D.K.; Smith, C.S.; Pearton, D.J.; Wells, D.N.; Broadhurst, R.; Donnison, M.; Pfeffer, P.L. Trophectoderm lineage determination in cattle. Dev. Cell 2011, 20, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. The strategy of the genes. In A Discussion of Some Aspects of Theoretical Biology; George Allen & Unwin, Ltd.: London, UK, 1957. [Google Scholar]
- Rossant, J.; Vijh, K.M. Ability of outside cells from preimplantation mouse embryos to form inner cell mass derivatives. Dev. Biol. 1980, 76, 475–482. [Google Scholar] [CrossRef]
- Tarkowski, A.K.; Suwinska, A.; Czolowska, R.; Ozdzenski, W. Individual blastomeres of 16- and 32-cell mouse embryos are able to develop into foetuses and mice. Dev. Biol. 2010, 348, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Suwinska, A.; Czolowska, R.; Ozdzenski, W.; Tarkowski, A.K. Blastomeres of the mouse embryo lose totipotency after the fifth cleavage division: Expression of Cdx2 and Oct4 and developmental potential of inner and outer blastomeres of 16- and 32-cell embryos. Dev. Biol. 2008, 322, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Niakan, K.K.; Han, J.; Pedersen, R.A.; Simon, C.; Pera, R.A.R. Human pre-implantation embryo development. Development 2012, 139, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J.; Lis, W.T. Potential of isolated mouse inner cell masses to form trophectoderm derivatives in vivo. Dev. Biol. 1979, 70, 255–261. [Google Scholar] [CrossRef]
- Szczepanska, K.; Stanczuk, L.; Maleszewski, M. Isolated mouse inner cell mass is unable to reconstruct trophectoderm. Differentiation 2011, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Grabarek, J.B.; Zyzynska, K.; Saiz, N.; Piliszek, A.; Frankenberg, S.; Nichols, J.; Hadjantonakis, A.K.; Plusa, B. Differential plasticity of epiblast and primitive endoderm precursors within the ICM of the early mouse embryo. Development 2012, 139, 129–139. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, C.; Cauffman, G.; Verloes, A.; Sterckx, J.; Devroey, P.; Tournaye, H.; Liebaers, I.; Van de Velde, H. Human trophectoderm cells are not yet committed. Hum. Reprod. 2013, 28, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Guillomot, M.; Guay, P. Ultrastructural features of the cell surfaces of uterine and trophoblastic epithelia during embryo attachment in the cow. Anat. Rec. 1982, 204, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Durruthy-Durruthy, J.; Wossidlo, M.; Pai, S.; Takahashi, Y.; Kang, G.; Omberg, L.; Chen, B.; Nakauchi, H.; Reijo Pera, R.; Sebastiano, V. Spatiotemporal Reconstruction of the Human Blastocyst by Single-Cell Gene-Expression Analysis Informs Induction of Naive Pluripotency. Dev. Cell 2016, 38, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Huss, M.; Tong, G.Q.; Wang, C.; Li Sun, L.; Clarke, N.D.; Robson, P. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev. Cell 2010, 18, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Edsgärd, D.; Reinius, B.; Deng, Q.; Panula, S.P.; Codeluppi, S.; Plaza Reyes, A.; Linnarsson, S.; Sandberg, R.; Lanner, F. Single-Cell RNA-Seq Reveals Lineage and X Chromosome Dynamics in Human Preimplantation Embryos. Cell 2016, 165, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhong, L.; Zhang, S.; Mu, H.; Xiang, J.; Yue, L.; Dai, Y.; Han, J. Bovine lineage specification revealed by single-cell gene expression analysis from zygote to blastocyst. Biol. Reprod. 2017, 97, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Okamoto, I.; Sasaki, K.; Yabuta, Y.; Iwatani, C.; Tsuchiya, H.; Seita, Y.; Nakamura, S.; Yamamoto, T.; Saitou, M. A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 2016, 537, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Stirparo, G.G.; Boroviak, T.; Guo, G.; Nichols, J.; Smith, A.; Bertone, P. Integrated analysis of single-cell embryo data yields a unified transcriptome signature for the human pre-implantation epiblast. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Blakeley, P.; Fogarty, N.M.; Del Valle, I.; Wamaitha, S.E.; Hu, T.X.; Elder, K.; Snell, P.; Christie, L.; Robson, P.; Niakan, K.K. Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development 2015, 142, 3151–3165. [Google Scholar] [CrossRef] [PubMed]
