Test of the Deception Hypothesis in Atlantic Mollies Poecilia mexicana—Does the Audience Copy a Pretended Mate Choice of Others?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Housing Conditions
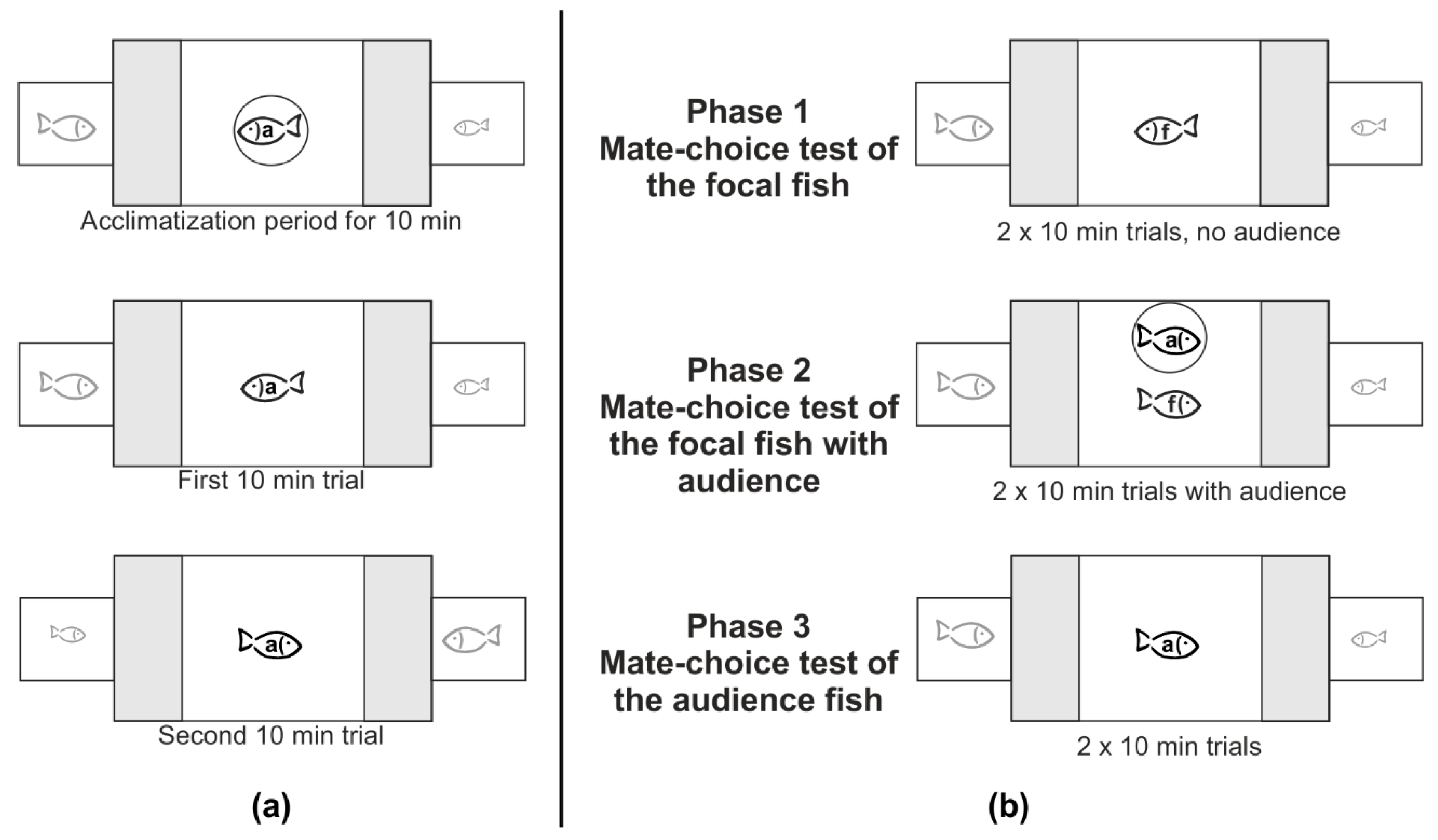
2.2. General Experimental Procedure
2.2.1. Single Mate-Choice Experiment of the Audience Fish
2.2.2. Social Mate-Choice Experiment
2.2.3. Controls for the Social Mate-Choice Test
2.2.4. Measuring Fish Body Length
2.3. Male Mate-Choice Experiments
2.4. Female Mate Choice Experiments
2.5. Ethical Statement
2.6. Data Analysis
3. Results
3.1. General Analysis
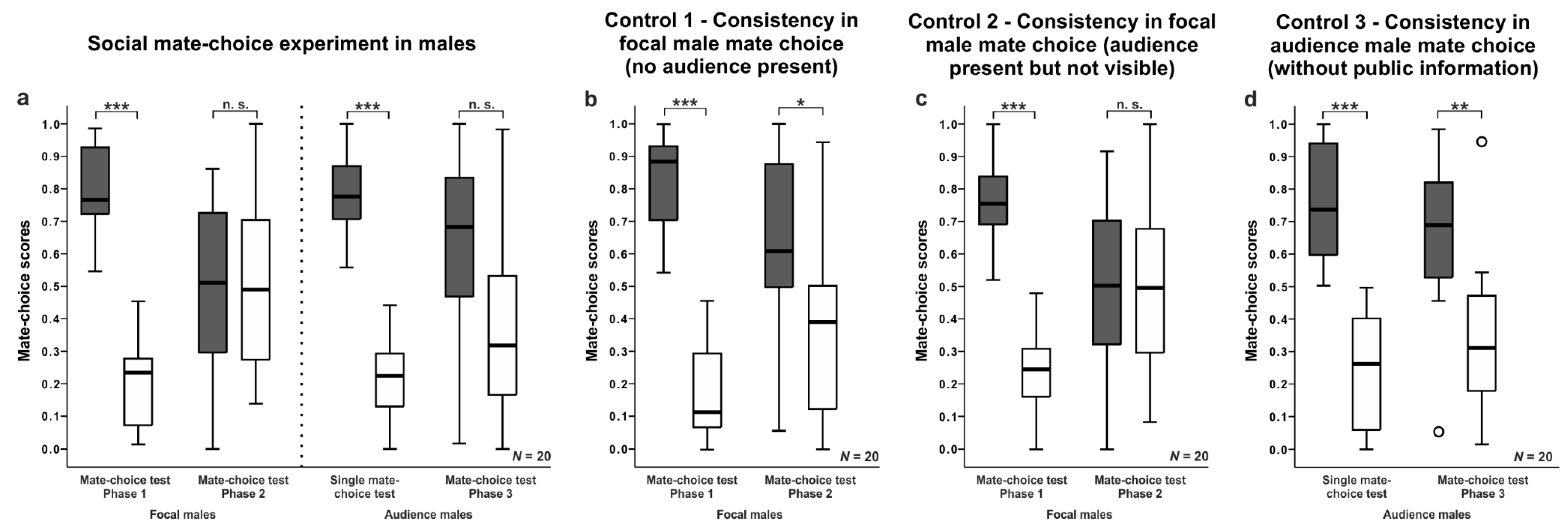
3.2. Male Mate-Choice Experiments
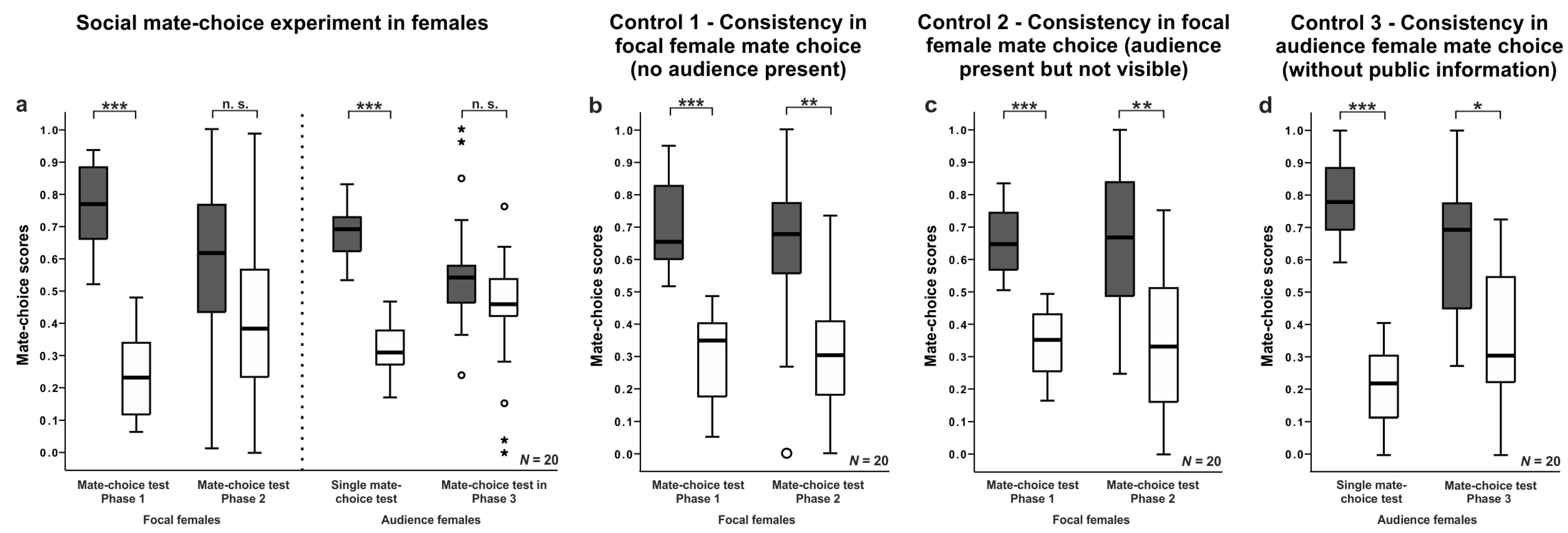
3.3. Female Mate-Choice Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darwin, C. The Descent of Man and Selection in Relation to Sex; John Murray: London, UK, 1888; Volume 1. [Google Scholar]
- Bakker, T.C.M. The study of intersexual selection using quantitative genetics. Behaviour 1999, 136, 1237–1266. [Google Scholar] [CrossRef]
- Bakker, T.C.M.; Pomiankowski, A. The genetic basis of female mate preferences. Evol. Biol. 1995, 8, 129–171. [Google Scholar] [CrossRef]
- Iwasa, Y.; Pomiankowski, A. Good parent and good genes models of handicap evolution. J. Theor. Biol. 1999, 200, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Mead, L.S.; Anorld, S.J. Quantitative genetic models of sexual selection. Trends Ecol. Evol. 2004, 19, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Simmons, L.W. Sexual selection and mate choice. Trends Ecol. Evol. 2006, 21, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Endler, J.A.; Basolo, A.L. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 1998, 13, 415–420. [Google Scholar] [CrossRef]
- Boughman, J.W.; Rundle, H.D.; Schluter, D. Parallel evolution of sexual isolation in sticklebacks. Evolution 2005, 59, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.; Farris, H.E.; Ryan, M.J.; Wilczynski, W. How cricket frog females deal with a noisy world: Habitat-related differences in auditory tuning. Behav. Ecol. 2005, 16, 571–579. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Fox, C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998, 13, 403–407. [Google Scholar] [CrossRef]
- Witte, K.; Ryan, M.J. Male body length influences mate-choice copying in the sailfin molly Poecilia latipinna. Behav. Ecol. 1998, 9, 534–539. [Google Scholar] [CrossRef]
- Freeberg, T.M.; Duncan, S.D.; Kast, T.L.; Enstrom, D.A. Cultural influences on female mate choice: An experimental test in cowbirds, Molothrus ater. Anim. Behav. 1999, 57, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.; Hirschler, U.; Curio, E. Sexual imprinting on a novel adornment influences mate preferences in the Javanese mannikin Lonchura leucogastroides. Ethology 2000, 106, 349–363. [Google Scholar] [CrossRef]
- Witte, K.; Caspers, B. Sexual imprinting on a novel blue ornament in zebra finches. Behaviour 2006, 143, 969–991. [Google Scholar] [CrossRef]
- Witte, K.; Nöbel, S. Learning and mate choice. In Fish Cognition and Behavior, 2nd ed.; Brown, C., Laland, K., Krause, J., Eds.; Wiley Blackwell: Oxford, UK, 2011; pp. 81–107. ISBN 978-1-444-33221-6. [Google Scholar]
