Effects of Sex Steroids on Fish Leukocytes
Abstract
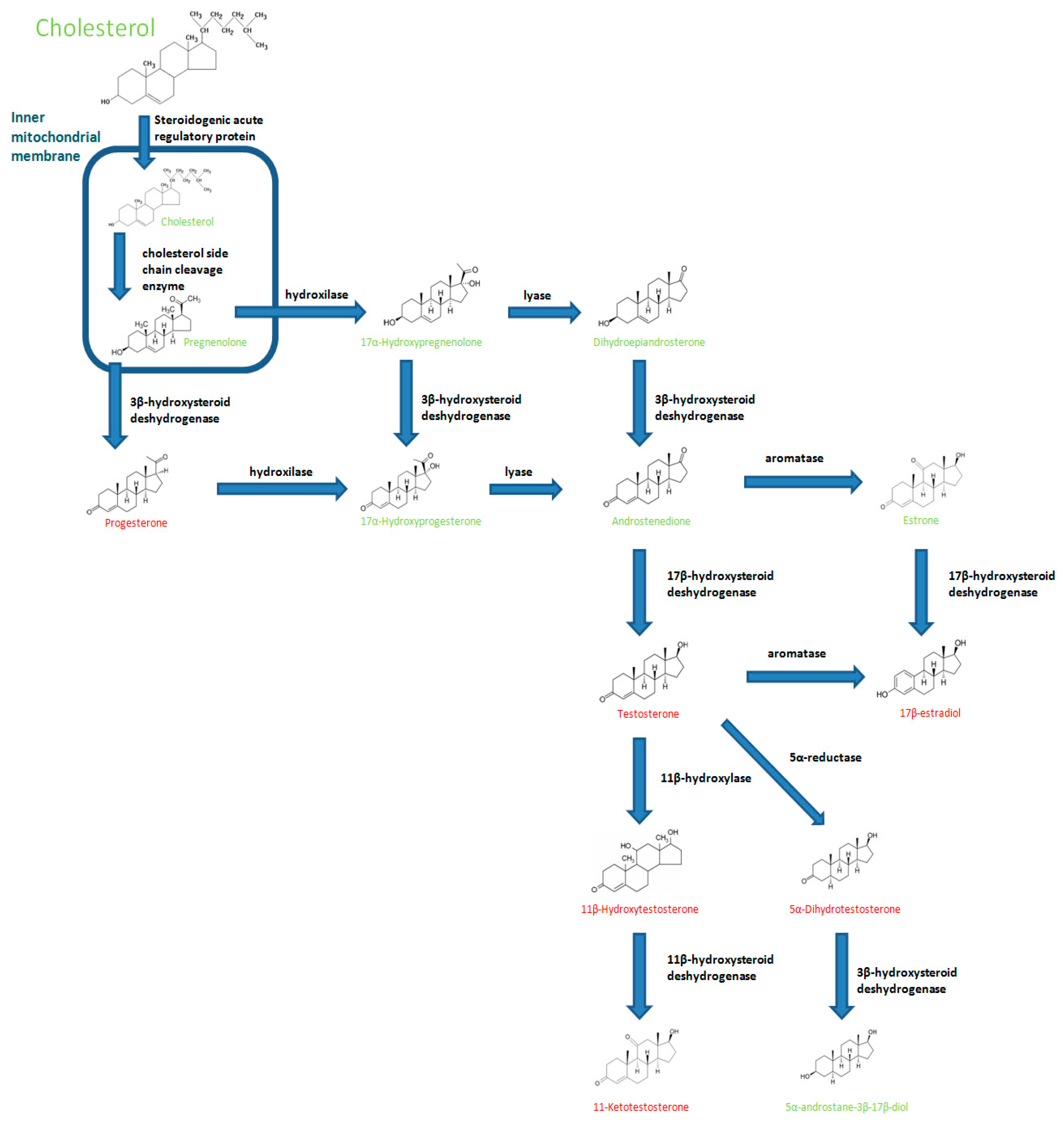
1. Introduction
2. Influence of Oestrogens on Fish Immune Responses
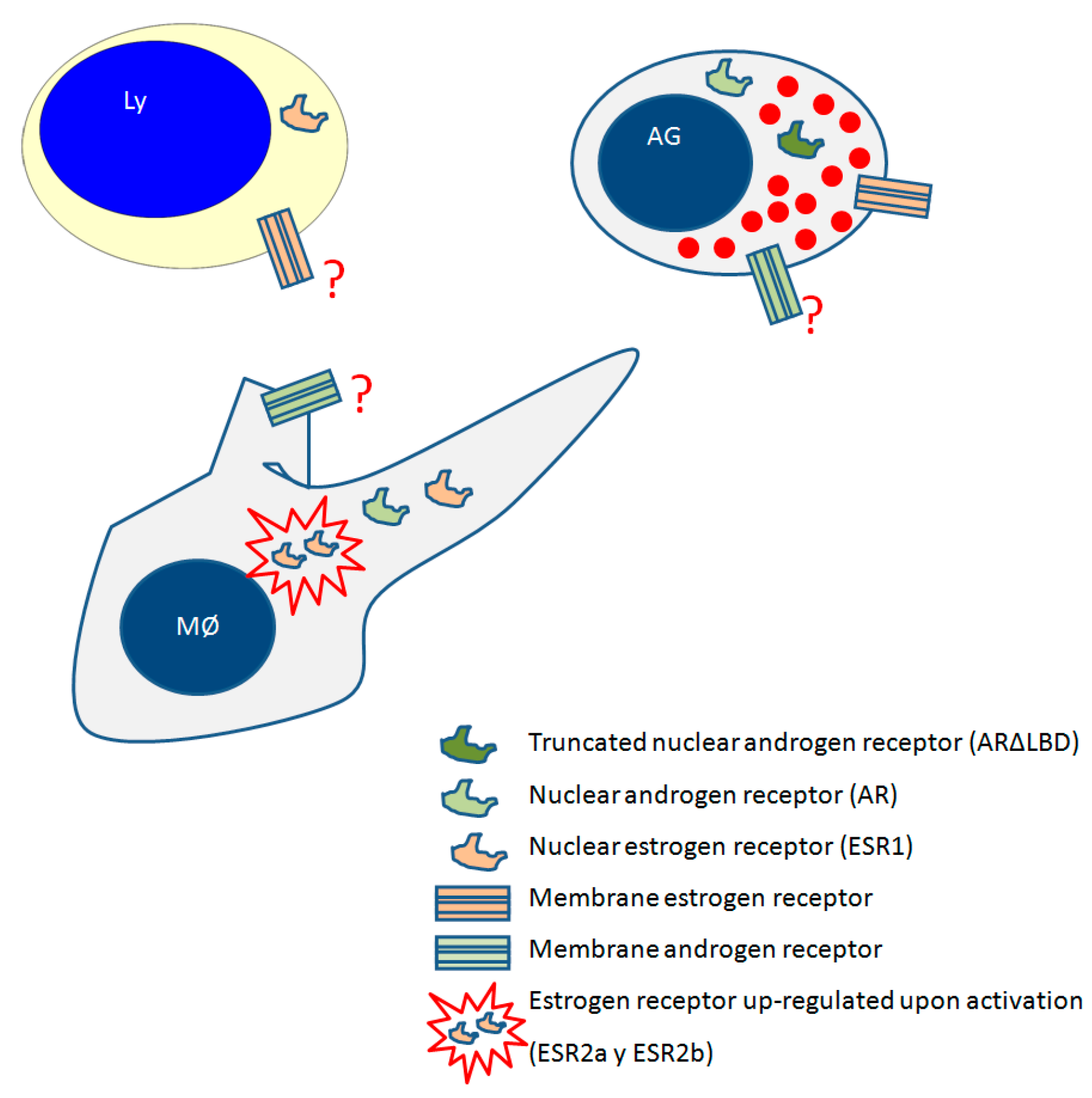
Oestrogen Receptor in Fish Leukocytes
3. Influence of Androgens on Fish Immune Responses
3.1. Testosterone
3.2. 11-Ketotestosterone
3.3. Other C-19 Steroid with Androgenic Function
3.4. Androgen Receptors in Fish Leukocytes
4. Influence of Progestins on Fish Immune Responses
Progestins Receptors in Fish Leukocytes
5. Immune System in the Fish Gonad
6. The Effect of Other Hormones on the Immune Function
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tokarz, J.; Möller, G.; Hrabě de Angelis, M.; Adamski, J. Steroids in teleost fishes: A functional point of view. Steroids 2015, 103, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Ansar Ahmed, S. The immune system is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 2015, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Mayer, I. Molecular biomarkers of endocrine disruption in small model fish. Mol. Cell. Endocrinol. 2008, 293, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Segner, H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hampl, R.; Kubátová, J.; Stárka, L. Steroids and endocrine disruptors-History, recent state of art and open questions. J. Steroid Biochem. Mol. Biol. 2016, 155, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Milla, S.; Depiereux, S.; Kestemont, P. The effects of estrogenic and androgenic endocrine disruptors on the immune system of fish: A review. Ecotoxicology 2011, 20, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Diotel, N.; Do Rego, J.L.; Anglade, I.; Vaillant, C.; Pellegrini, E.; Vaudry, H.; Kah, O. The brain of teleost fish, a source, and a target of sexual steroids. Front. Neurosci. 2011, 5, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Arukwe, A. Steroidogenic acute regulatory (StAR) protein and cholesterol side-chain cleavage (P450scc)-regulated steroidogenesis as an organ-specific molecular and cellular target for endocrine disrupting chemicals in fish. Cell Biol. Toxicol. 2008, 24, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A. Estrogen in the adult male reproductive tract: A review. Reprod. Biol. Endocrinol. 2003, 1, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pozo, E.; Arjona, F.J.; García-López, A.; García-Alcázar, A.; Meseguer, J.; García-Ayala, A. Sex steroids and metabolic parameter levels in a seasonal breeding fish (Sparus aurata L.). Gen. Comp. Endocrinol. 2008, 156, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Valero, Y.; Sánchez-Hernández, M.; García-Alcázar, A.; García-Ayala, A.; Cuesta, A.; Chaves-Pozo, E. Characterization of the annual regulation of reproductive and immune parameters on the testis of European sea bass. Cell Tissue Res. 2015, 362, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Martyniuk, C.J.; Bissegger, S.; Langlois, V.S.V.S. Reprint of “Current perspectives on the androgen 5 alpha-dihydrotestosterone (DHT) and 5 alpha-reductases in teleost fishes and amphibians”. Gen. Comp. Endocrinol. 2014, 203, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Borg, B. Androgens in teleost fishes. Comp. Biochem. Physiol. Part C Comp. 1994, 109, 219–245. [Google Scholar] [CrossRef]
- Miura, T.; Yamauchi, K.; Takahashi, H.; Nagahama, Y. The role of hormones in the acquisition of sperm motility in salmonid fish. J. Exp. Zool. 1992, 261, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Kambegawa, A.; Nagahama, Y. Involvement of gonadotrophin and steroid hormones in spermiation in the amago salmon, Oncorhynchus rhodurus, and goldfish, Carassius auratus. Gen. Comp. Endocrinol. 1985, 59, 24–30. [Google Scholar] [CrossRef]
- Miura, T.; Higuchi, M.; Ozaki, Y.; Ohta, T.; Miura, C. Progestin is an essential factor for the initiation of the meiosis in spermatogenetic cells of the eel. Proc. Natl. Acad. Sci. USA 2006, 103, 7333–7338. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L. Diverse roles for sex hormone-binding globulin in reproduction. Biol. Reprod. 2011, 85, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.J.; Thompson, K.D.; Morgan, A.L.; Gratacap, R.M.L.; Nikoskelainen, S. Seasonal variation and the immune response: A fish perspective. Fish Shellfish Immunol. 2007, 22, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Valero, Y.; García-Alcázar, A.; Esteban, M.A.; Cuesta, A.; Chaves-Pozo, E. Seasonal variations of the humoral immune parameters of European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2014, 39, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.A.; Cuesta, A.; Chaves-Pozo, E.; Meseguer, J. Influence of melatonin on the immune system of fish: A review. Int. J. Mol. Sci. 2013, 14, 7979–7999. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pozo, E.; Liarte, S.; Fernández-Alacid, L.; Abellán, E.; Meseguer, J.; Mulero, V.; García-Ayala, A. Pattern of expression of immune-relevant genes in the gonad of a teleost, the gilthead seabream (Sparus aurata L.). Mol. Immunol. 2008, 45, 2998–3011. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, D.L.; Blake, L.S.; Brodin, J.D.; Greene, K.J.; Knoebl, I.; Miracle, A.L.; Martinovic, D.; Ankley, G.T. Transcription of key genes regulating gonadal steroidogenesis in control and ketoconazole- or vinclozolin-exposed fathead minnows. Toxicol. Sci. 2007, 98, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Szwejser, E.; Verburg-van Kemenade, B.M.L.; Maciuszek, M.; Chadzinska, M. Estrogen-dependent seasonal adaptations in the immune response of fish. Horm. Behav. 2017, 88, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, G.K.; Jurutka, P.W.; Haussler, C.A.; Haussler, M.R. Steroid hormone receptors: Evolution, ligands, and molecular basis of biologic function. J. Cell. Biochem. 1999, 75, 110–122. [Google Scholar] [CrossRef]
