Variation in Mating Dynamics across Five Species of Leiobunine Harvestmen (Arachnida: Opliones)
Abstract
1. Introduction
2. Materials and Methods
3. Results
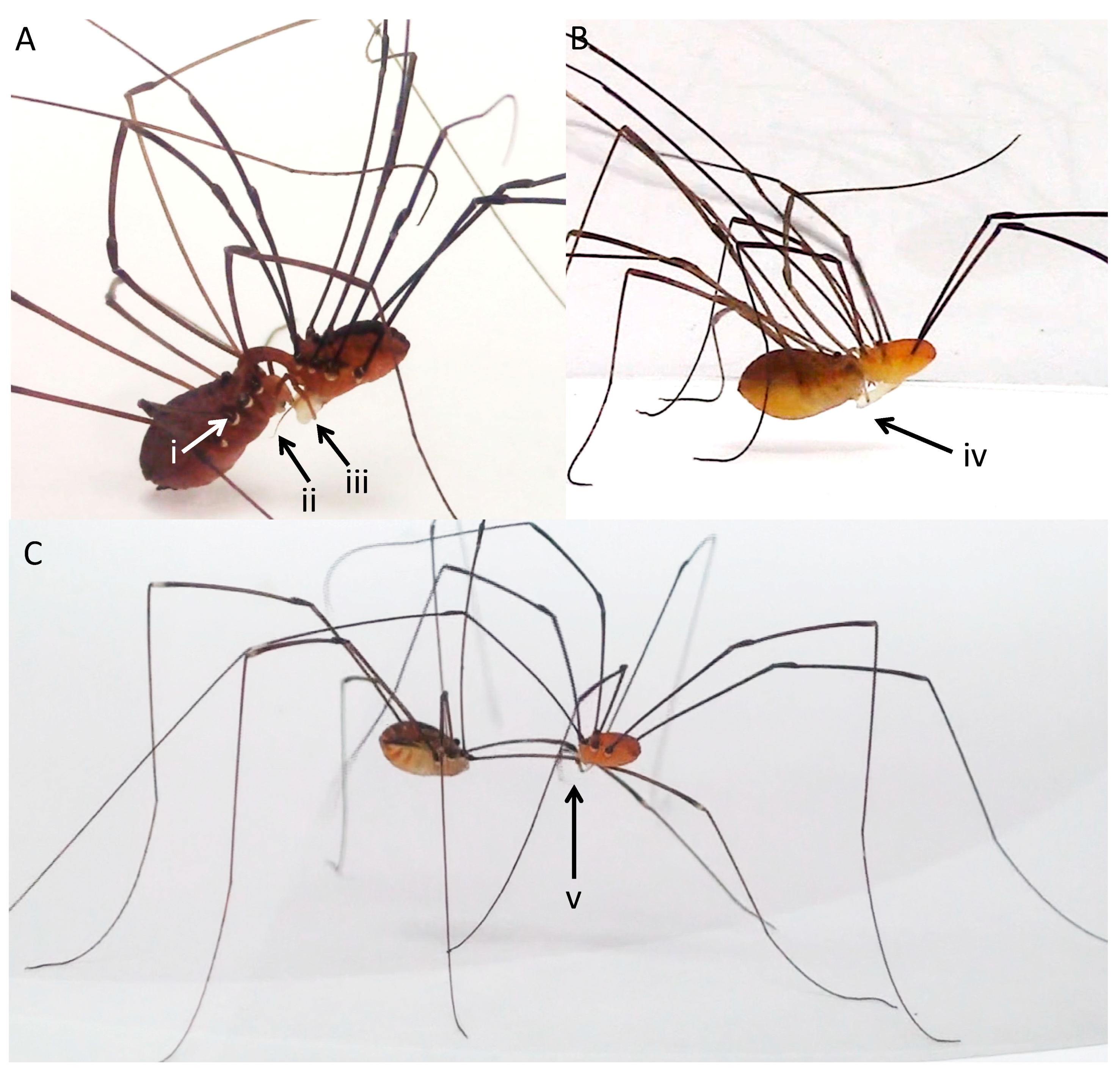
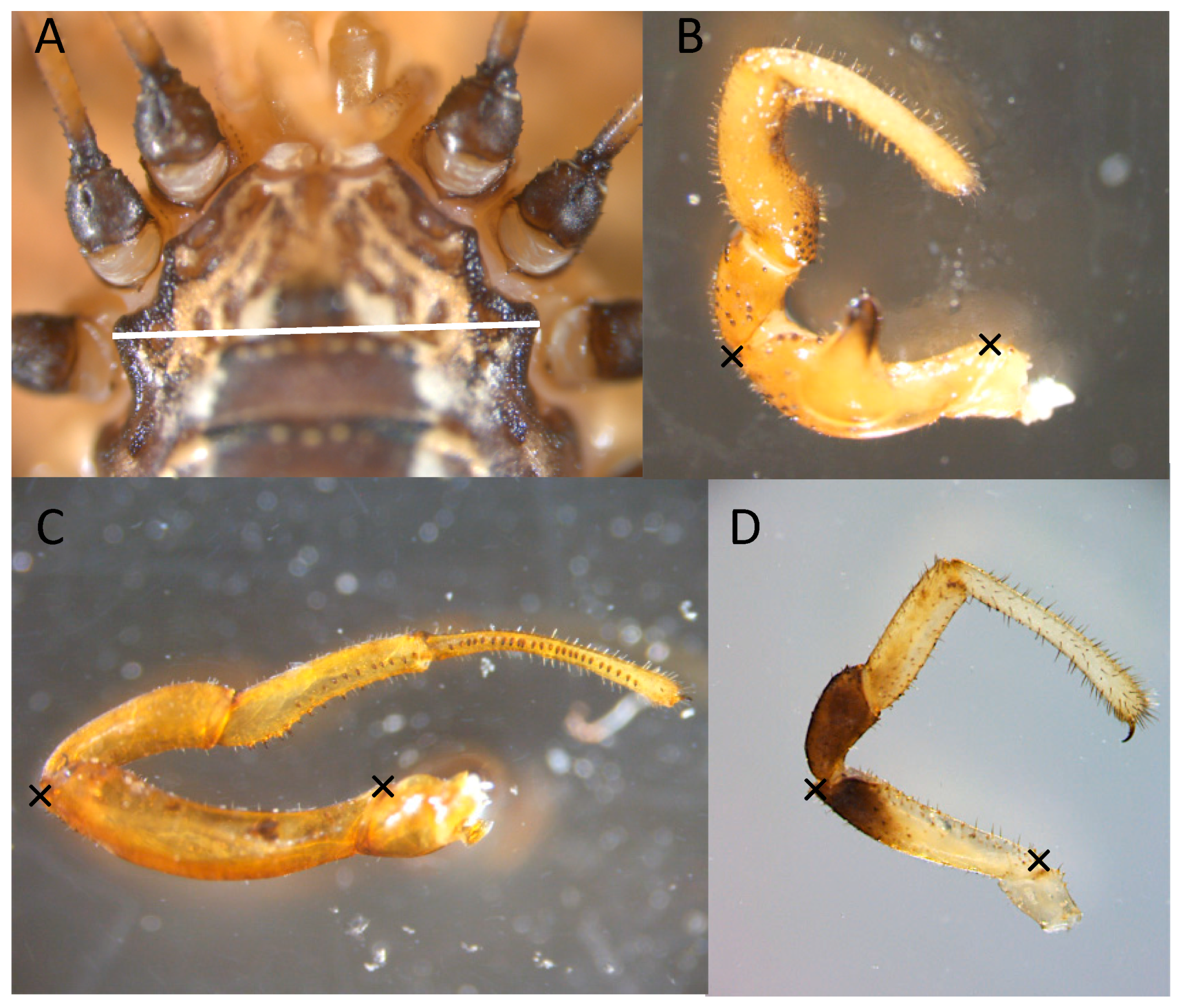
3.1. Morphology
3.2. Predictors of Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darwin, C. The Descent of Man and Selection in Relation to Sex; John Murray: London, UK, 1871. [Google Scholar]
- Eberhard, W.G. Sexual Selection and Animal Genitalia; Harvard University Press: Cambridge, MA, USA, 1985. [Google Scholar]
- West-Eberhard, M.J. Sexual selection, social competition, and speciation. Q. Rev. Biol. 1983, 58, 155–183. [Google Scholar] [CrossRef]
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Coyne, J.A.; Orr, H.A. Speciation; Sinauer Associates: Sunderland, MA, USA, 2004. [Google Scholar]
- Mendelson, T.C.; Shaw, K.L. Sexual behaviour: Rapid speciation in an arthropod. Nature 2005, 433, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.M.; Henry, C.S. Songs, reproductive isolation and speciation in cryptic species of insects: A case study using green lacewings. In Endless Forms: Species and Speciation; Howard, D., Berlocher, S., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 217–233. [Google Scholar]
- Cocroft, R.B.; Rodríguez, R.L.; Hunt, R.E. Host shifts, the evolution of communication and speciation in the Enchenopa binotata complex of treehoppers. In Specialization, Speciation, and Radiation: The Evolutionary Biology of Herbivorous Insects; Tilmon, K., Ed.; University of California Press: Oakland, CA, USA, 2008; pp. 88–100. [Google Scholar]
- Andersson, M.; Simmons, L.W. Sexual selection and mate choice. Trends Ecol. Evol. 2006, 21, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Arnqvist, G.; Rowe, L. Sexual Conflict; Princeton University Press: Princeton, NJ, USA, 2005. [Google Scholar]
- Parker, G.A. Sexual conflict over mating and fertilization: An overview. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, W.G. Female Control: Sexual Selection by Cryptic Female Choice; Princeton University Press: Princeton, NJ, USA; Chichester, UK, 1996; p. 501. [Google Scholar]