- Danchin, É.; Giraldeau, L.-A.; Valone, T.J.; Wagner, R.H. Public information: From nosy neighbors to cultural evolution. Science 2004, 305, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Bonnie, K.E.; Earley, R.L. Expanding the scope for social information use. Anim. Behav. 2007, 74, 171–181. [Google Scholar] [CrossRef]
- Valone, T.J. From eavesdropping on performance to copying the behavior of others: A review of public information use. Behav. Ecol. Sociobiol. 2007, 62, 1–14. [Google Scholar] [CrossRef]
- Schlupp, I.; Ryan, M.J. Male sailfin mollies (Poecilia latipinna) copy the mate choice of other males. Behav. Ecol. 1997, 8, 104–107. [Google Scholar] [CrossRef]
- Oliveira, R.F.; McGregor, P.K.; Latruffe, C. Know thine enemy: Fighting fish gather information from observing conspecific interactions. Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 1045–1049. [Google Scholar] [CrossRef]
- Bierbach, D.; Girndt, A.; Hamfler, S.; Klein, M.; Mücksch, F.; Penshorn, M.; Schwinn, M.; Zimmer, C.; Schlupp, I.; Streit, B.; et al. Male fish use prior knowledge about rivals to adjust their mate choice. Biol. Lett. 2011. [Google Scholar] [CrossRef] [PubMed]
- Westneat, D.F.; Walters, A.; McCarthy, T.M.; Hatch, M.I.; Hein, W.K. Alternative mechanisms of nonindependent mate choice. Anim. Behav. 2000, 59, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.; Kniel, N.; Kureck, I.M. Mate-choice copying: Status quo and where to go. Curr. Zool. 2015, 61, 1073–1081. [Google Scholar] [CrossRef]
- Witte, K.; Ueding, K. Sailfin molly females (Poecilia latipinna) copy the rejection of a male. Behav. Ecol. 2003, 14, 389–395. [Google Scholar] [CrossRef]
- Kniel, N.; Dürler, C.; Hecht, I.; Heinbach, V.; Zimmermann, L.; Witte, K. Novel mate preference through mate-choice copying in zebra finches: Sexes differ. Behav. Ecol. 2015, 26, 647–655. [Google Scholar] [CrossRef]
- Pruett-Jones, S. Independent versus nonindependent mate choice: Do females copy each other? Am. Nat. 1992, 140, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Vakirtzis, A. Mate choice copying and nonindependent mate choice: A critical review. Ann. Zool. Fenn. 2011, 48, 91–107. [Google Scholar] [CrossRef]
- Gierszewski, S.; Keil, M.; Witte, K. Mate-choice copying in sailfin molly females: Public information use from long-distance interactions between model and male. Behav. Ecol. Sociobiol. 2018, 72, 26–37. [Google Scholar] [CrossRef]
- Dugatkin, L.A. Interface between culturally based preferences and genetic preferences: Female mate choice in Poecilia reticulata. Proc. Natl. Acad. Sci. USA 1996, 93, 2770–2773. [Google Scholar] [CrossRef] [PubMed]
- Dugatkin, L.A. Genes, copying, and female mate choice: Shifting thresholds. Behav. Ecol. 1998, 9, 323–327. [Google Scholar] [CrossRef]
- Witte, K.; Noltemeier, B. The role of information in mate-choice copying in female sailfin mollies (Poecilia latipinna). Behav. Ecol. Sociobiol. 2002, 52, 194–202. [Google Scholar] [CrossRef]
- Godin, J.G.J.; Herdman, E.J.; Dugatkin, L.A. Social influences on female mate choice in the guppy, Poecilia reticulata: Generalized and repeatable trait-copying behaviour. Anim. Behav. 2005, 69, 999–1005. [Google Scholar] [CrossRef]
- Losey, G.S., Jr.; Stanton, F.G.; Telecky, T.M.; Tyler, W.A., III. Zoology 691 Graduate Seminar Class. Copying others, an evolutionarily stable strategy for mate choice: A model. Am. Nat. 1986, 128, 653–664. [Google Scholar] [CrossRef]
