The Mucosal Immune System of Teleost Fish
Abstract
:1. Introduction
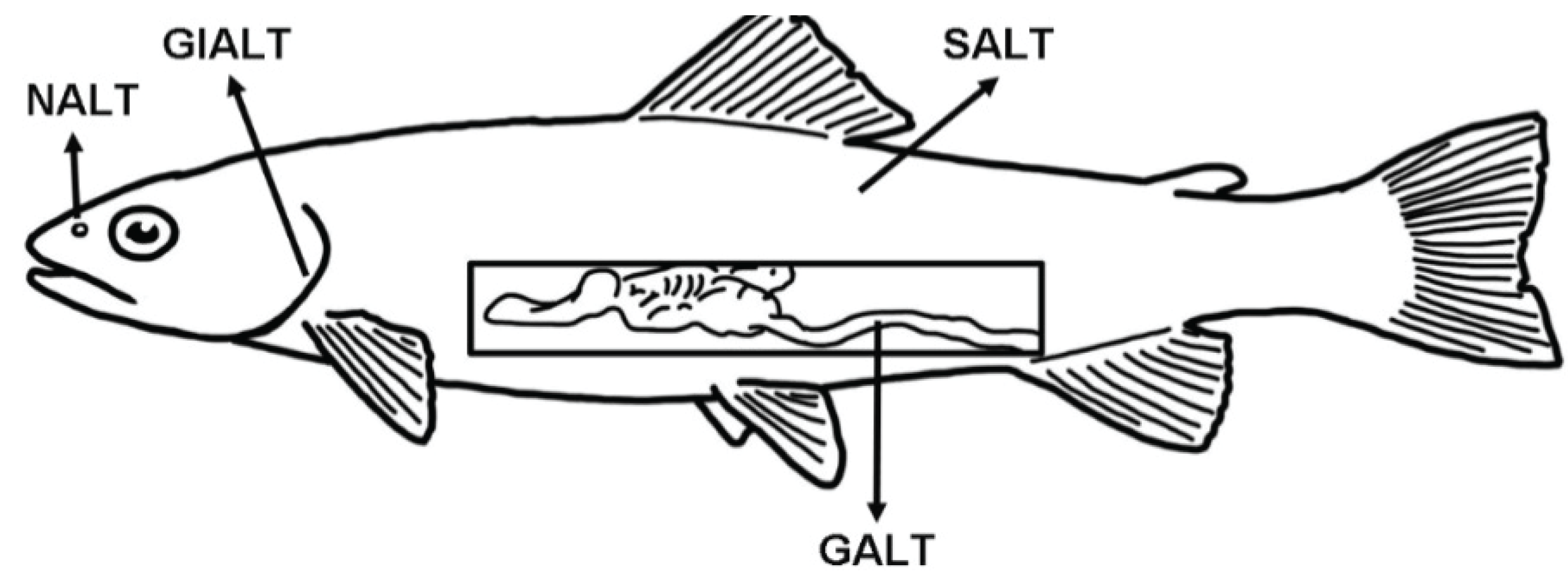
2. General Aspects of Teleost MALT Anatomy
| Characteristic | GALT | SALT | GIALT | NALT |
|---|---|---|---|---|
| Anatomical localization | Intestine | Skin | Gills | Olfactory organ |
| Organization | Diffuse only | Diffuse | Diffuse with one organized tissue in salmon (ILT) | Diffuse |
| Presence of goblet cells | Yes | Yes | Yes | Yes |
| Total % of B cells | 4-5% | 4-5% | ? | 35%–40% |
| Approximate IgT/IgM B cell ratio | 1:1 | 1:1 | ? | 1:1 |
| Expression of pIgR (protein level) | Yes | Yes | ? | Yes |
| Compartmentalized specific IgT responses against pathogens (protein level) | Yes | Yes | Not demonstrated | Not demonstrated |
| Abundant T cells | Yes | Yes | Yes | ? |
| Presence of bacterial microbiota | Yes | Yes | Yes | Yes |
| Microbiota coated by secretory immunoglobulins | Yes | Yes | ? | Yes |
3. Teleost Mucosal B Cells and Immunoglobulins
4. Teleost Mucosal T Cells
4.1. Mucosal CD8 T Cells
4.2. Mucosal CD4 T Cells
5. Adaptive Mucosal Immune Responses of Teleost Fish
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cooper, M.D.; Alder, M.N. The evolution of adaptive immune systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Kiyono, H. A marvel of mucosal T cells and secretory antibodies for the creation of first lines of defense. Cell. Mol. Life Sci. 2005, 62, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, K.Y.; Jang, Y.S. Mucosal immune system and M cell-targeting strategies for oral mucosal vaccination. Immune Netw. 2012, 12, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Duff, D.C.B. The oral immunization of trout against bacterium salmonicida. J. Immunol. 1942, 44, 87–94. [Google Scholar]
- Fletcher, T.C.; White, A. Antibody production in the plaice (Pleuronectes platessa L.) after oral and parenteral immunization with Vibrio anguillarum antigens. Aquaculture 1972, 1, 417–428. [Google Scholar] [CrossRef]
- Lobb, C.J.; Clem, L.W. Phylogeny of immunoglobulin structure and function. XI. Secretory immunoglobulins in the cutaneous mucus of the sheepshead, Archosargus probatocephalus. Dev. Comp. Immunol. 1981, 5, 587–596. [Google Scholar] [CrossRef]
- Lobb, C.J. Secretory immunity induced in catfish, Ictalurus punctatus, following bath immunization. Dev. Comp. Immunol. 1987, 11, 727–738. [Google Scholar] [CrossRef]
- Rombout, J.H.; Taverne, N.; van de Kamp, M.; Taverne-Thiele, A.J. Differences in mucus and serum immunoglobulin of carp (Cyprinus carpio L.). Dev. Comp. Immunol. 1993, 17, 309–317. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Salinas, I.; Sunyer, J.O. Recent findings on the structure and function of teleost IgT. Fish Shellfish Immunol. 2011, 31, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.H.; Yang, G.; Kiron, V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish Shellfish Immunol. 2014, 40, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S.; Six, A.; Magadan, S.; Castro, R.; Sunyer, J.O.; Boudinot, P. The astonishing diversity of Ig classes and B cell repertoires in teleost fish. Front. Immunol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Pettinello, R.; Dooley, H. The immunoglobulins of cold-blooded vertebrates. Biomolecules 2014, 4, 1045–1069. [Google Scholar] [CrossRef] [PubMed]