- Parker, G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970, 45, 525–567. [Google Scholar] [CrossRef]
- Simmons, L.W. Sperm Competition and Its Evolutionary Consequences in the Insects; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Brennan, P.L.R.; Prum, R.O. The limits of sexual conflict in the narrow sense: New insights from waterfowl biology. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2324–2338. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.M.; Langen, T.A. How do animals choose their mates? Trends Ecol. Evol. 1996, 11, 468–470. [Google Scholar] [CrossRef]
- Rodríguez, R.L. Mating Is a Give-and-Take of Influence and Communication between the Sexes. In Cryptic Female Choice in Arthropods: Patterns, Mechanisms and Prospects; Peretti, A.V., Aisenberg, A., Eds.; Springer: Basel, Switzerland, 2015; pp. 479–496. [Google Scholar]
- Fowler-Finn, K.D.; Triana, E.; Miller, O.G. Mating in the harvestman Leiobunum vittatum (Arachnida: Opiliones): From premating struggles to solicitous tactile engagement. Behaviour 2014, 151, 1663–1686. [Google Scholar] [CrossRef]
- Kvarnemo, C.; Simmons, L.W. Polyandry as a mediator of sexual selection before and after mating. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120042. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.D.; Marshall, D.; Cooley, J. Evolutionary perspectives on insect mating. In The Evolution of Mating Systems in Insects and Arachnids; Choe, J.C., Crespi, B.J., Eds.; Cambridge University Press: Cambridge, MA, USA, 1997; pp. 4–31. [Google Scholar]
- Clutton-Brock, T.; Parker, G.A. Sexual coercion in animal societies. Anim. Behav. 1995, 49, 1345–1365. [Google Scholar] [CrossRef]