- Gibson, R.M.; Höglund, J. Copying and sexual selection. Trends Ecol. Evol. 1992, 7, 229–232. [Google Scholar] [CrossRef]
- Servedio, M.R.; Kirkpatrick, M. The evolution of mate choice copying by indirect selection. Am. Nat. 1996, 148, 848–867. [Google Scholar] [CrossRef]
- Brooks, R. The importance of mate copying and cultural inheritance of mating preferences. Trends Ecol. Evol. 1998, 13, 45–46. [Google Scholar] [CrossRef]
- Nordell, S.E.; Valone, T.J. Mate choice copying as public information. Ecol. Lett. 1998, 1, 74–76. [Google Scholar] [CrossRef]
- Stöhr, S. Evolution of mate-choice copying: A dynamic model. Anim. Behav. 1998, 55, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Sirot, E. Mate-choice copying by females: The advantages of a prudent strategy. J. Evol. Biol. 2001, 14, 418–423. [Google Scholar] [CrossRef]
- Wade, M.J.; Pruett-Jones, S.G. Female copying increases the variance in male mating success. Proc. Natl. Acad. Sci. USA 1990, 87, 5749–5753. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.F. The evolutionary consequences of mate copying on male traits. Behav. Ecol. Sociobiol. 2001, 51, 33–40. [Google Scholar] [CrossRef]
- Kirkpatrick, M.; Dugatkin, L.A. Sexual selection and the evolutionary effects of dominance, coloration and courtship. Behav. Ecol. Sociobiol. 1994, 34, 443–449. [Google Scholar] [CrossRef]
- Varela, S.A.M.; Matos, M.; Schlupp, I. The role of mate-choice copying in speciation and hybridization. Biol. Rev. 2018, 93, 1304–1322. [Google Scholar] [CrossRef] [PubMed]
- Galef, B.G., Jr.; Lim, T.C.W.; Gilbert, G.S. Evidence of mate choice copying in Norway rats, Rattus norvegicus. Anim. Behav. 2008, 75, 1117–1123. [Google Scholar] [CrossRef]
- Kavaliers, M.; Choleris, E.; Agmo, A.; Braun, W.J.; Colwell, D.D.; Muglia, L.J.; Ogawa, S.; Pfaff, D.W. Inadvertent social information and the avoidance of parasitized male mice: A role for oxytocin. Proc. Natl. Acad. Sci. USA 2006, 103, 4293–4298. [Google Scholar] [CrossRef] [PubMed]
- Waynforth, D. Mate choice copying in humans. Hum. Nat. 2007, 18, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Yorzinski, J.L.; Platt, M.L. Same-sex gaze attraction influences mate-choice copying in humans. PLoS ONE 2010, 5, e9115. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.; Alatalo, R.V.; Gibson, R.M.; Lundberg, A. Mate-choice copying in black grouse. Anim. Behav. 1995, 49, 1627–1633. [Google Scholar] [CrossRef]
- Gibson, R.M.; Bradbury, J.W.; Vehrencamp, S.L. Mate choice in lekking sage grouse revisited: The roles of vocal display, female site fidelity, and copying. Behav. Ecol. 1991, 2, 165–180. [Google Scholar] [CrossRef]
- Galef, B.G., Jr.; White, D.J. Mate-choice copying in Japanese quail, Coturnix coturnix japonica. Anim. Behav. 1998, 55, 545–552. [Google Scholar] [CrossRef]
- White, D.J.; Galef, B.G., Jr. ‘Culture’ in quail: Social influences on mate choices of female Coturnix japonica. Anim. Behav. 2000, 59, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Swaddle, J.P.; Cathey, M.G.; Correll, M.; Hodkinson, B.P. Socially transmitted mate preferences in a monogamous bird: A non-genetic mechanism of sexual selection. Proc. R. Soc. Lond. B 2005, 272, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Drullion, D.; Dubois, F. Mate-choice copying by female zebra finches, Taeniopygia guttata: What happens when model females provide inconsistent information? Behav. Ecol. Sociobiol. 2008, 63, 269–276. [Google Scholar] [CrossRef]