- Thomas, P.; Dressing, G.; Pang, Y.; Berg, H.; Tubbs, C.; Benninghoff, A.; Doughty, K. Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids 2006, 71, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Whyte, S.K. The innate immune response of finfish-a review of current knowledge. Fish Shellfish Immunol. 2007, 23, 1127–1151. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.; Huttenhuis, H.; Picchietti, S.; Scapigliati, G. Phylogeny and ontogeny of fish leucocytes. Fish Shellfish Immunol. 2005, 19, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Abruzzini, A.F.; Ingram, L.O.; Clem, L.W. Temperature-mediated processes in teleost immunity: Homeoviscous adaptation in teleost lymphocytes. Proc. Soc. Exp. Biol. Med. 1982, 169, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Avtalion, R.R.; Clem, L.W. Environmental control of the immune response in fish. Crit. Rev. Environ. Control 1981, 11, 163–188. [Google Scholar] [CrossRef]
- Cuchens, M.A.; Clem, L.W. Phylogeny of lymphocyte heterogeneity. II. Differential effects of temperature on fish T-like and B-like cells. Cell. Immunol. 1977, 34, 219–230. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Esteban, M.A.; Cuesta, A.; Sun, Y.-Z. Prebiotics and fish immune response: A review of current knowledge and future perspectives. Rev. Fish. Sci. Aquac. 2015, 23, 315–328. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Makrinos, D.L.; Bowden, T.J. Natural environmental impacts on teleost immune function. Fish Shellfish Immunol. 2016, 53, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Crowhurst, M.O.; Layton, J.E.; Lieschke, G.J. Developmental biology of zebrafish myeloid cells. Int. J. Dev. Biol. 2002, 46, 483–492. [Google Scholar] [PubMed]
- Dos Santos, N.M.; Romano, N.; de Sousa, M.; Ellis, A.E.; Rombout, J.H.W. Ontogeny of B and T cells in sea bass (Dicentrarchus labrax, L.). Fish Shellfish Immunol. 2000, 10, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I.; Zhang, Y.-A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef] [PubMed]
- Trede, N.S.; Zapata, A.; Zon, L.I. Fishing for lymphoid genes. Trends Immunol. 2001, 22, 302–307. [Google Scholar] [CrossRef]
- Rubio-Godoy, M. Teleost fish immunology. Review. Rev. Mex. Cienc. Pecu. 2010, 1, 47–57. [Google Scholar]
- Parra, D.; Takizawa, F.; Sunyer, J.O. Evolution of B cell immunity. Annu. Rev. Anim. Biosci. 2013, 1, 65–97. [Google Scholar] [CrossRef] [PubMed]
- Flajnik, M.F. The last flag unfurled? A new immunoglobulin isotype in fish expressed in early development. Nat. Immunol. 2005, 6, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Coward, K.; Bromage, N.R.; Little, D.C. Inhibition of spawning and associated suppression of sex steroid levels during confinement in the substrate-spawning Tilapia zillii. J. Fish Biol. 1998, 52, 152–165. [Google Scholar] [CrossRef]
- Ohta, Y.; Flajnik, M. IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates. Proc. Natl. Acad. Sci. USA 2006, 103, 10723–10728. [Google Scholar] [CrossRef] [PubMed]
- Mashoof, S.; Criscitiello, M. Fish immunoglobulins. Biology 2016, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Acton, R.T.; Weinheimer, P.F.; Hall, S.J.; Niedermeier, W.; Shelton, E.; Bennett, J.C. Tetrameric immune macroglobulins in three orders of bony fishes. Proc. Natl. Acad. Sci. USA 1971, 68, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Bengtén, E.; Miller, N.W.; Clem, L.W.; Pasquier, L.D.; Warr, G.W. A novel chimeric Ig heavy chain from a teleost fish shares similarities to IgD (evolution). Immunology 1997, 94, 4593–4597. [Google Scholar]
- Danilova, N.; Bussmann, J.; Jekosch, K.; Steiner, L.A. The immunoglobulin heavy-chain locus in zebrafish: Identification and expression of a previously unknown isotype, immunoglobulin Z. Nat. Immunol. 2005, 6, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Edholm, E.S.; Bengten, E.; Wilson, M. Insights into the function of IgD. Dev. Comp. Immunol. 2011, 35, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-A.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Solem, S.T.; Stenvik, J. Antibody repertoire development in teleosts: A review with emphasis on salmonids and Gadus morhua L. Dev. Comp. Immunol. 2006, 30, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Maisey, K.; Imarai, M. Diversity of teleost leukocyte molecules: Role of alternative splicing. Fish Shellfish Immunol. 2011, 31, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Da’as, S.; Teh, E.M.; Dobson, J.T.; Nasrallah, G.K.; McBride, E.R.; Wang, H.; Neuberg, D.S.; Marshall, J.S.; Lin, T.J.; Berman, J.N. Zebrafish mast cells possess an FcεRI-like receptor and participate in innate and adaptive immune responses. Dev. Comp. Immunol. 