- Danielsson, I. Antagonistic pre- and post-copulatory sexual selection on male body size in a water strider (Gerris lacustris). Proc. R. Soc. Lond. Ser. B 2001, 268, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.M.; Hedin, M.; Shultz, J.W. Comparative Analyses of Reproductive Structures in Harvestmen (Opiliones) Reveal Multiple Transitions from Courtship to Precopulatory Antagonism. PLoS ONE 2013, 8, e66767. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.M.; Shultz, J.W. Biomechanical diversity of mating structures among harvestmen species is consistent with a spectrum of precopulatory strategies. PLoS ONE 2015, 10, e01378181. [Google Scholar] [CrossRef] [PubMed]
- Macías-Ordóñez, R.; Machado, G.; Pérez-González, A.; Shultz, J.W. Genitalic evolution in Opiliones. In The Evolution of Primary Sexual Character in Animals; Leonard, J., Cordoba-Aguilar, A., Eds.; Oxford University Press: Oxford, NY, USA, 2010. [Google Scholar]
- Burns, M.; Hedin, M.; Shultz, J.W. Molecular phylogeny of the leiobunine harvestment of eastern North American (Opiliones: Sclerosomatidae: Leiobuninae). Mol. Phylogenet. Evol. 2012, 63, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Fowler-Finn, K.D. Saint Louis University, Saint Louis, MO, USA. Unpublished work. 2014. [Google Scholar]
- Preston, B.T.; Stevenson, I.R.; Pemberton, J.M.; Coltman, D.W.; Wilson, K. Overt and covert competition in a promiscuous mammal: The importance of weaponry and testes size to male reproductive success. Proc. R. Soc. Lond. B 2003, 270, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Syriatowicz, A.; Brooks, R. Sexual responsiveness is condition-dependent in female guppies, but preference functions are not. BMC Ecol. 2004, 4, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Byrne, P.G.; Rice, W.R. Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 2006, 273, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.B.M.; Jennions, M.D. Costs influence male mate choice in a freshwater fish. Proc. R. Soc. Lond. B 2003, 270, S36–S38. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.B.; Lu, X. Male mate choice in the Andrew’s toad Bufo andrewsi: A preference for larger females. J. Ethol. 2009, 27, 413–417. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, B.; Guo, Y.; Raffa, K.F.; Sun, J. Gallery and acoustic traits related to female body size mediate male mate choice in a bark beetle. Anim. Behav. 2017, 125, 41–50. [Google Scholar] [CrossRef]