- Kniel, N.; Schmitz, J.; Witte, K. Quality of public information matters in mate-choice copying in female zebra finches. Front. Zool. 2015, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Kniel, N.; Müller, K.; Witte, K. The role of the model in mate-choice copying in female zebra finches. Ethology 2017, 123, 412–418. [Google Scholar] [CrossRef]
- Mery, F.; Varela, S.A.M.; Danchin, É.; Blanchet, S.; Parejo, D.; Coolen, I.; Wagner, R.H. Public versus personal information for mate copying in an invertebrate. Curr. Biol. 2009, 19, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Dagaeff, A.C.; Pocheville, A.; Nöbel, S.; Loyau, A.; Isabel, G.; Danchin, E. Drosophila mate copying correlates with atmospheric pressure in a speed learning situation. Anim. Behav. 2016, 121, 163–174. [Google Scholar] [CrossRef]
- Fowler-Finn, K.D.; Sullivan-Beckers, L.; Runck, A.M.; Hebets, E.A. The complexities of female mate choice and male polymorphisms: Elucidating the role of genetics, age, and mate-choice copying. Curr. Zool. 2015, 61, 1015–1035. [Google Scholar] [CrossRef]
- Dugatkin, L.A.; Godin, J.-G.J. Reversal of female mate choice by copying in the guppy (Poecilia reticulata). Proc. R. Soc. Lond. B 1992, 249, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Dugatkin, L.A.; Godin, J.-G.J. Female mate copying in the guppy (Poecilia reticulata): Age-dependent effects. Behav. Ecol. 1993, 4, 289–292. [Google Scholar] [CrossRef]
- Amlacher, J.; Dugatkin, L.A. Preference for older over younger models during mate-choice copying in young guppies. Ethol. Ecol. Evol. 2005, 17, 161–169. [Google Scholar] [CrossRef]
- Vukomanovic, J.; Rodd, F.H. Size-dependent female mate copying in the guppy (Poecilia reticulata): Large females are role models but small ones are not. Ethology 2007, 113, 579–586. [Google Scholar] [CrossRef]
- Godin, J.-G.J.; Hair, K.P.E. Mate-choice copying in free-ranging Trinidadian guppies (Poecilia reticulata). Behaviour 2009, 146, 1443–1461. [Google Scholar] [CrossRef]
- Auld, H.L.; Godin, J.-G.J. Sexual voyeurs and copiers: Social copying and the audience effect on male mate choice in the guppy. Behav. Ecol. Sociobiol. 2015, 69, 1795–1807. [Google Scholar] [CrossRef]
- Schlupp, I.; Marler, C.; Ryan, M.J. Benefit to male sailfin mollies of mating with heterospecific females. Science 1994, 263, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.; Ryan, M.J. Mate choice copying in the sailfin molly, Poecilia latipinna, in the wild. Anim. Behav. 2002, 63, 943–949. [Google Scholar] [CrossRef]
- Witte, K.; Massmann, R. Female sailfin mollies, Poecilia latipinna, remember males and copy the choice of others after 1 day. Anim. Behav. 2003, 65, 1151–1159. [Google Scholar] [CrossRef]
- Hill, S.E.; Ryan, M.J. The role of model female quality in the mate choice copying behavior of sailfin mollies. Biol. Lett. 2006, 2, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Heubel, K.U.; Hornhardt, K.; Ollmann, T.; Parzefall, J.; Ryan, M.J.; Schlupp, I. Geographic variation in female mate-copying in the species complex of a unisexual fish, Poecilia formosa. Behaviour 2008, 145, 1041–1064. [Google Scholar] [CrossRef]
