The Cell as the First Niche Construction
Abstract
:1. Introduction
2. Compartmentalization of Physiologic Traits—Endosymbiosis
3. Niche Construction + Epigenetics = Evolution
4. Phenotypic Variation as Agency for Epigenetic Inheritance
5. Niche Construction + Epigenetics= Primacy of the Unicellular State
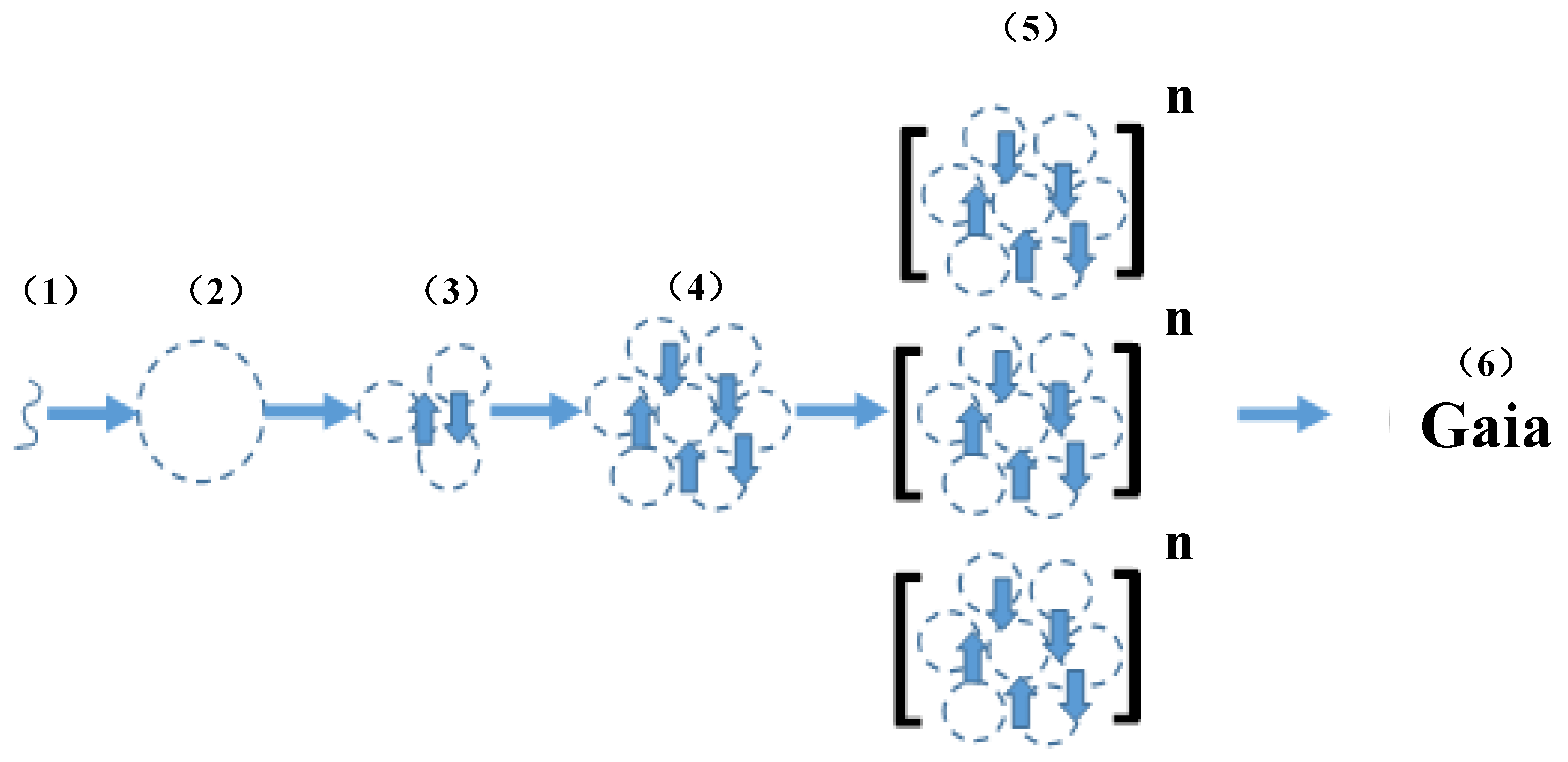
6. Gaia Theory = Niche Construction + Epigenetics
7. Niche Construction Controversy
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Torday, J.S. A central theory of biology. Med. Hypotheses 2015, 85, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Laland, K.N.; Odling-Smee, F.J.; Feldman, M.W. Evolutionary consequences of niche construction and their implications for ecology. Proc. Natl. Acad. Sci. USA 1999, 96, 10242–10247. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Vrba, E.S. Exaptation—A missing term in the science of form. Paleobiology 1982, 8, 4–15. [Google Scholar] [CrossRef]
- Mann, C. Lynn Margulis: Science’s Unruly Earth Mother. Science 1991, 252, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.; Odling-Smee, J. Integrating Ecology and Evolution: Niche Construction and Ecological Engineering; Springer Dordrecht: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Burggren, W.W. Epigenetics as a source of variation in comparative animal physiology—or—Lamarck is lookin’ pretty good these days. J. Exp. Biol. 2014, 217, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S. Evolutionary biology redux. Perspect. Biol. Med. 2013, 56, 455–484. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Dworkin, J.P.; Sandford, S.A.; Bernstein, M.P.; Allamandola, L.J. The first cell membranes. Astrobiology 2002, 2, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.; Allen, J.F.; Martin, W. How did LUCA make a living? Chemiosmosis in the origin of life. Bioessays 2010, 32, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, E. What Is LIFE—The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Torday, J.S. Homeostasis as the Mechanism of Evolution. Biology (Basel) 2015, 4, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Berner, R.A. Atmospheric oxygen over Phanerozoic time. Proc. Natl. Acad. Sci. USA 1999, 96, 10955–10957. [Google Scholar] [CrossRef] [PubMed]
- Romer, A.S. The Vertebrate Story; University of Chicago Press: Chicago, IL, USA, 1949. [Google Scholar]
- Falkowski, P.G.; Katz, M.E.; Milligan, A.J.; Fennel, K.; Cramer, B.S.; Aubry, M.P.; Berner, R.A.; Novacek, M.J.; Zapol, W.M. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science 2005, 309, 2202–2204. [Google Scholar] [CrossRef] [PubMed]
- Berner, R.A.; Vandenbrooks, J.M.; Ward, P.D. Evolution. Oxygen and evolution. Science 2007, 316, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Sammels, E.; Parys, J.B.; Missiaen, L.; de Smedt, H.; Bultynck, G. Intracellular Ca2+ storage in health and disease: A dynamic equilibrium. Cell Calcium 2010, 47, 297–314. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C. Evolution of the peroxisome. Annu. N. Y. Acad. Sci. 1969, 168, 369–381. [Google Scholar] [CrossRef]