- Sato, N.; Yoshida, M.; Kasugai, T. Impact of cryptic female choice on insemination success: Larger sized and longer copulating male squid ejaculate more, but females influence insemination success by removing spermatangia. Evolution 2017, 71, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Laird, G.; Gwynne, D.T.; Andrade, M.C.B. Extreme repeated mating as a counter-adaptation to sex conflict? Proc. R. Soc. Lond. Ser. B 2004, 271, S402–S404. [Google Scholar] [CrossRef] [PubMed]
- Boggs, C.L. Male nuptial gifts: Phenotypic consequences and evolutionary implications. In Insect Reproduction; Leather, S.R., Hardie, J., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 215–242. [Google Scholar]
- Bukowski, T.C.; Christenson, T.E. Determinants of sperm release and storage in a spiny orb-weaving spider. Anim. Behav. 1997, 53, 381–395. [Google Scholar] [CrossRef]
- Snow, L.S.E.; Andrade, M.C.B. Pattern of sperm transfer in redback spiders: Implications for sperm competition and male sacrifice. Behav. Ecol. 2004, 15, 785–792. [Google Scholar] [CrossRef]
- Linn, C.D.; Molina, Y.; Difatta, J.; Christenson, T.E. The adaptive advantage of prolonged mating: A test of alternative hypotheses. Anim. Behav. 2007, 74, 481–485. [Google Scholar] [CrossRef]
- Willemart, R.H.; Farine, J.P.; Peretti, A.V.; Gnaspini, P. Behavioral roles of the sexually dimorphic structures in the male harvestman, Phalangium opilio (Opiliones, Phalangiidae). Can. J. Zool. 2006, 84, 1763–1774. [Google Scholar] [CrossRef]
- Williams, G.C. Natural selection, the cost of reproduction, and a refinement of Lack’s principle. Am. Nat. 1966, 100, 687–690. [Google Scholar] [CrossRef]
- Pianka, E.R.; Parker, W.S. Age-specific reproductive tactics. Am. Nat. 1975, 109, 453–464. [Google Scholar] [CrossRef]
- Poizat, G.; Rosecchi, E.; Crivelli, A.J. Empirical evidence of a trade-off between reproductive effort and expectation of future reproduction in female three-spined sticklebacks. Proc. R. Soc. Lond. Ser. B 1999, 266, 1543–1548. [Google Scholar] [CrossRef]