- Boroviak, T.; Loos, R.; Lombard, P.; Okahara, J.; Behr, R.; Sasaki, E.; Nichols, J.; Smith, A.; Bertone, P. Lineage-Specific Profiling Delineates the Emergence and Progression of Naive Pluripotency in Mammalian Embryogenesis. Dev. Cell 2015, 35, 366–382. [Google Scholar] [CrossRef] [PubMed]
- Home, P.; Kumar, R.P.; Ganguly, A.; Saha, B.; Milano-Foster, J.; Bhattacharya, B.; Ray, S.; Gunewardena, S.; Paul, A.; Camper, S.A.; et al. Genetic redundancy of GATA factors in the extraembryonic trophoblast lineage ensures the progression of preimplantation and postimplantation mammalian development. Development 2017, 144, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Ramsköld, D.; Reinius, B.; Sandberg, R. Single-Cell RNA-Seq Reveals Dynamic, Random Monoallelic Gene Expression in Mammalian Cells. Science 2014, 343, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Degrelle, S.A.; Campion, E.; Cabau, C.; Piumi, F.; Reinaud, P.; Richard, C.; Renard, J.P.; Hue, I. Molecular evidence for a critical period in mural trophoblast development in bovine blastocysts. Dev. Biol. 2005, 288, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Pearton, D.J.; Broadhurst, R.; Donnison, M.; Pfeffer, P.L. Elf5 regulation in the Trophectoderm. Dev. Biol. 2011, 360, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Valdez Magana, G.; Rodriguez, A.; Zhang, H.; Webb, R.; Alberio, R. Paracrine effects of embryo-derived FGF4 and BMP4 during pig trophoblast elongation. Dev. Biol. 2014, 387, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Niakan, K.K.; Eggan, K. Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev. Biol. 2013, 375, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Madeja, Z.E.; Sosnowski, J.; Hryniewicz, K.; Warzych, E.; Pawlak, P.; Rozwadowska, N.; Plusa, B.; Lechniak, D. Changes in sub-cellular localisation of trophoblast and inner cell mass specific transcription factors during bovine preimplantation development. BMC Dev. Biol. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Bou, G.; Liu, S.; Sun, M.; Zhu, J.; Xue, B.; Guo, J.; Zhao, Y.; Qu, B.; Weng, X.; Wei, Y.; et al. CDX2 is essential for cell proliferation and polarity in porcine blastocysts. Development 2017, 144, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Piliszek, A.; Barłowska, K.; Madeja, Z.; Pawlak, P.; Plusa, B. Differentiation of trophectoderm in rabbit embryos is initiated in the absence of Gata3 and Cdx2. Mech. Dev. 2017, 145, S79. [Google Scholar] [CrossRef]
- Ralston, A.; Rossant, J. Cdx2 acts downstream of cell polarization to cell-autonomously promote trophectoderm fate in the early mouse embryo. Dev. Biol. 2008, 313, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Gentile, L.; Fuchikami, T.; Sutter, J.; Psathaki, K.; Esteves, T.C.; Araúzo-Bravo, M.J.; Ortmeier, C.; Verberk, G.; Abe, K.; et al. Initiation of trophectoderm lineage specification in mouse embryos is independent of Cdx2. Development 2010, 137, 4159–4169. [Google Scholar] [CrossRef] [PubMed]
- Strumpf, D.; Mao, C.A.; Yamanaka, Y.; Ralston, A.; Chawengsaksophak, K.; Beck, F.; Rossant, J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 2005, 132, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Jedrusik, A.; Cox, A.; Wicher, K.; Glover, D.M.; Zernicka-Goetz, M. Maternal-zygotic knockout reveals a critical role of Cdx2 in the morula to blastocyst transition. Dev. Biol. 2015, 398, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Sritanaudomchai, H.; Sparman, M.; Tachibana, M.; Clepper, L.; Woodward, J.; Gokhale, S.; Wolf, D.; Hennebold, J.; Hurlbut, W.; Grompe, M.; et al. CDX2 in the formation of the trophectoderm lineage in primate embryos. Dev. Biol. 2009, 335, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Goissis, M.D.; Cibelli, J.B. Functional characterization of CDX2 during bovine preimplantation development in vitro. Mol. Reprod. Dev. 2014, 81, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N.; Takahashi, K.; Emura, N.; Fujii, T.; Hirayama, H.; Kageyama, S.; Hashizume, T.; Sawai, K. The Necessity of OCT-4 and CDX2 for Early Development and Gene Expression Involved in Differentiation of Inner Cell Mass and Trophectoderm Lineages in Bovine Embryos. Cell Reprogr. 2016, 18, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, N.; Yamamoto, S.; Kiyonari, H.; Sato, H.; Sawada, A.; Ota, M.; Nakao, K.; Sasaki, H. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech. Dev. 2008, 125, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.J.; DePamphilis, M.L. Regulation of gene expression at the beginning of mammalian development and the TEAD family of transcription factors. Dev. Genet. 1998, 22, 43–55. [Google Scholar] [CrossRef]