- Torday, J.S.; Rehan, V.K. Evolutionary Biology, Cell-Cell Communication and Complex Disease; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Martin, W.F.; Sousa, F.L.; Lane, N. Evolution. Energy at life’s origin. Science 2014, 344, 1092–1093. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.; Dayhoff, M. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science 1998, 199, 395–403. [Google Scholar] [CrossRef]
- Lane, N.; Martin, W.F. The origin of membrane bioenergetics. Cell 2012, 151, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.L.; Thiergart, T.; Landan, G.; Nelson-Sathi, S.; Pereira, I.A.; Allen, J.F.; Lane, N.; Martin, W.F. Early bioenergetic evolution. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-W. What is (Schrödinger’s) Negentropy? Mod. Trends BiothermoKinetics 1994, 3, 50–61. [Google Scholar]
- Torday, J.S.; Rehan, V.K. Exploiting cellular-developmental evolution as the scientific basis for preventive medicine. Med. Hypotheses 2009, 72, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S. The cell as the mechanistic basis for evolution. Wiley Interdiscip. Rev. Syst. Biol. Med. 2015, 7, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.F.; Carrier, D.R. The coupled evolution of breathing and locomotion as a game of leapfrog. Physiol. Biochem. Zool. 2006, 79, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Miller, W.B., Jr. Phenotype as agent for epigenetic inheritance. Biology (Basel) 2016. submitted. [Google Scholar]
- Oró, J.; Kimball, A.P. Synthesis of purines under possible primitive earth conditions. I. Adenine from hydrogen cyanide. Arch. Biochem. Biophys. 1961, 94, 217–227. [Google Scholar] [CrossRef]
- Darwin, C. The Various Contrivances by Which Orchids Are Fenilised by Insects; John Murray: London, UK, 1877. [Google Scholar]
- Roux, E. The concept of function in modern physiology. J. Physiol. 2014, 592, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- Butler, S. Life and Habit; Dutton: London, UK, 1911. [Google Scholar]
- Pincheira-Donoso, D.; Hunt, J. Fecundity selection theory: Concepts and evidence. Biol. Rev. Camb. Philos. Soc. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stulp, G.; Barrett, L. Evolutionary perspectives on human height variation. Biol. Rev. Camb. Philos. Soc. 2016, 91, 206–234. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, J. Gaia as seen through the atmosphere (1967). Atmosph. Environ. 1972, 6, 579–514. [Google Scholar] [CrossRef]
- Lovelock, J.E.; Margulis, L. Atmospheric homeostasis by and for the biosphere: The gaia hypothesis. Tellus 1974, 26, 2–10. [Google Scholar] [CrossRef]