- Alcock, J. Postinsemination associations between males and females in insects: The mate-guarding hypothesis. Annu. Rev. Entomol. 1994, 39, 1–21. [Google Scholar] [CrossRef]
- Dickinson, J.L. Trade-offs between postcopulatory riding and mate location in the blue milkweed beetle. Behav. Ecol. 1995, 6, 280–286. [Google Scholar] [CrossRef]
- Machado, G.; Macías-Ordóñez, G. Reproduction. In Harvestmen: The biology of Opiliones; Pinto-da-Rocha, R., Machado, G., Giribet, G., Eds.; Harvard University Press: Cambridge, MA, USA, 2007; pp. 414–454. [Google Scholar]
- Harts, A.M.; Kokko, H. Understanding promiscuity: When is seeking additional mates better than guarding an already found one? Evolution 2013, 67, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A.; Simmons, L.W. A model of constant random sperm displacement during mating: Evidence from Scatophaga. Proc. R. Soc. Lond. B 1991, 246, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.L. Prolonged mating in the milkweed leaf beetle Labidomera clivicollis clivicollis (Coleoptera: Chrysomelidae): A test of the “sperm-loading” hypothesis. Behav. Ecol. Sociobiol. 1986, 18, 331–338. [Google Scholar] [CrossRef]
- Macías-Ordóñez, R. The Mating System of Leiobunum vittatum Say 1821 (Arachnida: Opiliones: Palpatores): Resource Defense Polygyny in the Striped Harvestman; Lehigh University: Bethlehem, PA, USA, 1997. [Google Scholar]
- Macías-Ordóñez, R. Touchy harvestmen. Nat. Hist. 2000, 109, 58–67. [Google Scholar]
- Fowler-Finn, K.D. (Saint Louis University, Saint Louis, MO, USA). Personal communication, 2014.
- Kahn, P.C.; Cao, D.C.; Burns, M.M.; Boyer, S.L. Nuptial gift chemistry reveals convergent evolution correlated with antagonism in mating systems of harvestmen (Arachnida, Opiliones). Ecol. Evol. in press.
- Fowler-Finn, K.D. Saint Louis University, Saint Louis, MO, USA. Boyer, S.L. Macalester College, St Paul, MN, USA. Unpublished work, 2013, 2016.