- Vassilev, A.; Kaneko, K.J.; Shu, H.; Zhao, Y.; DePamphilis, M.L. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001, 15, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Carey, T.S.; Ganguly, A.; Wilson, C.A.; Paul, S.; Knott, J.G. Transcription factor AP-2gamma induces early Cdx2 expression and represses HIPPO signaling to specify the trophectoderm lineage. Development 2015, 142, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Home, P.; Ray, S.; Dutta, D.; Bronshteyn, I.; Larson, M.; Paul, S. GATA3 is selectively expressed in the trophectoderm of peri-implantation embryo and directly regulates Cdx2 gene expression. J. Biol. Chem. 2009, 284, 28729–28737. [Google Scholar] [CrossRef] [PubMed]
- Rayon, T.; Menchero, S.; Nieto, A.; Xenopoulos, P.; Crespo, M.; Cockburn, K.; Cañon, S.; Sasaki, H.; Hadjantonakis, A.-K.; de la Pompa, J.L.; et al. Notch and Hippo Converge on Cdx2 to Specify the Trophectoderm Lineage in the Mouse Blastocyst. Dev. Cell 2014, 30, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Toyooka, Y.; Shimosato, D.; Strumpf, D.; Takahashi, K.; Yagi, R.; Rossant, J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 2005, 123, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Murohashi, M.; Nakamura, T.; Tanaka, S.; Ichise, T.; Yoshida, N.; Yamamoto, T.; Shibuya, M.; Schlessinger, J.; Gotoh, N. An FGF4-FRS2alpha-Cdx2 axis in trophoblast stem cells induces Bmp4 to regulate proper growth of early mouse embryos. Stem Cells 2010, 28, 113–121. [Google Scholar] [PubMed]
- Ralston, A.; Cox, B.J.; Nishioka, N.; Sasaki, H.; Chea, E.; Rugg-Gunn, P.; Guo, G.; Robson, P.; Draper, J.S.; Rossant, J. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development 2010, 137, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Sakurai, T.; Someya, Y.; Konno, T.; Ideta, A.; Aoyagi, Y.; Imakawa, K. Regulation of Trophoblast-Specific Factors by GATA2 and GATA3 in Bovine Trophoblast CT-1 Cells. J. Reprod. Dev. 2011, 57, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Negron-Perez, V.M.; Zhang, Y.; Hansen, P.J. Single-cell gene expression of the bovine blastocyst. Reproduction 2017, 154, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Winger, Q.; Huang, J.; Auman, H.J.; Lewandoski, M.; Williams, T. Analysis of transcription factor AP-2 expression and function during mouse preimplantation development. Biol. Reprod. 2006, 75, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Kuckenberg, P.; Buhl, S.; Woynecki, T.; van Furden, B.; Tolkunova, E.; Seiffe, F.; Moser, M.; Tomilin, A.; Winterhager, E.; Schorle, H. The transcription factor TCFAP2C/AP-2gamma cooperates with CDX2 to maintain trophectoderm formation. Mol. Cell Biol. 2010, 30, 3310–3320. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kwon, J.W.; Choi, I.; Kim, N.H. Expression and function of transcription factor AP-2gamma in early embryonic development of porcine parthenotes. Reprod. Fertil. Dev. 2016, 28, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Zhang, H.; Tang, W.W.C.; Irie, N.; Withey, S.; Klisch, D.; Sybirna, A.; Dietmann, S.; Contreras, D.A.; Webb, R.; et al. Principles of early human development and germ cell program from conserved model systems. Nature 2017, 546, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.L. Clonal analysis of early mammalian development. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1985, 312, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.L.; Rossant, J. Investigation of the fate of 4-5 day post-coitum mouse inner cell mass cells by blastocyst injection. J. Embryol. Exp. Morphol. 1979, 52, 141–152. [Google Scholar] [PubMed]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Beddington, R.S.; Robertson, E.J. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development 1989, 105, 733–737. [Google Scholar] [PubMed]