2011, 35, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Utke, K.; Somamoto, T.; Kollner, B.; Ototake, M.; Nakanishi, T. Cytotoxic activities of fish leucocytes. Fish Shellfish Immunol. 2006, 20, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Quiniou, S.M.A.; Boudinot, P. Processing of fish Ig heavy chain transcripts: Diverse splicing patterns and unusual nonsense mediated decay. Dev. Comp. Immunol. 2011, 35, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Mutoloki, S.; Jørgensen, J.B.; Evensen, Ø. The adaptive immune response in fish. In Fish Vaccination; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 104–115. ISBN 9781118806913. [Google Scholar]
- Secombes, C. Will advances in fish immunology change vaccination strategies? Fish Shellfish Immunol. 2008, 25, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Takizawa, F.; Fischer, U.; Dijkstra, J. Along the axis between type 1 and type 2 immunity; principles conserved in evolution from fish to mammals. Biology 2015, 4, 814–859. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar]
- Nelson, E.R.; Habibi, H.R. Estrogen receptor function and regulation in fish and other vertebrates. Gen. Comp. Endocrinol. 2013, 192, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Iwanowicz, L.R.; Ottinger, C.A. Estrogens, estrogen receptors and their role as immunoregulators in fish. In Fish Defenses; Zaccone, G., Meseguer, J., García-Ayala, A., Kapoor, B.G., Eds.; Science Publisher: Enfield, UK, 2009; Volume 1, pp. 277–322. ISBN 978-1-57808-327-5. [Google Scholar]
- Liarte, S.; Chaves-Pozo, E.; Abellán, E.; Meseguer, J.; Mulero, V.; Canario, A.V.; García-Ayala, A. Estrogen-responsive genes in macrophages of the bony fish gilthead seabream: A transcriptomic approach. Dev. Comp. Immunol. 2011, 35, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Cabas, I.; Liarte, S.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. 17alpha-Ethynylestradiol alters the immune response of the teleost gilthead seabream (Sparus aurata L.) both in vivo and in vitro. Dev. Comp. Immunol. 2012, 36, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Cabas, I.; Chaves-Pozo, E.; Arizcun, M.; Meseguer, J.; Mulero, V.; García-Ayala, A. Natural and synthetic estrogens modulate the inflammatory response in the gilthead seabream (Sparus aurata L.) through the activation of endothelial cells. Mol. Immunol. 2011, 48, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Chaves-Pozo, E.; Abellán, E.; Meseguer, J.; Mulero, V.; García-Ayala, A. 17beta-Estradiol regulates gilthead seabream professional phagocyte responses through macrophage activation. Dev. Comp. Immunol. 2011, 35, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pozo, E.; Pelegrín, P.; Mulero, V.; Meseguer, J.; García-Ayala, A. A role for acidophilic granulocytes in the testis of the gilthead seabream (Sparus aurata L., Teleostei). J. Endocrinol. 2003, 179, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Law, W.Y.; Chen, W.H.; Song, Y.L.; Dufour, S.; Chang, C.F. Differential in vitro suppressive effects of steroids on leukocyte phagocytosis in two teleosts, tilapia and common carp. Gen. Comp. Endocrinol. 2001, 121, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Belosevic, M. The in vitro effects of estradiol and cortisol on the function of a long-term goldfish macrophage cell line. Dev. Comp. Immunol. 1995, 19, 327–336. [Google Scholar] [CrossRef]
- Watanuki, H.; Yamaguchi, T.; Sakai, M. Suppression in function of phagocytic cells in common carp Cyprinus carpio L. injected with estradiol, progesterone or 11-ketotestosterone. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 132, 407–413. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Watanuki, H.; Sakai, M. Effects of estradiol, progesterone and testosterone on the function of carp, Cyprinus carpio, phagocytes in vitro. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 129, 49–55. [Google Scholar] [CrossRef]
- Chaves-Pozo, E.; Liarte, S.; Vargas-Chacoff, L.; García-López, A.; Mulero, V.; Meseguer, J.; Mancera, J.M.; García-Ayala, A. 17Beta-estradiol triggers postspawning in spermatogenically active gilthead seabream (Sparus aurata L.) males. Biol. Reprod. 2007, 76, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.Y.; Dong, H.X. Effects of estradiol-17beta on immunocompetence in rainbow trout (Oncorhynchus mykiss). Acta Zool. Sin. 2001, 47, 285–291. [Google Scholar]