| Species | Correlation | p-Value | n |
|---|---|---|---|
| Sacculate | |||
| L. aldrichi | 0.64 | 0.0011 | 23 |
| L. politum | 0.53 | 0.0516 | 14 |
| L. ventricosum | 0.29 | 0.2031 | 21 |
| Non-sacculate | |||
| L. calcar | 0.34 | 0.0624 | 31 |
| L. vittatum | 0.53 | 0.0004 | 41 |
| Cephalothorax Width (mm) | Weight (g) | Cephalothorax Width Dimorphism | Weight Dimorphism | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||
| Sacculate | |||||||
| L. aldrichi | mean | 2.406 | 2.483 | 0.0212 | 0.0342 | 0.97 | 0.62 |
| S.E. | 0.025 | 0.025 | 0.0006 | 0.0012 | |||
| L. politum | mean | 2.788 | 2.946 | 0.0132 | 0.0270 | 0.95 | 0.49 |
| S.E. | 0.0293 | 0.042 | 0.00054 | 0.0011 | |||
| L. ventricosum | mean | 3.140 | 3.453 | 0.0485 | 0.1150 | 0.91 | 0.42 |
| S.E. | 0.021 | 0.036 | 0.0001 | 0.0062 | |||
| Non-sacculate | |||||||
| L. calcar | mean | 3.839 | 3.838 | 0.1131 | 0.171 | 1.00 | 0.66 |
| S.E. | 0.020 | 0.035 | 0.0023 | 0.0044 | |||
| L. vittatum | mean | 2.966 | 3.061 | 0.0467 | 0.0854 | 0.97 | 0.55 |
| S.E. | 0.016 | 0.015 | 0.0008 | 0.0016 | |||
| Sacculate Species | Non-Sacculate Species | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. aldrichi | L. politum | L. ventricosum | L. calcar | L. vittatum | ||||||||||||
| (7/23 Mated) | (11/14 Mated) | (18/21 Mated) | (22/31 Mated) | (20/41 Mated) | ||||||||||||
| Χ2(df) | p | n | Χ2(df) | p | n | Χ2(df) | p | n | Χ2(df) | p | n | Χ2(df) | p | n | ||
| Precopulatory: | ||||||||||||||||
| Attempt y/n | female size | 7.6(1,4) | 0.0060 | 23 | All attempted | 0.0(1,4) | 0.9960 | 21 | 5.9(1,4) | 0.0153 | 31 | 0.1(1,4) | 0.7686 | 41 | ||
| male size | 0.0(1,4) | 1.0000 | 0.0(1,4) | 0.9988 | 0.0(1,4) | 0.8592 | 0.8(1,4) | 0.3865 | ||||||||
| female x male size | 4.0(1,4) | 0.0451 | 0.0(1,4) | 0.9182 | 0.1(1,4) | 0.7364 | 1.3(1,4) | 0.2458 | ||||||||
| male pedipalp | 0.0(1,4) | 0.9983 | 0.0(1,4) | 0.9991 | 0.3(1,4) | 0.6069 | 1.5(1,4) | 0.2252 | ||||||||
| Resist y/n | female size | 1.5(1,4) | 0.2276 | 22 | 1.5(1,4) | 0.2278 | 14 | 0.2(1,4) | 0.6261 | 20 | 1.8(1,4) | 0.1789 | 24 | 1.5(1,4) | 0.2266 | 39 |
| male size | 0.0(1,4) | 0.8777 | 2.8(1,4) | 0.0928 | 0.1(1,4) | 0.7881 | 2.6(1,4) | 0.1052 | 2.1(1,4) | 0.1466 | ||||||
| female x male size | 0.0(1,4) | 0.8402 | 2.3(1,4) | 0.1283 | 12.0(1,4) | 0.0005 | 1.7(1,4) | 0.1911 | 1.2(1,4) | 0.2655 | ||||||
| male pedipalp | 1.3(1,4) | 0.2631 | 2.0(1,4) | 0.1594 | 0.9(1,4) | 0.3556 | 0.4(1,4) | 0.5406 | 8.5(1,4) | 0.0036 | ||||||
| First attempt successful | female size | 1.9(1,4) | 0.1647 | 22 | 1.5(1,4) | 0.2200 | 14 | 0.7(1,4) | 0.4129 | 20 | 4.9(1,4) | 0.0273 | 24 | 6.6(1,4) | 0.0102 | 39 |