- Munger, L.; Cruz, A.; Applebaum, S. Mate choice copying in female humpback limia (Limia nigrofasciata, Family Poeciliidae). Ethology 2004, 110, 563–573. [Google Scholar] [CrossRef]
- Grant, J.W.A.; Green, L.D. Mate copying versus preference for actively courting males by female Japanese medaka (Oryzia latipes). Behav. Ecol. 1996, 7, 165–167. [Google Scholar] [CrossRef]
- Howard, R.D.; Martens, R.S.; Innis, S.A.; Drenvich, J.M.; Hale, J. Mate choice and mate competition influence male body size in Japanese medaka. Anim. Behav. 1998, 55, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Frommen, J.G.; Rahn, A.K.; Schroth, S.H.; Waltschyk, N.; Bakker, T.C.M. Mate-choice copying when both sexes face high costs of reproduction. Evol. Ecol. 2009, 23, 435–446. [Google Scholar] [CrossRef]
- Goulet, D.; Goulet, T.L. Nonindependent mating in a coral reef damselfish: Evidence of mate choice copying in the wild. Behav. Ecol. 2006, 17, 998–1003. [Google Scholar] [CrossRef]
- Alonzo, S.H. Female mate choice copying affects sexual selection in wild populations of the ocellated wrasse. Anim. Behav. 2008, 75, 1715–1723. [Google Scholar] [CrossRef]
- Widemo, M.S. Male but not female pipefish copy mate choice. Behav. Ecol. 2006, 17, 255–259. [Google Scholar] [CrossRef]
- Di Bitetti, M.S. Food-associated calls and audience effects in tufted capuchin monkeys, Cebus apella nigritus. Anim. Behav. 2005, 69, 911–919. [Google Scholar] [CrossRef]
- Leaver, L.A.; Hopewell, L.; Caldwell, C.; Mallarky, L. Audience effects on food caching in grey squirrels (Sciurus carolinensis): Evidence for pilferage avoidance strategies. Anim. Cogn. 2007, 10, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, A.; Cherry, M.I.; Manser, M.B. The audience effect in a facultatively social mammal, the yellow mongoose, Cynictis penicillata. Anim. Behav. 2008, 75, 943–949. [Google Scholar] [CrossRef]
- Makowicz, A.; Plath, M.; Schlupp, I. Male guppies (Poecilia reticulata) adjust their mate choice behaviour to the presence of an audience. Behaviour 2010, 147, 1657–1674. [Google Scholar] [CrossRef]
- Tachon, G.; Murray, A.M.; Gray, D.A.; Cade, W.H. Agonistic displays and the benefits of fighting in the field cricket, Gryllus bimaculatus. J. Insect Behav. 1999, 12, 533–543. [Google Scholar] [CrossRef]
- Fitzsimmons, L.P.; Bertram, S.M. Playing to an audience: The social environment influences aggression and victory displays. Biol. Lett. 2013, 9, 20130449. [Google Scholar] [CrossRef] [PubMed]
- Vignal, C.; Mathevon, N.; Mottin, S. Audience drive male songbird responses to partner’s voice. Nature 2004, 430, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Baltz, A.P.; Clark, A.B. Limited evidence for an audience effect in budgerigars, Melopsittacus undulatus. Anim. Behav. 1994, 47, 460–462. [Google Scholar] [CrossRef]
- Ung, D.; Amy, M.; Leboucher, G. Heaven it’s my wife! Male canaries conceal extra-pair courtships but increase aggressions when their mate watches. PLoS ONE 2011, 6, e22686. [Google Scholar] [CrossRef] [PubMed]
- Dubois, F.; Belzile, A. Audience effect alters male mating preferences in zebra finches (Taeniopygia guttata). PLoS ONE 2012, 7, e43697. [Google Scholar] [CrossRef] [PubMed]