- Lenton, T.M. Gaia and natural selection. Nature 1998, 394, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Owen, T.; Cess, R.D.; Ramanathan, V. Earth: An enhanced carbon dioxide greenhouse to compensate for reduced solar luminosity. Nature 1979, 277, 640–642. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Biogeochemistry: An Analysis of Global Change; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Christiansen, E.H.; Hamblin, W.K. Dynamic Earth; Jones & Bartlett Learning: Burlington, MA, USA, 2014. [Google Scholar]
- Lovelock, J. The Vanishing Face of Gaia; Basic Books: New York, NY, USA, 2009. [Google Scholar]
- Torday, J.S.; Miller, W.B.J. Man is Integral with nature. Minding Nat. 2015, 8, 36–44. [Google Scholar]
- Barrow, J.D.; Tipler, F.J. The Anthropic Cosmological Principle; Oxford University Press: Oxford, UK, 1988. [Google Scholar]
- Heylighen, F. Stigmergy as a Universal Coordination Mechanism: Components, Varieties and Applications; Springer: New York, NY, USA, 2016. [Google Scholar]
- O’Malley, A.J.; Elwert, F.; Rosenquist, J.N.; Zaslavsky, A.M.; Christakis, N.A. Estimating peer effects in longitudinal dyadic data using instrumental variables. Biometrics 2014, 70, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Gould, S. Wonderful Life: The Burgess Shale and the Nature of History; W. W. Norton and Company: New York, NY, USA, 1990. [Google Scholar]
- Scott-Phillips, T.C.; Laland, K.N.; Shuker, D.M.; Dickins, T.E.; West, S.A. The niche construction perspective: A critical appraisal. Evolution 2014, 68, 1231–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laland, K.; Uller, T.; Feldman, M.; Sterelny, K.; Müller, G.B.; Moczek, A.; Jablonka, E.; Odling-Smee, J.; Wray, G.A.; Hoekstra, H.E.; et al. Does evolutionary theory need a rethink? Nature 2014, 514, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Bohr, N. The Quantum Postulate and the Recent Development of Atomic Theory. Nature 1928, 121, 580–590. [Google Scholar] [CrossRef]
- Nicholson, D.J. The machine conception of the organism in development and evolution: A critical analysis. Stud. Hist. Philos. Biol. Biomed. Sci. 2014, 48, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T. The Structure of Scientific Revolutions; The University of Chicago Press: Chicago, IL, USA, 1962. [Google Scholar]
- Torday, J.S. Pleiotropy as the mechanism for evolving novelty: Same signal, different result. Biology (Basel) 2015, 4, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S. Heterochrony as Diachronically Modified Cell-Cell Interactions. Biology (Basel) 2016, 5. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torday, J.S. The Cell as the First Niche Construction. Biology 2016, 5, 19. https://doi.org/10.3390/biology5020019
Torday JS. The Cell as the First Niche Construction. Biology. 2016; 5(2):19. https://doi.org/10.3390/biology5020019
Chicago/Turabian StyleTorday, John S. 2016. "The Cell as the First Niche Construction" Biology 5, no. 2: 19. https://doi.org/10.3390/biology5020019
APA StyleTorday, J. S. (2016). The Cell as the First Niche Construction. Biology, 5(2), 19. https://doi.org/10.3390/biology5020019