| male size | 0.0(1,4) | 0.9732 | 4.5(1,4) | 0.0335 | 1.0(1,4) | 0.3096 | 1.1(1,4) | 0.2947 | 0.9(1,4) | 0.3332 | ||||||
| female x male size | 0.3(1,4) | 0.5991 | 4.4(1,4) | 0.0350 | 1.3(1,4) | 0.2526 | 3.3(1,4) | 0.0698 | 2.4(1,4) | 0.1179 | ||||||
| male pedipalp | 0.3(1,4) | 0.6107 | 5.1(1,4) | 0.0242 | 0.3(1,4) | 0.6055 | 0.3(1,4) | 0.5544 | 0.3(1,4) | 0.5714 | ||||||
| Secure y/n | female size | 2.8(1,4) | 0.0952 | 22 | 4.1(1,4) | 0.0432 | 14 | 0.0(1,4) | 0.9849 | 20 | 4.9(1,4) | 0.0273 | 24 | 9.9(1,4) | 0.0017 | 41 |
| male size | 0.0(1,4) | 0.9575 | 0.0(1,4) | 0.9181 | 0.0(1,4) | 0.9916 | 1.1(1,4) | 0.2947 | 0.5(1,4) | 0.4979 | ||||||
| female x male size | 0.4(1,4) | 0.5413 | 0.0(1,4) | 0.8359 | 80.1(1,4) | <0.0001 | 3.3(1,4) | 0.0698 | 4.4(1,4) | 0.0361 | ||||||
| male pedipalp | 0.2(1,4) | 0.6785 | 1.7(1,4) | 0.1910 | 23.8(1,4) | <0.0001 | 0.3(1,4) | 0.5544 | 0.3(1,4) | 0.5837 | ||||||
| Copulatory: | ||||||||||||||||
| Mate y/n | female size | 3.4(1,4) | 0.0669 | 23 | 4.1(1,4) | 0.0432 | 14 | 0.1(1,4) | 0.7477 | 21 | 11.7(1,4) | 0.0006 | 31 | 6.1(1,4) | 0.0136 | 41 |
| male size | 0.0(1,4) | 0.9806 | 0.0(1,4) | 0.9181 | 2.3(1,4) | 0.1292 | 0.3(1,4) | 0.5567 | 0(1,4) | 0.8329 | ||||||
| female x male size | 0.4(1,4) | 0.5508 | 0.0(1,4) | 0.8359 | 2.1(1,4) | 0.1464 | 2.1(1,4) | 0.1487 | 1.3(1,4) | 0.2570 | ||||||
| male pedipalp | 0.2(1,4) | 0.6765 | 1.7(1,4) | 0.1910 | 0.7(1,4) | 0.3998 | 1.1(1,4) | 0.2995 | 0.6(1,4) | 0.4481 | ||||||
| Length Intromission | fem size | 0.1(1,4) | 0.8352 | 6 | 0.0(1,4) | 0.8484 | 11 | 1.8(1,4) | 0.2047 | 18 | 0.0(1,4) | 0.9347 | 22 | 1.8(1,4) | 0.2048 | 20 |
| male size | 0.0(1,4) | 0.9780 | 0.0(1,4) | 0.9965 | 4.5(1,4) | 0.0534 | 3.4(1,4) | 0.0837 | 11.7(1,4) | 0.0038 | ||||||
| female x male size | 0.1(1,4) | 0.7946 | 0.0(1,4) | 0.9214 | 7.1(1,4) | 0.0194 | 0.8(1,4) | 0.3912 | 7.2(1,4) | 0.0170 | ||||||
| male pedipalp | 0.0(1,4) | 0.9629 | 0.7(1,4) | 0.4219 | 0.7(1,4) | 0.4070 | 0.5(1,4) | 0.4857 | 0.2(1,4) | 0.7030 | ||||||
| Postcopulatory: | ||||||||||||||||
| Guard y/n | female size | All guarded | 6 | 0.0(1,4) | 0.9083 | 11 | 2.4(1,4) | 0.1221 | 18 | 0.1(1,4) | 0.7790 | 21 | 3.7(1,4) | 0.0542 | 20 | |
| male size | 4.8(1,4) | 0.0282 | 2.3(1,4) | 0.1295 | 2.8(1,4) | 0.0971 | 0.5(1,4) | 0.4717 | ||||||||
| female x male size | 0.2(1,4) | 0.6770 | 0.7(1,4) | 0.4064 | 0.5(1,4) | 0.4898 | 4.5(1,4) | 0.0332 | ||||||||
| male pedipalp | 0.0(1,4) | 0.9083 | 1.1(1,4) | 0.2865 | 1.7(1,4) | 0.1986 | 0.6(1,4) | 0.4492 | ||||||||