- Thilagam, H.; Gopalakrishnan, S.; Qu, H.D.; Bo, J.; Wang, K.J. 17beta estradiol induced ROS generation, DNA damage and enzymatic responses in the hepatic tissue of Japanese sea bass. Ecotoxicology 2010, 19, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Thilagam, H.; Gopalakrishnan, S.; Bo, J.; Wang, K.-J.J. Effect of 17beta-estradiol on the immunocompetence of Japanese sea bass (Lateolabrax japonicus). Environ. Toxicol. Chem. 2009, 28, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Seemann, F.; Knigge, T.; Rocher, B.; Minier, C.; Monsinjon, T. 17β-Estradiol induces changes in cytokine levels in head kidney and blood of juvenile sea bass (Dicentrarchus labrax L., 1758). Mar. Environ. Res. 2013, 87–88, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wenger, M.; Sattler, U.; Goldschmidt-Clermont, E.; Segner, H. 17Beta-estradiol affects the response of complement components and survival of rainbow trout (Oncorhynchus mykiss) challenged by bacterial infection. Fish Shellfish Immunol. 2011, 31, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, A.; Vargas-Chacoff, L.; García-López, A.; Arjona, F.J.; Martínez-Rodríguez, G.; Meseguer, J.; Mancera, J.M.; Esteban, M.A. Effect of sex-steroid hormones, testosterone and estradiol, on humoral immune parameters of gilthead seabream. Fish Shellfish Immunol. 2007, 23, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.Y.; Suzuki, Y.; Aida, K. Effects of steroid hormones on immunoglobulin M (IgM) in rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 1999, 20, 155–162. [Google Scholar] [CrossRef]
- Hou, Y.; Suzuki, Y.; Aida, K. Effects of steroids on the antibody producing lymphocytes in rainbow trout. Fish. Sci. 1999, 65, 850–855. [Google Scholar] [CrossRef][Green Version]
- Saha, N.R.; Usami, T.; Suzuki, Y. In vitro effects of steroid hormones on IgM-secreting cells and IgM secretion in common carp (Cyprinus carpio). Fish Shellfish Immunol. 2004, 17, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Iwanowicz, L.R.; Stafford, J.L.; Patiño, R.; Bengten, E.; Miller, N.W.; Blazer, V.S. Channel catfish (Ictalurus punctatus) leukocytes express estrogen receptor isoforms ERα and ERβ2 and are functionally modulated by estrogens. Fish Shellfish Immunol. 2014, 40, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Belosevic, M. Estradiol increases susceptibility of goldfish to Trypanosoma danilewskyi. Dev. Comp. Immunol. 1994, 18, 377–387. [Google Scholar] [CrossRef]
- Hintemann, T.; Schneider, C.; Schöler, H.F.; Schneider, R.J. Field study using two immunoassays for the determination of estradiol and ethinylestradiol in the aquatic environment. Water Res. 2006, 40, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Aerni, H.R.; Gerritsen, A.; Gibert, M.; Giger, W.; Hylland, K.; Jurgens, M.; Nakari, T.; Pickering, A.; Suter, M.J.; et al. Comparing steroid estrogen, and nonylphenol content across a range of European sewage plants with different treatment and management practices. Water Res. 2005, 39, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Soffker, M.; Tyler, C.R.; Söffker, M.; Tyler, C.R. Endocrine disrupting chemicals and sexual behaviors in fish—A critical review on effects and possible consequences. Crit. Rev. Toxicol. 2012, 42, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Rodenas, M.C.; Cabas, I.; Abellán, E.; Meseguer, J.; Mulero, V.; García-Ayala, A. Tamoxifen persistently disrupts the humoral adaptive immune response of gilthead seabream (Sparus aurata L.). Dev. Comp. Immunol. 2015, 53, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Rodenas, M.C.; Cabas, I.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. Selective estrogen receptor modulators differentially alter the immune response of gilthead seabream juveniles. Fish Shellfish Immunol. 2016, 52, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Gómez González, N.E.; Cabas, I.; Rodenas, M.C.; Arizcun, M.; Mulero, V.; García Ayala, A. 17α-Ethynylestradiol alters the peritoneal immune response of gilthead seabream. Dev. Comp. Immunol. 2017, 76, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Briceño, P.; Águila-Martínez, S.; Liarte, S.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. In situ forming microparticle implants for delivery of sex steroids in fish: Modulation of the immune response of gilthead seabream by testosterone. Steroids 2013, 78, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Águila, S.; Castillo-Briceño, P.; Sánchez, M.; Cabas, I.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. Specific and non-overlapping functions of testosterone and 11-ketotestosterone in the regulation of professional phagocyte responses in the teleost fish gilthead seabream. Mol. Immunol. 2013, 53, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Vainikka, A.; Jokinen, E.I.; Kortet, R.; Paukku, S.; Pirhonen, J.; Ratala, M.J.; Taskinen, J. Effects of testosterone and b-glucan on immune function in tench. J. Fish Biol. 2005, 66, 348–361. [Google Scholar] [CrossRef]
- Slater, C.H.; Schreck, C.B. Testosterone alters the immune response of chinook salmon, Oncorhynchus tshawytscha. Gen. Comp. Endocrinol. 1993, 89, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.; Kalbe, M.; Langefors, A.; Mayer, I.; Milinski, M.; Hasselquist, D. An experimental test of the immunocompetence handicap hypothesis in a teleost fish: 11-ketotestosterone suppresses innate immunity in three-spined sticklebacks. Am. Nat. 2007, 170, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Nagler, J.J.; Cavileer, T.; Sullivan, J.; Cyr, D.G.; Rexroad, C., 3rd. The complete nuclear estrogen receptor family in the rainbow trout: Discovery of the novel ERalpha2 and both ERbeta isoforms. Gene 2007, 392, 164–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xia, Z.; Gale, W.L.; Chang, X.; Langenau, D.; Patino, R.; Maule, A.G.; Densmore, L.D. Phylogenetic sequence analysis, recombinant expression, and tissue distribution of a channel catfish estrogen receptor beta. Gen. Comp. Endocrinol. 