- Hoi, H.; Griggio, M. Bearded reedlings adjust their pair-bond behaviour in relation to the sex and attractiveness of unpaired conspecifics. PLoS ONE 2012, 7, e32806. [Google Scholar] [CrossRef] [PubMed]
- Kniel, N.; Bender, S.; Witte, K. Sex-specific audience effect in the context of mate choice in zebra finches. PLoS ONE 2016, 11, e0147130. [Google Scholar] [CrossRef] [PubMed]
- Townsend, S.W.; Zuberbuhler, K. Audience effects in chimpanzee copulation calls. Commun. Integr. Biol. 2009, 2, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Laporte, M.N.C.; Zuberbühler, K. Vocal greeting behavior in wild chimpanzee females. Anim. Behav. 2010, 80, 467–473. [Google Scholar] [CrossRef]
- Overduin-Devries, A.M.; Massen, J.J.M.; Spruijt, B.M.; Sterck, E.H.M. Sneaky monkeys: An audience effect of male rhesus macaques (Macaca mulatta) on sexual behavior. Am. J. Primatol. 2012, 74, 217–228. [Google Scholar] [CrossRef]
- Pollick, A.S.; Gouzoules, H.; De Waal, F.B.M. Audience effects on food calls in captive brown capuchin monkeys, Cebus apella. Anim. Behav. 2005, 70, 1273–1281. [Google Scholar] [CrossRef]
- Matos, R.J.; McGregor, P.K. The effect of the sex of an audience on male-male displays of Siamese fighting fish (Betta splendens). Behaviour 2002, 139, 1211–1221. [Google Scholar] [CrossRef]
- Herb, B.M.; Biron, S.A.; Kidd, M.R. Courtship by subordinate male Siamese fighting fish, Betta splendens: Their response to eavesdropping and naïve females. Behaviour 2003, 140, 71–78. [Google Scholar] [CrossRef]
- Dzieweczynski, T.L.; Greaney, N.E.; Mannion, K.L. Who’s watching me: Female Siamese fighting fish alter their interactions in response to an audience. Ethology 2014, 120, 855–862. [Google Scholar] [CrossRef]
- Dzieweczynski, T.L.; Rowland, W.J. Behind closed doors: Use of visual cover by courting male three-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 2004, 68, 465–471. [Google Scholar] [CrossRef]
- Makowicz, A.M.; Plath, M.; Schlupp, I. Using video playback to study the effect of an audience on male mating behavior in the Sailfin molly (Poecilia latipinna). Behav. Process. 2010, 85, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Nöbel, S.; Witte, K. Public information influences sperm transfer to females in sailfin molly males. PLoS ONE 2013, 8, e53865. [Google Scholar] [CrossRef] [PubMed]
- Plath, M.; Schlupp, I. Misleading mollies: The effect of an audience on the expression of mating preferences. Commun. Integr. Biol. 2008, 1, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Plath, M.; Blum, D.; Schlupp, I.; Tiedemann, R. Audience effect alters mating preferences in a livebearing fish, the Atlantic molly, Poecilia mexicana. Anim. Behav. 2008, 75, 21–29. [Google Scholar] [CrossRef]
- Ziege, M.; Mahlow, K.; Hennige-Schulz, C.; Kronmarck, C.; Tiedemann, R.; Streit, B.; Plath, M. Audience effects in the Atlantic molly (Poecilia mexicana)-prudent male mate choice in response to perceived sperm competition risk? Front. Zool. 2009, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Castellano, S.; Friard, O.; Pilastro, A. The audience effect and the role of deception in the expression of male mating preferences. Anim. Behav. 2016, 115, 273–282. [Google Scholar] [CrossRef]