- Boroviak, T.; Loos, R.; Bertone, P.; Smith, A.; Nichols, J. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat. Cell Biol. 2014, 16, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Smith, A. Naive and Primed Pluripotent States. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Guo, G.; Loos, R.; Nichols, J.; Ficz, G.; Krueger, F.; Oxley, D.; Santos, F.; Clarke, J.; Mansfield, W.; et al. Resetting Transcription Factor Control Circuitry toward Ground-State Pluripotency in Human. Cell 2014, 158, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, T.W.; Powell, B.E.; Wang, H.; Mitalipova, M.; Faddah, D.A.; Reddy, J.; Fan, Z.P.; Maetzel, D.; Ganz, K.; Shi, L.; et al. Systematic Identification of Culture Conditions for Induction and Maintenance of Naive Human Pluripotency. Cell Stem Cell 2014, 15, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Ávila-González, D.; García-López, G.; García-Castro, I.L.; Flores-Herrera, H.; Molina-Hernández, A.; Portillo, W.; Díaz, N.F. Capturing the ephemeral human pluripotent state. Dev. Dyn. 2016, 245, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.J.; Martello, G.; Yordanov, B.; Emmott, S.; Smith, A.G. Defining an essential transcription factor program for naive pluripotency. Science 2014, 344, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Scholer, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef]
- Frum, T.; Halbisen, M.A.; Wang, C.; Amiri, H.; Robson, P.; Ralston, A. Oct4 cell-autonomously promotes primitive endoderm development in the mouse blastocyst. Dev. Cell 2013, 25, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Avilion, A.A.; Nicolis, S.K.; Pevny, L.H.; Perez, L.; Vivian, N.; Lovell-Badge, R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003, 17, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, M.; Maeda, M.; Yamanaka, S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003, 113, 631–642. [Google Scholar] [CrossRef]
- Frankenberg, S.; Gerbe, F.; Bessonnard, S.; Belville, C.; Pouchin, P.; Bardot, O.; Chazaud, C. Primitive endoderm differentiates via a three-step mechanism involving Nanog and RTK signaling. Dev. Cell 2011, 21, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Elling, U.; Klasen, C.; Eisenberger, T.; Anlag, K.; Treier, M. Murine inner cell mass-derived lineages depend on Sall4 function. Proc. Natl. Acad. Sci. USA 2006, 103, 16319–16324. [Google Scholar] [CrossRef] [PubMed]
- Chiplunkar, A.R.; Curtis, B.C.; Eades, G.L.; Kane, M.S.; Fox, S.J.; Haar, J.L.; Lloyd, J.A. The Kruppel-like factor 2 and Kruppel-like factor 4 genes interact to maintain endothelial integrity in mouse embryonic vasculogenesis. BMC Dev. Biol. 2013, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Yonemura, S.; Takada, S. Grainyhead-related transcription factor is required for duct maturation in the salivary gland and the kidney of the mouse. Development 2006, 133, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Sladek, R.; Bader, J.A.; Matthyssen, A.; Rossant, J.; Giguere, V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature 1997, 388, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, K.M.; Lewandoski, M.; Campbell, K.; Joyner, A.L.; Rubenstein, J.L.; Martinez, S.; Martin, G.R. Specification of the anterior hindbrain and establishment of a normal mid/hindbrain organizer is dependent on Gbx2 gene function. Development 1997, 124, 2923–2934. [Google Scholar] [PubMed]
- Chen, L.; Yabuuchi, A.; Eminli, S.; Takeuchi, A.; Lu, C.W.; Hochedlinger, K.; Daley, G.Q. Cross-regulation of the Nanog and Cdx2 promoters. Cell Res. 2009, 19, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Tsubooka, N.; Ichisaka, T.; Okita, K.; Takahashi, K.; Nakagawa, M.; Yamanaka, S. Roles of Sall4 in the generation of pluripotent stem cells from blastocysts and fibroblasts. Genes Cells 2009, 14, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Kotkamp, K.; Mossner, R.; Allen, A.; Onichtchouk, D.; Driever, W. A Pou5f1/Oct4 dependent Klf2a, Klf2b, and Klf17 regulatory sub-network contributes to EVL and ectoderm development during zebrafish embryogenesis. Dev. Biol. 2014, 385, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ren, Y.; Li, H.; Wang, H. ESRRB plays a crucial role in the promotion of porcine cell reprograming. J. Cell Physiol. 