2000, 118, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Caviola, E.; Dalla Valle, L.; Belvedere, P.; Colombo, L. Characterisation of three variants of estrogen receptor beta mRNA in the common sole, Solea solea L. (Teleostei). Gen. Comp. Endocrinol. 2007, 153, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Paiola, M.; Knigge, T.; Picchietti, S.; Duflot, A.; Guerra, L.; Pinto, P.I.S.; Scapigliati, G.; Monsinjon, T. Oestrogen receptor distribution related to functional thymus anatomy of the European sea bass, Dicentrarchus labrax. Dev. Comp. Immunol. 2017, 77, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Szwejser, E.; Maciuszek, M.; Casanova-Nakayama, A.; Segner, H.; Verburg-van Kemenade, B.M.L. A role for multiple estrogen receptors in immune regulation of common carp. Dev. Comp. Immunol. 2017, 66, 61–72. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, A.; Liarte, S.; Gómez-González, N.E.; Cabas, I.; Meseguer, J.; García-Ayala, A.; Mulero, V. Estrogen receptor 2b deficiency impairs the antiviral response of zebrafish. Dev. Comp. Immunol. 2015, 53, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Szego, C.M.; Davis, J.S. Adenosine 3′,5′-monophosphate in rat uterus: Acute elevation by estrogen. Proc. Natl. Acad. Sci. USA 1967, 58, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Korach, K.S.; Moss, R.L. Rapid action of 17β-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology 1999, 140, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Ropero, A.B.; Laribi, O.; Maillet, M.; Fuentes, E.; Soria, B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc. Natl. Acad. Sci. USA 2000, 97, 11603–11608. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Bosch, M.A.; Tobias, S.C.; Grandy, D.K.; Scanlan, T.S.; Ronnekleiv, O.K.; Kelly, M.J. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 2003, 23, 9529–9540. [Google Scholar] [PubMed]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005, 146, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Arterburn, J.B.; Sklar, L.A. GPR30: AG protein-coupled receptor for estrogen. Mol. Cell. Endocrinol. 2007, 265–266, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Morini, M.; Peñaranda, D.S.; Vílchez, M.C.; Tveiten, H.; Lafont, A.-G.; Dufour, S.; Pérez, L.; Asturiano, J.F. The expression of nuclear and membrane estrogen receptors in the European eel throughout spermatogenesis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 203, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cabas, I.; Rodenas, M.C.; Abellán, E.; Meseguer, J.; Mulero, V.; García-Ayala, A. Estrogen signaling through the G protein-coupled estrogen receptor regulates granulocyte activation in fish. J. Immunol. 2013, 191, 4628–4639. [Google Scholar] [CrossRef] [PubMed]
- Diamante, G.; Menjivar-Cervantes, N.; Leung, M.S.; Volz, D.C.; Schlenk, D. Contribution of G protein-coupled estrogen receptor 1 (GPER) to 17β-estradiol-induced developmental toxicity in zebrafish. Aquat. Toxicol. 2017, 186, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Mangiamele, L.A.; Gómez, J.R.; Curtis, N.J.; Thompson, R.R. GPER/GPR30, a membrane estrogen receptor, is expressed in the brain and retina of a social fish (Carassius auratus) and colocalizes with isotocin. J. Comp. Neurol. 2017, 525, 252–270. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Alyea, R.; Pang, Y.; Peyton, C.; Dong, J.; Berg, A.H. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids 2010, 75, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Patiño, R.; Xia, Z.; Gale, W.L.; Wu, C.; Maule, A.G.; Chang, X. Novel transcripts of the estrogen receptor alpha gene in channel catfish. Gen. Comp. Endocrinol. 2000, 120, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Slater, C.H.; Fitzpatrick, M.S.; Schreck, C.B. Characterization of an androgen receptor in salmonid lymphocytes: Possible link to androgen-induced immunosuppression. Gen. Comp. Endocrinol. 1995, 100, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, M.; Piferrer, F. Sea bass (Dicentrarchus labrax) androgen receptor: CDNA cloning, tissue-specific expression, and mRNA levels during early development and sex differentiation. Mol. Cell. Endocrinol. 2005, 237, 37–48. [Google Scholar] [CrossRef] [PubMed]
- De Waal, P.P.; Wang, D.S.; Nijenhuis, W.A.; Schulz, R.W.; Bogerd, J. Functional characterization and expression analysis of the androgen receptor in zebrafish (Danio rerio) testis. Reproduction 2008, 136, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, M.; Arizcun, M.; García-Alcázar, A.; Sarropoulou, E.; Mulero, V.; García-Ayala, A. Fish granulocytes express a constitutively active androgen receptor variant. Dev. Comp. Immunol. 2014, 45, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.A.; Dufour, S.; Karlsen, O.; Norberg, B.; et al. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef] [PubMed]
- Mouriec, K.; Gueguen, M.M.; Manuel, C.; Percevault, F.; Thieulant, M.L.; Pakdel, F.; Kah, O. Androgens upregulate cyp19a1b (aromatase B) gene expression in the brain of zebrafish (Danio rerio) through estrogen receptors. Biol. Reprod. 2009, 80, 889–896. [Google Scholar] [CrossRef] [PubMed]