- Haugarvoll, E.; Bjerkas, I.; Nowak, B.F.; Hordvik, I.; Koppang, E.O. Identification and characterization of a novel intraepithelial lymphoid tissue in the gills of Atlantic salmon. J. Anat. 2008, 213, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Koppang, E.O.; Fischer, U.; Moore, L.; Tranulis, M.A.; Dijkstra, J.M.; Köllner, B.; Aune, L.; Jirillo, E.; Hordvik, I. Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J. Anat. 2010, 217, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Aas, I.B.; Austbø, L.; König, M.; Syed, M.; Falk, K.; Hordvik, I.; Koppang, E.O. Transcriptional characterization of the T cell population within the salmonid interbranchial lymphoid tissue. J. Immunol. 2014, 193, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Kiyono, H.; Pabst, R.; Russell, M.W. Terminology: Nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I.; Zhang, Y.A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef] [PubMed]
- Huttenhuis, H.B.; Romano, N.; van Oosterhoud, C.N.; Taverne-Thiele, A.J.; Mastrolia, L.; van Muiswinkel, W.B.; Rombout, J.H. The ontogeny of mucosal immune cells in common carp (Cyprinus carpio L.). Anat. Embryol. 2006, 211, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Flajnik, M. All GOD’s creatures got dedicated mucosal immunity. Nat. Immunol. 2010, 11, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Fagarasan, S.; Kawamoto, S.; Kanagawa, O.; Suzuki, K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2009, 28, 243–273. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.-E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Parra, D.; Gómez, D.; Salinas, I.; Zhang, Y.A.; von Gersdorff Jørgensen, L.; Heinecke, R.D.; Buchmann, K.; LaPatra, S.; Sunyer, J.O. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef] [PubMed]
- Tacchi, L.; Musharrafieh, R.; Larragoite, E.T.; Crossey, K.; Erhardt, E.B.; Martin, S.A.M.; LaPatra, S.E.; Salinas, I. Nasal immunity is an ancient arm of the mucosal immune system of vertebrates. Nat. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.H.; Joosten, P.H.; Engelsma, M.Y.; Vos, A.P.; Taverne, N.; Taverne-Thiele, J.J. Indications for a distinct putative T cell population in mucosal tissue of carp (Cyprinus carpio L.). Dev. Comp. Immunol. 1998, 22, 63–77. [Google Scholar] [CrossRef]
- Ramirez-Gomez, F.; Greene, W.; Rego, K.; Hansen, J.D.; Costa, G.; Kataria, P.; Bromage, E.S. Discovery and characterization of secretory IgD in rainbow trout: Secretory IgD is produced through a novel splicing mechanism. J. Immunol. 2012, 188, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Bromage, E.S.; Kaattari, I.M.; Zwollo, P.; Kaattari, S.L. Plasmablast and plasma cell production and distribution in trout immune tissues. J. Immunol. 2004, 173, 7317–7323. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Sun, B.; Luo, Y.-P.; Zhang, Y.; Ni, P. Distribution of IgM, IgD and IgZ in mandarin fish, Siniperca chuatsi lymphoid tissues and their transcriptional changes after Flavobacterium columnare stimulation. Aquaculture 2009, 288, 14–21. [Google Scholar] [CrossRef]
- Toda, H.; Saito, Y.; Koike, T.; Takizawa, F.; Araki, K.; Yabu, T.; Somamoto, T.; Suetake, H.; Suzuki, Y.; Ototake, M.; et al. Conservation of characteristics and functions of CD4 positive lymphocytes in a teleost fish. Dev. Comp. Immunol. 2011, 35, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Bernard, D.; Lefranc, M.P.; Six, A.; Benmansour, A.; Boudinot, P. T cell diversity and TcR repertoires in teleost fish. Fish Shellfish Immunol. 