- Bierbach, D.; Kronmarck, C.; Hennige-Schulz, C.; Stadler, S.; Plath, M. Sperm competition risk affects male mate choice copying. Behav. Ecol. Sociobiol. 2011, 65, 1699–1707. [Google Scholar] [CrossRef]
- Bierbach, D.; Sommer-Trembo, C.; Hanisch, J.; Wolf, M.; Plath, M. Personality affects mate choice: Bolder males show stronger audience effects under high competition. Behav. Ecol. 2015, 26, 1314–1325. [Google Scholar] [CrossRef]
- Plath, M.; Kromuszczynski, K.; Tiedemann, R. Audience effect alters male but not female mating preferences. Behav. Ecol. Sociobiol. 2009, 63, 381–390. [Google Scholar] [CrossRef]
- Meffe, G.K.; Snelson, F.F. Ecology and Evolution of Livebearing Fishes (Poeciliidae); Prentice Hall: Englewood Cliffs, NJ, USA, 1989; ISBN 978-0132227209. [Google Scholar]
- Jeswiet, S.B.; Godin, J.-G.J. Validation of a method for quantifying male mating preferences in the guppy (Poecilia reticulata). Ethology 2011, 117, 422–429. [Google Scholar] [CrossRef]
- Bischoff, R.J.; Gould, J.L.; Rubenstein, D.I. Tail size and female choice in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 1985, 17, 253–255. [Google Scholar] [CrossRef]
- Forsgren, E. Predation risk affects mate choice in the gobiid fish. Am. Nat. 1992, 140, 1041–1049. [Google Scholar] [CrossRef]
- Berglund, A. Risky sex: Male pipefishes mate at random in the presence of a predator. Anim. Behav. 1993, 46, 169–175. [Google Scholar] [CrossRef]
- Kodric-Brown, A. Female choice of multiple male criteria in guppies: Interacting effects of dominance, coloration and courtship. Behav. Ecol. Sociobiol. 1993, 32, 415–420. [Google Scholar] [CrossRef]
- Walling, C.A.; Royle, N.J.; Lindstrom, J.; Metcalfe, N.B. Do female association preferences predict the likelihood of reproduction? Behav. Ecol. Sociobiol. 2010, 64, 541–548. [Google Scholar] [CrossRef]
- Dosen, L.D.; Montgomerie, R. Mate preferences by male guppies (Poecilia reticulata) in relation to the risk of sperm competition. Behav. Ecol. Sociobiol. 2004, 55, 266–271. [Google Scholar] [CrossRef]
- Hoysak, D.J.; Godin, J.G.J. Repeatability of Male Mate Choice in the Mosquitofish, Gambusia holbrooki. Ethology 2007, 113, 1007–1018. [Google Scholar] [CrossRef]
- Akaike, H. Annals of the Institute of Statistical Mathematics; Springer: Berlin, Germany, 1969. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2011; ISBN 978-1412975148. [Google Scholar]
- Marler, C.A.; Ryan, M.J. Origin and maintenance of a female mating preference. Evolution 1997, 51, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Plath, M.; Seggel, U.; Burmeister, H.; Heubel, K.U.; Schlupp, I. Choosy males from the underground: Male mating preferences in surface- and cave-dwelling Atlantic mollies Poecilia mexicana. Naturwissenschaften 2006, 93, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P. False alarm calls as a means of resource usurpation in the Great tit Parus major. Ethology 1988, 79, 25–30. [Google Scholar] [CrossRef]
- Bshary, R.; Grutter, A.S. Asymmetric cheating opportunities and partner control in a cleaner fish mutualism. Anim. Behav. 2002, 63, 547–555. [Google Scholar] [CrossRef]
- Flower, T.P.; Gribble, M.; Ridley, A.R. Deception by flexible alarm mimicry in an African bird. Science 2014, 344, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P. Deceptive use of alarm calls by male swallows, Hirundo rustica: A new paternity guard. Behav. Ecol. 1990, 1, 1–6. [Google Scholar] [CrossRef]