- García-García, M.; Sánchez-Hernández, M.; García-Hernández, M.P.; García-Ayala, A.; Chaves-Pozo, E. Role of 5α-dihydrotestosterone in testicular development of gilthead seabream following finasteride administration. J. Steroid Biochem. Mol. Biol. 2017, 174, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Folstad, I.; Karter, A.J. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992, 139, 603–622. [Google Scholar] [CrossRef]
- Slater, C.H.; Schreck, C.B. Physiological levels of testosterone kill salmonid leukocytes in vitro. Gen. Comp. Endocrinol. 1997, 106, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.R.; Usami, T.; Suzuki, Y. A double staining flow cytometric assay for the detection of steroid induced apoptotic leucocytes in common carp (Cyprinus carpio). Dev. Comp. Immunol. 2003, 27, 351–363. [Google Scholar] [CrossRef]
- Margiotta-Casaluci, L.; Sumpter, J.P. 5alpha-Dihydrotestosterone is a potent androgen in the fathead minnow (Pimephales promelas). Gen. Comp. Endocrinol. 2011, 171, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Senthilkumaran, B. Dynamic expression of 11β-hydroxylase during testicular development, recrudescence and after hCG induction, in vivo and in vitro in catfish, Clarias batrachus. Gen. Comp. Endocrinol. 2015, 211, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R. In vitro metabolism of steroid hormones in the liver and in blood cells of male rainbow trout (Salmo gairdneri Richardson). Gen. Comp. Endocrinol. 1986, 64, 312–319. [Google Scholar] [CrossRef]
- Sperry, T.S.; Thomas, P. Characterization of two nuclear androgen receptors in Atlantic croaker: Comparison of their biochemical properties and binding specificities. Endocrinology 1999, 140, 1602–1611. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikeuchi, T.; Todo, T.; Kobayashi, T.; Nagahama, Y. Two subtypes of androgen and progestogen receptors in fish testes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 449–455. [Google Scholar] [CrossRef]
- Louie, M.C.; Sevigny, M.B. Steroid hormone receptors as prognostic markers in breast cancer. Am. J. Cancer Res. 2017, 7, 1617–1636. [Google Scholar] [PubMed]
- Kobayashi, T.; Sakai, N.; Adachi, S.; Asahina, K.; Iwasawa, H.; Nagahama, Y. 17alpha,20alpha-Dihydroxy-4-pregnen-3-one is the naturally occurring spermiation-inducing hormone in the testis of a frog, Rana nigromaculata. Endocrinology 1993, 133, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Goldfien, G.A.; Barragan, F.; Chen, J.; Takeda, M.; Irwin, J.C.; Perry, J.; Greenblatt, R.M.; Smith-McCune, K.K.; Giudice, L.C. Progestin-containing contraceptives alter expression of host defense-related genes of the endometrium and cervix. Reprod. Sci. 2015, 22, 814–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huijbregts, R.P.H.; Michel, K.G.; Hel, Z. Effect of progestins on immunity: Medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception 2014, 90, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, C.; Neumann, N.; Knopf, K.; Wuertz, S.; Kloas, W. Progestogens cause immunosuppression of stimulated carp (Cyprinus carpio L.) leukocytes in vitro. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2009, 150, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 2008, 29, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Verburg-van Kemenade, B.M.; Van der Aa, L.M.; Chadzinska, M. Neuroendocrine-immune interaction: Regulation of inflammation via G-protein coupled receptors. Gen. Comp. Endocrinol. 2013, 188, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Verburg-van Kemenade, B.M.L.; Ribeiro, C.M.S.; Chadzinska, M. Neuroendocrine-immune interaction in fish: Differential regulation of phagocyte activity by neuroendocrine factors. Gen. Comp. Endocrinol. 2011, 172, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, M.; Chaves-Pozo, E.; Cabas, I.; Mulero, V.; García-Ayala, A.; García-Alcázar, A. Testosterone implants modify the steroid hormone balance and the gonadal physiology of gilthead seabream (Sparus aurata L.) males. J. Steroid Biochem. Mol. Biol. 2013, 138, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P. Immune privilege of the testis: Meaning, mechanisms, and manifestations. In Infection, Immune Homeostasis and Immune Privilege; Stein-Streilein, J., Ed.; Springer: Basel, Switzerland, 2012. [Google Scholar]
- Chaves-Pozo, E.; Mulero, V.; Meseguer, J.; García-Ayala, A. Professional phagocytic granulocytes of the bony fish gilthead seabream display functional adaptation to testicular microenvironment. J. Leukoc. Biol. 2005, 78, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P. Testicular leukocytes: What are they doing? Rev. Reprod. 1997, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, B.S.; Prasad, S.; Carino, C.; Skinner, S.M. The ovary as an immune target. J. Soc. Gynecol. Investig. 2001, 8, S43–S48. [Google Scholar] [PubMed]
- Chaves-Pozo, E.; Liarte, S.; García-Ayala, A. Immune and reproductive interaction: An essential clue for understanding gonad functions in gilthead seabream. In Recent Advances in Fish Reproductive Biology; García-Ayala, A., Meseguer, J., Chaves-Pozo, E., Eds.; Research Signpost: Kerala, India, 2010; ISBN 978-81-308-0397-5. [Google Scholar]