2018, 233, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Platero-Luengo, A.; Sakurai, M.; Sugawara, A.; Gil, M.A.; Yamauchi, T.; Suzuki, K.; Bogliotti, Y.S.; Cuello, C.; Morales Valencia, M.; et al. Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell 2017, 168, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Piliszek, A.; Madeja, Z.E.; Plusa, B. Suppression of ERK signalling abolishes primitive endoderm formation but does not promote pluripotency in rabbit embryo. Development 2017, 144, 3719–3730. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Lei, L.; Liu, S.; Xue, B.; Wang, J.; Wang, J.; Shen, J.; Duan, L.; Shen, X.; Cong, Y.; et al. Morphological changes and germ layer formation in the porcine embryos from days 7-13 of development. Zygote 2015, 23, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg, S.; Shaw, G.; Freyer, C.; Pask, A.J.; Renfree, M.B. Early cell lineage specification in a marsupial: A case for diverse mechanisms among mammals. Development 2013, 140, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Lavial, F.; Acloque, H.; Bertocchini, F.; Macleod, D.J.; Boast, S.; Bachelard, E.; Montillet, G.; Thenot, S.; Sang, H.M.; Stern, C.D.; et al. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development 2007, 134, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kajikawa, E.; Kurokawa, D.; Noro, M.; Iwai, T.; Yonemura, S.; Kobayashi, K.; Kiyonari, H.; Aizawa, S. Conserved and divergent expression patterns of markers of axial development in reptilian embryos: Chinese soft-shell turtle and Madagascar ground gecko. Dev. Biol. 2016, 415, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Tapia, N.; Reinhardt, P.; Duemmler, A.; Wu, G.; Arauzo-Bravo, M.J.; Esch, D.; Greber, B.; Cojocaru, V.; Rascon, C.A.; Tazaki, A.; et al. Reprogramming to pluripotency is an ancient trait of vertebrate Oct4 and Pou2 proteins. Nat. Commun. 2012, 3, 1279. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, T.W.; Costa, Y.; Radzisheuskaya, A.; van Oosten, A.L.; Lavial, F.; Pain, B.; Castro, L.F.; Silva, J.C. Reprogramming capacity of Nanog is functionally conserved in vertebrates and resides in a unique homeodomain. Development 2011, 138, 4853–4865. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.H.; Wu, Q.; Chew, J.L.; Vega, V.B.; Zhang, W.; Chen, X.; Bourque, G.; George, J.; Leong, B.; Liu, J.; et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006, 38, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Soufi, A.; Garcia, M.F.; Jaroszewicz, A.; Osman, N.; Pellegrini, M.; Zaret, K.S. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 2015, 161, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.; Zimmer, D.; Jauch, R. Diversity among POU transcription factors in chromatin recognition and cell fate reprogramming. Cell Mol. Life Sci. 2018, 75, 1587–1612. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Levasseur, D.N.; Orkin, S.H. Requirement of Nanog dimerization for stem cell self-renewal and pluripotency. Proc. Natl. Acad. Sci. USA 2008, 105, 6326–6331. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, N.M.E.; McCarthy, A.; Snijders, K.E.; Powell, B.E.; Kubikova, N.; Blakeley, P.; Lea, R.; Elder, K.; Wamaitha, S.E.; Kim, D.; et al. Genome editing reveals a role for OCT4 in human embryogenesis. Nature 2017, 550, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Simmet, K.; Zakhartchenko, V.; Philippou-Massier, J.; Blum, H.; Klymiuk, N.; Wolf, E. OCT4/POU5F1 is required for NANOG expression in bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2770–2775. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; D’Alessio, A.C.; Taranova, O.V.; Hong, K.; Sowers, L.C.; Zhang, Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010, 466, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Dejosez, M.; Krumenacker, J.S.; Zitur, L.J.; Passeri, M.; Chu, L.F.; Songyang, Z.; Thomson, J.A.; Zwaka, T.P. Ronin is essential for embryogenesis and the pluripotency of mouse embryonic stem cells. Cell 2008, 133, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, C.; Yamanaka, Y.; Pawson, T.; Rossant, J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell 2006, 10, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Kuijk, E.W.; van Tol, L.T.; Van de Velde, H.; Wubbolts, R.; Welling, M.; Geijsen, N.; Roelen, B.A. The roles of FGF and MAP kinase signaling in the segregation of the epiblast and hypoblast cell lineages in bovine and human embryos. Development 2012, 139, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Plusa, B.; Piliszek, A.; Frankenberg, S.; Artus, J.; Hadjantonakis, A.K. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development 2008, 135, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Artus, J.; Piliszek, A.; Hadjantonakis, A.K. The primitive endoderm lineage of the mouse blastocyst: Sequential transcription factor activation and regulation of differentiation by Sox17. Dev. Biol. 2011, 350, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Bessonnard, S.; De Mot, L.; Gonze, D.; Barriol, M.; Dennis, C.; Goldbeter, A.; Dupont, G.; Chazaud, C. Gata6, Nanog and Erk signaling control cell fate in the inner cell mass through a tristable regulatory network. Development 2014, 141, 3637–3648. [Google Scholar] [CrossRef] [PubMed]
- Schrode, N.; Saiz, N.; Di Talia, S.; Hadjantonakis, A.-K. GATA6 Levels Modulate Primitive Endoderm Cell Fate Choice and Timing in the Mouse Blastocyst. Dev. Cell 2014, 29, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Wamaitha, S.E.; del Valle, I.; Cho, L.T.Y.; Wei, Y.; Fogarty, N.M.E.; Blakeley, P.; Sherwood, R.I.; Ji, H.; Niakan, K.K. Gata6 potently initiates reprograming of pluripotent and differentiated cells to extraembryonic endoderm stem cells. Genes Dev. 2015, 29, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Saiz, N.; Williams, K.M.; Seshan, V.E.; Hadjantonakis, A.K. Asynchronous fate decisions by single cells collectively ensure consistent lineage composition in the mouse blastocyst. Nat. Commun. 2016, 7, 13463. [Google Scholar] [CrossRef] [PubMed]
- Xenopoulos, P.; Kang, M.; Puliafito, A.; Di Talia, S.; Hadjantonakis, A.K. Heterogeneities in Nanog Expression Drive Stable Commitment to Pluripotency in the Mouse Blastocyst. Cell Rep. 2015, 10, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Krawchuk, D.; Honma-Yamanaka, N.; Anani, S.; Yamanaka, Y. FGF4 is a limiting factor controlling the proportions of primitive endoderm and epiblast in the ICM of the mouse blastocyst. Dev. Biol. 2013, 384, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Piliszek, A.; Artus, J.; Hadjantonakis, A.K. FGF4 is required for lineage restriction and salt-and-pepper distribution of primitive endoderm factors but not their initial expression in the mouse. Development 2013, 140, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Garg, V.; Hadjantonakis, A.-K. Lineage Establishment and Progression within the Inner Cell Mass of the Mouse Blastocyst Requires FGFR1 and FGFR2. Dev. Cell 2017, 41, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Molotkov, A.; Mazot, P.; Brewer, J.R.; Cinalli, R.M.; Soriano, P. Distinct Requirements for FGFR1 and FGFR2 in Primitive Endoderm Development and Exit from Pluripotency. Dev. Cell 2017, 41, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Lanner, F.; Rossant, J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 2010, 137, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Le Bin, G.C.; Munoz-Descalzo, S.; Kurowski, A.; Leitch, H.; Lou, X.; Mansfield, W.; Etienne-Dumeau, C.; Grabole, N.; Mulas, C.; Niwa, H.; et al. Oct4 is required for lineage priming in the developing inner cell mass of the mouse blastocyst. Development 2014, 141, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, D.-C.; Schöler, H.R.; Dailey, L.; Basilico, C. Modulation of the Activity of Multiple Transcriptional Activation Domains by the DNA Binding Domains Mediates the Synergistic Action of Sox2 and Oct-3 on the Fibroblast Growth Factor-4Enhancer. J. Biol. Chem. 2000, 275, 23387–23397. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Huber, W.; Tsumura, A.; Kang, M.; Xenopoulos, P.; Kurimoto, K.; Oleś, A.K.; Araúzo-Bravo, M.J.; Saitou, M.; Hadjantonakis, A.-K.; et al. Cell-to-cell expression variability followed by signal reinforcement progressively segregates early mouse lineages. Nat. Cell Biol. 2013, 16, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.A.; Teo, R.T.; Li, H.; Robson, P.; Glover, D.M.; Zernicka-Goetz, M. Origin and formation of the first two distinct cell types of the inner cell mass in the mouse embryo. Proc. Natl. Acad. Sci. USA 2010, 107, 6364–6369. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.A.; Graham, S.J.; Jedrusik, A.; Zernicka-Goetz, M. The differential response to Fgf signalling in cells internalized at different times influences lineage segregation in preimplantation mouse embryos. Open Biol. 2013, 3, 130104. [Google Scholar] [CrossRef] [PubMed]