- Alatalo, R.V.; Carlson, A.; Lundberg, A.; Ulfstrand, S. The conflict between male polygamy and female monogamy: The case of the pied flycatcher, Ficedula hypoleuca. Am. Nat. 1981, 177, 738–753. [Google Scholar] [CrossRef]
- Alatalo, R.V.; Lundberg, A.; Rätti, O. Male polyterritoriality, and imperfect female choice in the pied flycatcher Ficedula hypoleuca. Behav. Ecol. 1990, 1, 171–177. [Google Scholar] [CrossRef]
- Evans, J.P.; Pilastro, A. Postcopulatory sexual selection. In Ecology and Evolution of Poeciliid Fishes; Evans, J.P., Pilastro, A., Schlupp, I., Eds.; Chicago University Press: Chicago, IL, USA, 2011; pp. 197–208. [Google Scholar]
- Foran, C.M.; Ryan, M.J. Female-female competition in a unisexual/bisexual complex of mollies. Copeia 1994, 2, 504–508. [Google Scholar] [CrossRef]
- Makowicz, A.M.M.; Schlupp, I. Effects of Female-Female Aggression in a Sexual/Unisexual Species Complex. Ethology 2013, 21, 903–914. [Google Scholar] [CrossRef]
- Wong, B.B.M.; McCarthy, M. Prudent male mate choice under perceived sperm competition risk in the eastern mosquito fish. Behav. Ecol. 2009, 20, 278–282. [Google Scholar] [CrossRef]
- Órfao, I.; Ojanguren, A.F.; Barbosa, M.; Vicente, L.; Varela, S.A.M.; Magurran, A.E. How pre- and postcopulatory sexual selection influence male mating decisions in a promiscuous species. Anim. Behav. 2018, 136, 147–157. [Google Scholar] [CrossRef]
- Parzefall, J. Zur vergleichenden Ethologie verschiedener Mollienesia-Arten einschliesslich einer Höhlenform von Mollienesia sphenops. Behaviour 1969, 33, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Capron de Caprona, M.D.; Ryan, M.J. Conspecific mate recognition in swordtails, Xiphophorus nigrensis and X. pygmaeus (Poeciliidae): Olfactory and visual cues. Anim. Behav. 1990, 39, 290–296. [Google Scholar] [CrossRef]
- Wong, B.B.M.; Fisher, H.S.; Rosenthal, G.G. Species recognition by male swordtails via chemical cues. Behav. Ecol. 2005, 16, 818–822. [Google Scholar] [CrossRef]
- Schlupp, I.; Parzefall, J.; Schartl, M. Male mate choice in mixed bisexual/unisexual breeding complexes of Poecilia (Teleostei: Poeciliidae). Ethology 2010, 88, 215–222. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witte, K.; Baumgärtner, K.; Röhrig, C.; Nöbel, S. Test of the Deception Hypothesis in Atlantic Mollies Poecilia mexicana—Does the Audience Copy a Pretended Mate Choice of Others? Biology 2018, 7, 40. https://doi.org/10.3390/biology7030040
Witte K, Baumgärtner K, Röhrig C, Nöbel S. Test of the Deception Hypothesis in Atlantic Mollies Poecilia mexicana—Does the Audience Copy a Pretended Mate Choice of Others? Biology. 2018; 7(3):40. https://doi.org/10.3390/biology7030040
Chicago/Turabian StyleWitte, Klaudia, Katharina Baumgärtner, Corinna Röhrig, and Sabine Nöbel. 2018. "Test of the Deception Hypothesis in Atlantic Mollies Poecilia mexicana—Does the Audience Copy a Pretended Mate Choice of Others?" Biology 7, no. 3: 40. https://doi.org/10.3390/biology7030040
APA StyleWitte, K., Baumgärtner, K., Röhrig, C., & Nöbel, S. (2018). Test of the Deception Hypothesis in Atlantic Mollies Poecilia mexicana—Does the Audience Copy a Pretended Mate Choice of Others? Biology, 7(3), 40. https://doi.org/10.3390/biology7030040