2011, 31, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, F.; Dijkstra, J.M.; Kotter, P.; Korytáˇr, T.; Kock, H.; Köllner, B.; Jaureguiberry, B.; Nakanishi, T.; Fischer, U. The expression of CD8 discriminates distinct T cell subsets in teleost fish. Dev. Comp. Immunol. 2011, 35, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Boardman, T.; Warner, C.; Ramirez-Gomez, F.; Matrisciano, J.; Bromage, E. Characterization of an anti-rainbow trout (Oncorhynchus mykiss) CD3ε monoclonal antibody. Vet. Immunol. Immunopathol. 2012, 145, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Nuñez Ortiz, N.; Gerdol, M.; Stocchi, V.; Marozzi, C.; Randelli, E.; Bernini, C.; Buonocore, F.; Picchietti, S.; Papeschi, C.; Sood, N.; et al. T cell transcripts and T cell activities in the gills of the teleost fish sea bass (Dicentrarchus labrax). Dev. Comp. Immunol. 2014, 47, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Umesaki, Y.; Honda, K. Microbiotal influence on T cell subset development. Semin. Immunol. 2011, 23, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Abelli, L.; Picchietti, S.; Romano, N.; Mastrolia, L.; Scapigliati, G. Immunohistochemistry of gut-associated lymphoid tissue of the sea bass Dicentrarchus labrax (L.). Fish Shellfish Immunol. 1997, 7, 235–245. [Google Scholar] [CrossRef]
- Araki, K.; Suetake, H.; Kikuchi, K.; Suzuki, Y. Characterization and expression analysis of CD3ε and CD3γ/δ in fugu, Takifugu rubripes. Immunogenetics 2005, 57, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.; Six, A.; Rigottier-Gois, L.; Messiaen, S.; Chilmonczyk, S.; Quillet, E.; Boudinot, P.; Benmansour, A. Phenotypic and functional similarity of gut intraepithelial and systemic T cells in a teleost fish. J. Immunol. 2006, 176, 3942–3949. [Google Scholar] [CrossRef] [PubMed]
- Picchietti, S.; Guerra, L.; Bertoni, F.; Randelli, E.; Belardinelli, M.C.; Buonocore, F.; Fausto, A.M.; Rombout, J.H.; Scapigliati, G.; Abelli, L. Intestinal T cells of Dicentrarchus labrax (L.): Gene expression and functional studies. Fish Shellfish Immunol. 2011, 30, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Trichet, V.V.; Legrand-Frossi, C.; Frippiat, J.-P. Comparison between intestinal and non-mucosal immune functions of rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol. 2013, 33, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Chettri, J.K.; Raida, M.K.; Kania, P.W.; Buchmann, K. Differential immune response of rainbow trout (Oncorhynchus mykiss) at early developmental stages (larvae and fry) against the bacterial pathogen Yersinia ruckeri. Dev. Comp. Immunol. 2012, 36, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Kania, P.; Larsen, T.B.; Ingerslev, H.C.; Buchmann, K. Baltic salmon activates immune relevant genes in fin tissue when responding to Gyrodactylus salaris infection. Dis. Aquat. Org. 2007, 76, 81–85. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.N.; Secombes, C.J. Isolation of rainbow trout (Oncorhynchus mykiss) intestinal intraepithelial lymphocytes (IEL) and measurement of their cytotoxic activity. Fish Shellfish Immunol. 1997, 7, 527–541. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, G.L.; Fu, J.P.; Nie, P. Characterization and expression of CD8 molecules in mandarin fish Siniperca chuatsi. J. Fish Biol. 2013, 82, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Goodman, T.; Lefrançois, L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature 1988, 333, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Viney, J.; MacDonald, T.T.; Spencer, J. Gamma/delta T cells in the gut epithelium. Gut 1990, 31, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Bergstresser, P.R.; Sullivan, S.; Streilein, J.W.; Tigelaar, R.E. Origin and function of Thy-1+ dendritic epidermal cells in mice. J. Investig. Dermatol. 