- Loir, M.; Sourdaine, P.; Mendis-Handagama, S.M.; Jegou, B. Cell-cell interactions in the testis of teleosts and elasmobranchs. Microsc. Res. Tech. 1995, 32, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Chaves-Pozo, E.; García-Alcázar, A.; Mulero, V.; Meseguer, J.; García-Ayala, A. Testicular involution prior to sex change in gilthead seabream is characterized by a decrease in DMRT1 gene expression and by massive leukocyte infiltration. Reprod. Biol. Endocrinol. 2007, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- García-García, M.; Liarte, S.; Gómez-González, N.E.; García-Alcázar, A.; Pérez-Sánchez, J.; Meseguer, J.; Mulero, V.; García-Ayala, A.; Chaves-Pozo, E. Cimetidine disrupts the renewal of testicular cells and the steroidogenesis in a hermaphrodite fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 189, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Cabas, I.; Chaves-Pozo, E.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. Dietary intake of 17alpha-ethinylestradiol promotes leukocytes infiltration in the gonad of the hermaphrodite gilthead seabream. Mol. Immunol. 2011, 48, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.; Luque, A.; Abós, B.; Castro, R.; González-Torres, L.; Tafalla, C. Molecular characterization of three novel chemokine receptors in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2013, 34, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Lutton, B.V.; Callard, I.P. Morphological relationships and leukocyte influence on steroid production in the epigonal organ-ovary complex of the skate, Leucoraja erinacea. J. Morphol. 2008, 269, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Tazumi, Y.; Nakamura, O.; Watanabe, T. Intraovarian cavity leucocytes of viviparous fish, Neoditrema ransonneti (Perciformes, Embiotocidae). Zool. Sci. 2004, 21, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pozo, E.; Castillo-Briceño, P.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. A role for matrix metalloproteinases in granulocyte infiltration and testicular remodelation in a seasonal breeding teleost. Mol. Immunol. 2008, 45, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Munro, E.S.; Ellis, A.E. A comparison between non-destructive and destructive testing of Atlantic salmon, Salmo salar L., broodfish for IPNV—Destructive testing is still the best at time of maturation. J. Fish Dis. 2008, 31, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pozo, E.; Montero, J.; Cuesta, A.; Tafalla, C. Viral hemorrhagic septicemia and infectious pancreatic necrosis viruses replicate differently in rainbow trout gonad and induce different chemokine transcription profiles. Dev. Comp. Immunol. 2010, 34, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pozo, E.; Zou, J.; Secombes, C.J.; Cuesta, A.; Tafalla, C. The rainbow trout (Oncorhynchus mykiss) interferon response in the ovary. Mol. Immunol. 2010, 47, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Valero, Y.; Arizcun, M.; Esteban, M.A.; Bandín, I.; Olveira, J.G.; Patel, S.; Cuesta, A.; Chaves-Pozo, E. Nodavirus colonizes and replicates in the testis of gilthead seabream and European sea bass modulating its immune and reproductive functions. PLoS ONE 2015, 10, e0145131. [Google Scholar] [CrossRef] [PubMed]
- Valero, Y.; Morcillo, P.; Meseguer, J.; Buonocore, F.; Esteban, M.A.; Chaves-Pozo, E.; Cuesta, A. Characterization of the interferon pathway in the teleost fish gonad against the vertically transmitted viral nervous necrosis virus. J. Gen. Virol. 2015, 96, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Goikoetxea, A.; Todd, E.V.; Gemmell, N.J. Stress and sex: Does cortisol mediate sex change in fish? Reproduction 2017, 154, R149–R160. [Google Scholar] [CrossRef] [PubMed]
- Szwejser, E.; Pijanowski, L.; Maciuszek, M.; Ptak, A.; Wartalski, K.; Duda, M.; Segner, H.; Verburg-van Kemenade, B.M.L.; Chadzinska, M. Stress differentially affects the systemic and leukocyte estrogen network in common carp. Fish Shellfish Immunol. 2017, 68, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, B.T.; Stefansson, S.O.; Mccormick, S.D. Environmental endocrinology of salmon smoltification. Gen. Comp. Endocrinol. 2011, 170, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Ohta, H.; Yamauchi, K. Serum thyroxine, estradiol-17β, and testosterone profiles during the parr-smolt transformation of masu salmon, Oncorhynchus masou. Fish Physiol. Biochem. 1993, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Sex Steroids | Leukocytes | Treatment | Effects | Fish Species | References |
|---|---|---|---|---|---|
| E2 | Head kidney cells | In vivo | Decrease of IL1β and TNFα transcription and IL1β production | European sea bass | [76] |
| In vitro | Increase of IL1β production | Gilthead seabream | [67] | ||
| In vitro | Inhibition of ROIs production activity | Gilthead seabream | [67] | ||
| Head kidney acidophilic granulocytes | In vivo | Migration from head kidney to peripheral tissues | Gilthead seabream | [72] | |
| Macrophages | In vivo | Inhibition of ROIs production activity | Rainbow trout | [73] | |