- Schröter, C.; Rué, P.; Mackenzie, J.P.; Martinez Arias, A. FGF/MAPK signaling sets the switching threshold of a bistable circuit controlling cell fate decisions in embryonic stem cells. Development 2015, 142, 4205–4216. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.M.; Hamazaki, T.; Hankowski, K.E.; Terada, N. A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells 2007, 25, 2534–2542. [Google Scholar] [CrossRef] [PubMed]
- Cauffman, G.; De Rycke, M.; Sermon, K.; Liebaers, I.; Van de Velde, H. Markers that define stemness in ESC are unable to identify the totipotent cells in human preimplantation embryos. Hum. Reprod. 2009, 24, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Allegrucci, C.; Alberio, R. Modulation of Pluripotency in the Porcine Embryo and iPS Cells. PLoS ONE 2012, 7, e49079. [Google Scholar] [CrossRef] [PubMed]
- Roode, M.; Blair, K.; Snell, P.; Elder, K.; Marchant, S.; Smith, A.; Nichols, J. Human hypoblast formation is not dependent on FGF signalling. Dev. Biol. 2012, 361, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Deglincerti, A.; Croft, G.F.; Pietila, L.N.; Zernicka-Goetz, M.; Siggia, E.D.; Brivanlou, A.H. Self-organization of the in vitro attached human embryo. Nature 2016, 533, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Denicol, A.C.; Block, J.; Kelley, D.E.; Pohler, K.G.; Dobbs, K.B.; Mortensen, C.J.; Ortega, M.S.; Hansen, P.J. The WNT signaling antagonist Dickkopf-1 directs lineage commitment and promotes survival of the preimplantation embryo. FASEB J. 2014, 28, 3975–3986. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Berg, D.; Beaumont, S.; Standley, N.T.; Wells, D.N.; Pfeffer, P.L. Simultaneous gene quantitation of multiple genes in individual bovine nuclear transfer blastocysts. Reproduction 2007, 133, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.; Huang, B.; Oback, B. Inhibition of MAP2K and GSK3 signaling promotes bovine blastocyst development and epiblast-associated expression of pluripotency factors. Biol. Reprod. 2013, 88, 74. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kunath, T.; Hadjantonakis, A.K.; Nagy, A.; Rossant, J. Promotion of trophoblast stem cell proliferation by FGF4. Science 1998, 282, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Artus, J.; Kang, M.; Cohen-Tannoudji, M.; Hadjantonakis, A.K. PDGF signaling is required for primitive endoderm cell survival in the inner cell mass of the mouse blastocyst. Stem Cells. 2013, 31, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Artus, J.; Panthier, J.J.; Hadjantonakis, A.K. A role for PDGF signaling in expansion of the extra-embryonic endoderm lineage of the mouse blastocyst. Development 2010, 137, 3361–3372. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.; Il’kova, G.; Rehak, P.; Czikkova, S.; Baran, V.; Koppel, J. Inhibitory effect of IGF-I on induced apoptosis in mouse preimplantation embryos cultured in vitro. Theriogenology 2004, 61, 745–755. [Google Scholar] [CrossRef]
- Spanos, S.; Becker, D.L.; Winston, R.M.; Hardy, K. Anti-apoptotic action of insulin-like growth factor-I during human preimplantation embryo development. Biol. Reprod. 