1985, 85, 85s–90s. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, F.; Castro, R.; Randelli, E.; Lefranc, M.P.; Six, A.; Kuhl, H.; Reinhardt, R.; Facchiano, A.; Boudinot, P.; Scapigliati, G. Diversity, molecular characterization and expression of T cell receptor G in a teleost fish, the sea bass (Dicentrarchus labrax, L). PLoS ONE 2012, 7, e47957. [Google Scholar] [CrossRef] [PubMed]
- Brugman, S.; Witte, M.; Scholman, R.C.; Klein, M.R.; Boes, M.; Nieuwenhuis, E.E.S. T lymphocyte-dependent and -independent regulation of cxcl8 expression in Zebrafish intestines. J. Immunol. 2014, 192, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, F.; Koppang, E.O.; Ohtani, M.; Nakanishi, T.; Hashimoto, K.; Fischer, U.; Dijkstra, J.M. Constitutive high expression of interleukin-4/13A and GATA-3 in gill and skin of salmonid fishes suggests that these tissues form Th2-skewed immune environments. Mol. Immunol. 2011, 48, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Allez, M.; Mayer, L. Regulatory T cells. Peace keepers in the gut. Inflamm. Bowel Dis. 2006, 10, 666–676. [Google Scholar] [CrossRef]
- Van Wijk, F.; Cheroutre, H. Intestinal T cells: Facing the mucosal immune dilemma with synergy and diversity. Semin. Immunol. 2009, 21, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wei, H.; Zhao, T.; Wang, X.; Zhang, A.; Zhou, H. Characterization of Foxp3 gene from grass carp (Ctenopharyngodon idellus): A rapamycin-inducible transcription factor in teleost immune system. Dev. Comp. Immunol. 2012, 38, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Monte, M.M.; Huang, W.; Boudinot, P.; Martin, S.A.M.; Secombes, C.J. Identification of two FoxP3 genes in rainbow trout (Oncorhynchus mykiss) with differential induction patterns. Mol. Immunol. 2010, 47, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C.W.; Santacruz, N.; Peterson, D.A.; Stappenbeck, T.S.; Hsieh, C.S. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011, 478, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Holmkvist, P.; Roepstorff, K.; Uronen-Hansson, H.; Sandén, C.; Gudjonsson, S.; Patschan, O.; Grip, O.; Marsal, J.; Schmidtchen, A.; Hornum, L.; et al. A major population of mucosal memory CD4+ T cells, coexpressing IL-18Rα and DR3, display innate lymphocyte functionality. Mucosal Immunol. 2015, 8, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.L.; Farber, D.L. Mucosal resident memory CD4 T cells in protection and immunopathology. Front. Immunol. 2014, 5, 331. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Turner, D.; Pham, Q.; Wherry, E.J.; Lefrancois, L.; Farber, D.L. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011, 187, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Plant, K.P.; LaPatra, S.E. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Raida, M.K.; Buchmann, K. Bath vaccination of rainbow trout (Oncorhynchus mykiss Walbaum) against Yersinia ruckeri: Effects of temperature on protection and gene expression. Vaccine 2008, 26, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.M.; Kania, P.W.; Heinecke, R.D.; Skjoedt, K.; Rasmussen, K.J.; Buchmann, K. Cellular and humoral factors involved in the response of rainbow trout gills to Ichthyophthirius multifiliis infections: Molecular and immunohistochemical studies. Fish Shellfish Immunol. 