| Blood macrophages | In vivo | Increases of ROIs production activity | Japanese sea bass | [75] | |
| Phagocytes | In vivo | Inhibition of NO production | Common carp | [70] | |
| In vivo | Inhibition of ROIs production activity | Common carp | [70] | ||
| Head kidney macrophages | In vitro | Inhibition of chemotaxis against endotoxin | Goldfish | [69] | |
| In vitro | Impartment of the immune-related gene expression pattern | Gilthead seabream | [63] | ||
| In vitro | Non-effect on ROIs and NO production | Common carp | [71] | ||
| In vitro | Non-effect on ROIs and NO production | Goldfish | [69] | ||
| In vitro | Inhibition of the phagocytic capability | Common carp | [71] | ||
| In vitro | Inhibition of the phagocytic capability | Goldfish | [69] | ||
| Peripheral blood leukocytes | In vitro | Suppression of mitogenic activity | Goldfish | [83] | |
| In vitro | Suppression of mitogenic activity | Channel catfish | [82] | ||
| In vivo | Impairment of mitogenic activity | Goldfish | [83] | ||
| IgM-secreting cells | In vivo | Impairment of mitogenic activity | Goldfish | [83] | |
| In vivo | Decreases on IgM production | Gilthead seabream | [78] | ||
| In vivo | Decreases on IgM production | Rainbow trout | [73] | ||
| In vivo | Increases on IgM production | Japanese sea bass | [75] | ||
| Head kidney leukocytes | In vivo | Increases of IL1β and TLRs transcription | Gilthead seabream | [90] | |
| In vitro and in vivo | Increases on ROIs production | Gilthead seabream | [90,91] | ||
| In vitro | Non-effect on ROIs production | Tilapia | [68] | ||
| In vitro | Increases on phagocytosis | Common carp | [68] | ||
| In vitro | Gilthead seabream | [91] | |||
| Acidophilic granulocytes | In vitro | Increases of IL1β and TLRs transcription | Gilthead seabream | [91] | |
| Blood leukocytes | In vivo | Non-effect on lysozyme activity | Tench | [92] | |
| In vivo | Non-effect on ROIs production | Tench | [92] | ||
| IgM-secreting cells | In vitro | Decreases in number in blood, head-kidney, spleen and skin | Chinook salmon | [93] | |
| In vitro | Rainbow trout | [80] | |||
| In vitro | Reduction in spleen | Common carp | [81] | ||
| Non-effect in head-kidney | |||||
| Non-effect in blood | |||||
| 11KT | Head-kidney macrophages | In vivo | Inhibition of ROIs production | Common carp | [70] |
| In vivo | Inhibition of phagocytosis | Common carp | [70] | ||
| In vitro | Increases of TLRs and IL1β transcription | Gilthead seabream | [91] | ||
| Blood leukocytes | In vitro | Non-effect on phagocytosis | Common carp | [68] | |
| In vitro | Tilapia | [68] | |||
| Head kidney phagocytes | In vitro | Activation of ROIs production | Gilthead seabream | [67] | |
| In vitro | Increases pro-IL1β accumulation | Gilthead seabream | [67] | ||
| In vivo | Inhibition of ROIs production | Three-spine sticklebacks | [94] | ||
| Head kidney acidophilic granulocytes | In vitro | Decreases of TLRs transcription | Gilthead seabream | [91] | |
| IgM-secreting cells | In vivo and in vitro | Decreases production | Rainbow trout | [79,80] | |
| In vitro | Decreases in number in blood, head-kidney, spleen and skin | Rainbow trout | [80] |
| Sex Steroid | Receptor | Tissue or Cells | Fish Specie | References |
|---|---|---|---|---|
| E2 | ESR1 | Spleen, blood and head-kidney cells | Channel catfish | [113] |
| Macrophages | Gilthead seabream | [66] | ||
| Common carp | [99] | |||
| Neutrophils | Common carp | [99] | ||
| Lymphocytes | Gilthead seabream | [66] | ||
| Common carp | [99] | |||
| Thymocytes | European sea bass | [98] | ||
| Mast cells | European sea bass | [98] | ||
| Peritoneal leukocytes | Gilthead seabream | [89] | ||
| ESR2 | Spleen | Channel catfish | [113] | |
| Spleen and head-kidney | Common sole | [97] | ||
| Macrophages | Common carp | [99] | ||
| Neutrophils | Common carp | [99] | ||
| Lymphocytes | Common carp | [99] | ||
| Thymocytes | European sea bass | [98] | ||
| Mast cells | European sea bass | [98] | ||
| GPER | Macrophages | Common carp | [99] | |
| Acidophilic granulocytes | Gilthead seabream | [109] | ||
| Peritoneal leukocytes | Gilthead seabream | [89] | ||
| Androgens | AR | Head-kidney | salmonids | [114] |
| European sea bass | [115] | |||
| Zebrafish | [116] | |||
| Liver | European sea bass | [115] | ||
| Zebrafish | [116] | |||
| Spleen | European sea bass | [115] | ||
| Zebrafish | [116] | |||
| Macrophages | Gilthead seabream | [91] | ||
| Acidophilic granulocytes | Gilthead seabream | [91] | ||
| ARΔLBD variant | Acidophilic granulocytes | Gilthead seabream | [117] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves-Pozo, E.; García-Ayala, A.; Cabas, I. Effects of Sex Steroids on Fish Leukocytes. Biology 2018, 7, 9. https://doi.org/10.3390/biology7010009
Chaves-Pozo E, García-Ayala A, Cabas I. Effects of Sex Steroids on Fish Leukocytes. Biology. 2018; 7(1):9. https://doi.org/10.3390/biology7010009
Chicago/Turabian StyleChaves-Pozo, Elena, Alfonsa García-Ayala, and Isabel Cabas. 2018. "Effects of Sex Steroids on Fish Leukocytes" Biology 7, no. 1: 9. https://doi.org/10.3390/biology7010009
APA StyleChaves-Pozo, E., García-Ayala, A., & Cabas, I. (2018). Effects of Sex Steroids on Fish Leukocytes. Biology, 7(1), 9. https://doi.org/10.3390/biology7010009