| Length postcopulatory contact | female size | 58.9(1,4) | 0.0825 | 6 | 0.9(1,4) | 0.3887 | 11 | 7.0(1,4) | 0.0200 | 18 | 0.6(1,4) | 0.4661 | 21 | 1.3(1,4) | 0.2746 | 20 |
| male size | 33.4(1,4) | 0.1090 | 7.0(1,4) | 0.0384 | 3.0(1,4) | 0.1053 | 0.5(1,4) | 0.4961 | 0.0(1,4) | 0.9463 | ||||||
| female x male size | 35.7(1,4) | 0.1055 | 3.1(1,4) | 0.1288 | 1.6(1,4) | 0.2235 | 0.3(1,4) | 0.5941 | 2.2(1,4) | 0.1602 | ||||||
| male pedipalp | 28.8(1,4) | 0.1172 | 3.5(1,4) | 0.1115 | 4.2(1,4) | 0.0600 | 1.5(1,4) | 0.2427 | 0.8(1,4) | 0.3935 | ||||||
| Sacculate | Non-Sacculate | |||||
|---|---|---|---|---|---|---|
| L. aldrichi | L. politum | L. ventricosum | L. calcar | L. vittatum | ||
| Precopulatory | Attempt y/n |  (One did not attempt) (One did not attempt) | — | — |  | — |
| Resist y/n | — | — | 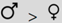 | — | — | |
| First attempt successful | — | 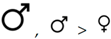 | — | 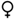 | 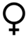 | |
| Secure y/n | — |  | — |  |  | |
| Copulatory | Mate y/n |  |  | — | 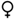 | |
| Length intromission | — | — |  | — | 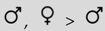 | |
| Postcopulatory | Guard y/n | — | 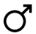 | — | — | 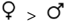 |
| Postcopulatory contact | — |  |  | — | — | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fowler-Finn, K.D.; Boyer, S.L.; Ikagawa, R.; Jeffries, T.; Kahn, P.C.; Larsen, E.M.; Lee, D.; Smeester, M. Variation in Mating Dynamics across Five Species of Leiobunine Harvestmen (Arachnida: Opliones). Biology 2018, 7, 36. https://doi.org/10.3390/biology7020036
Fowler-Finn KD, Boyer SL, Ikagawa R, Jeffries T, Kahn PC, Larsen EM, Lee D, Smeester M. Variation in Mating Dynamics across Five Species of Leiobunine Harvestmen (Arachnida: Opliones). Biology. 2018; 7(2):36. https://doi.org/10.3390/biology7020036
Chicago/Turabian StyleFowler-Finn, Kasey D., Sarah L. Boyer, Raine Ikagawa, Timothy Jeffries, Penelope C. Kahn, Eva M. Larsen, Daniel Lee, and Morgan Smeester. 2018. "Variation in Mating Dynamics across Five Species of Leiobunine Harvestmen (Arachnida: Opliones)" Biology 7, no. 2: 36. https://doi.org/10.3390/biology7020036
APA StyleFowler-Finn, K. D., Boyer, S. L., Ikagawa, R., Jeffries, T., Kahn, P. C., Larsen, E. M., Lee, D., & Smeester, M. (2018). Variation in Mating Dynamics across Five Species of Leiobunine Harvestmen (Arachnida: Opliones). Biology, 7(2), 36. https://doi.org/10.3390/biology7020036