2011, 30, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, R.D.; Buchmann, K. Inflammatory response of rainbow trout Oncorhynchus mykiss (Walbaum, 1792) larvae against Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2013, 34, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Makesh, M.; Ponnerassery, S.S.; Cain, K.D. Systemic and mucosal immune response of rainbow trout to immunization with an attenuated Flavobacterium psychrophilum vaccine strain by different routes. Fish Shellfish Immunol. 2015, 44, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Austbø, L.; Aas, I.B.; König, M.; Chioma Weli, S.; Syed, M.; Falk, K.; Koppang, E.O. Transcriptional response of immune genes in gills and the interbranchial lymphoid tissue of Atlantic salmon challenged with infectious salmon anaemia virus. Dev. Comp. Immunol. 2014, 45, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, N.A.; Rodríguez-Saint-Jean, S.; Pérez-Prieto, S.I.; Aquilino, C.; Tafalla, C. Modulation of genes related to the recruitment of immune cells in the digestive tract of trout experimentally infected with infectious pancreatic necrosis virus (IPNV) or orally vaccinated. Dev. Comp. Immunol. 2014, 44, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Aquilino, A.; Castro, R.; Fischer, U.; Tafalla, C. Transcriptomic responses in rainbow trout gills upon infection with viral hemorrhagic septicemia virus (VHSV). Dev. Comp. Immunol. 2014, 44, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.; Takano, T.; Sakai, T.; Matsuyama, T.; Nakayasu, C. Vibrio anguillarum bacterin uptake via the gills of Japanese flounder and subsequent immune responses. Fish Shellfish Immunol. 2013, 35, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Norte dos Santos, C.C.; Adams, M.B.; Leef, M.J.; Nowak, B.F. Changes in the interbranchial lymphoid tissue of Atlantic salmon (Salmo salar) affected by amoebic gill disease. Fish Shellfish Immunol. 2014, 41, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Chettri, J.K.; Kuhn, J.A.; Jaafar, R.M.; Kania, P.W.; Møller, O.S.; Buchmann, K. Epidermal response of rainbow trout to Ichthyobodo necator: Immunohistochemical and gene expression studies indicate a Th1-/Th2-like switch. J. Fish Dis. 2014, 37, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Tadiso, T.M.; Krasnov, A.; Skugor, S.; Afanasyev, S.; Hordvik, I.; Nilsen, F. Gene expression analyses of immune responses in Atlantic salmon during early stages of infection by salmon louse (Lepeophtheirus salmonis) revealed bi-phasic responses coinciding with the copepod-chalimus transition. BMC Genom. 2011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, B.; Wu, H.; Gao, L.; Liu, Q.; Wang, Q.; Xiao, J.; Zhang, Y. Th17-like immune response in fish mucosal tissues after administration of live attenuated Vibrio anguillarum via different vaccination routes. Fish Shellfish Immunol. 2014, 37, 229–238. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas, I. The Mucosal Immune System of Teleost Fish. Biology 2015, 4, 525-539. https://doi.org/10.3390/biology4030525
Salinas I. The Mucosal Immune System of Teleost Fish. Biology. 2015; 4(3):525-539. https://doi.org/10.3390/biology4030525
Chicago/Turabian StyleSalinas, Irene. 2015. "The Mucosal Immune System of Teleost Fish" Biology 4, no. 3: 525-539. https://doi.org/10.3390/biology4030525
APA StyleSalinas, I. (2015). The Mucosal Immune System of Teleost Fish. Biology, 4(3), 525-539. https://doi.org/10.3390/biology4030525