2000, 63, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.T.; Southgate, J.; Brison, D.R.; Leese, H.J. Regulation of apoptosis in the bovine blastocyst by insulin and the insulin-like growth factor (IGF) superfamily. Mol. Reprod. Dev. 2002, 62, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, C.J.; Salvador, I.; Cebrian-Serrano, A.; Lopera, R.; Silvestre, M.A. Effect of supplementation of different growth factors in embryo culture medium with a small number of bovine embryos on in vitro embryo development and quality. Animal 2013, 7, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Navarrete Santos, A.; Ramin, N.; Tonack, S.; Fischer, B. Cell lineage-specific signaling of insulin and insulin-like growth factor I in rabbit blastocysts. Endocrinology 2008, 149, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Forrester-Gauntlett, B.; Turner, P.; Henderson, H.; Oback, B. Signal Inhibition Reveals JAK/STAT3 Pathway as Critical for Bovine Inner Cell Mass Development. Biol. Reprod. 2015, 93, 132. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.L.; Weis, W.I. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat. Struct. Mol. Biol. 2005, 12, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Martello, G.; Sugimoto, T.; Diamanti, E.; Joshi, A.; Hannah, R.; Ohtsuka, S.; Gottgens, B.; Niwa, H.; Smith, A. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell 2012, 11, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ten Berge, D.; Kurek, D.; Blauwkamp, T.; Koole, W.; Maas, A.; Eroglu, E.; Siu, R.K.; Nusse, R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat. Cell Biol. 2011, 13, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Biechele, S.; Cockburn, K.; Lanner, F.; Cox, B.J.; Rossant, J. Porcn-dependent Wnt signaling is not required prior to mouse gastrulation. Development 2013, 140, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
| Term and/or Abbreviation | Equivalent (Species) | Description |
|---|---|---|
| conceptus | Refers to all tissue derived from the zygote (embryonic and extraembryonic). | |
| embryo | embryo proper | Before gastrulation equivalent to conceptus. From gastrulation stages refers to the embryonic parts of a conceptus that will give rise to the foetus as opposed to the extraembryonic membranes and placenta. |
| Epiblast (epi) | Derived from ICM, progenitor population of the three germ layers as well as the amniotic ectoderm and primordial germ cells. | |
| Hypoblast (HB) | Primitive endoderm (mouse) | Cells differentiated from the ICM not contributing to the epiblast. The hypoblast will give rise to the inner layer of the yolk sac and, in primates, to extraembryonic mesenchyme. |
| Inner cell mass (ICM) | Pluriblast (marsupial) | Cells giving rise to epiblast and hypoblast. |
| primitive endoderm | HB, Hypoblast | Primitive endoderm is an alternative name to hypoblast and not to be confused with true (definitive) endoderm. |
| Polar trophoblast (pTB) | Rauber’s Layer (e.g., cow, pig, rabbit, horse) | Trophoblast overlying the ICM or epiblast. |
| Trophoblast (TB) | TE (mouse) | Extraembryonic layer: cells giving rise to the conceptus-derived part of the chorionic membrane and subsequently the foetal part of the placenta. |
| Trophectoderm (TE) | TB | During blastocyst stages, before overt differentiation, the trophoblast epithelium is often termed trophectoderm. |
| Species: | Mouse | Rabbit | Human | Cynomolgus | Cattle |
|---|---|---|---|---|---|
| Early (cavity visible, <30% vol) | From 32 cells | From 64 cells | From 35 cells | From 50 cells | From 64 cells |
| E3.25 | E3 (“VI”) | E4–early E5 | E5–6 | E6 | |
| Mid (ca 30–70%) | <64 cells | >128 cells | 64–100 cells | 100–130 cells | |
| E3.5 | E3.25 (“VII”) | late E5 | E7 | ||
| Late (max cavity zona enclosed) | >64 cells | >256 cells | 128–256 cells | 200–300 cells | 140–200 cells |
| E3.75 | E3.5 (“VIII”) | early E6 | E7-8 | E7 | |
| Hatched (Hypo forming layer) | >100 cells | >512 cells | >256 cells | 300–600 cells | >250 cells |
| E4.25 | E3.75 (“IX”) | late E6 | E8-9 | E8 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeffer, P.L. Building Principles for Constructing a Mammalian Blastocyst Embryo. Biology 2018, 7, 41. https://doi.org/10.3390/biology7030041
Pfeffer PL. Building Principles for Constructing a Mammalian Blastocyst Embryo. Biology. 2018; 7(3):41. https://doi.org/10.3390/biology7030041
Chicago/Turabian StylePfeffer, Peter L. 2018. "Building Principles for Constructing a Mammalian Blastocyst Embryo" Biology 7, no. 3: 41. https://doi.org/10.3390/biology7030041
APA StylePfeffer, P. L. (2018). Building Principles for Constructing a Mammalian Blastocyst Embryo. Biology, 7(3), 41. https://doi.org/10.3390/biology7030041





