Antibody Affinity Maturation in Fishes—Our Current Understanding
Abstract
:1. Introduction
2. Earlier Research—Fishes Have Somatic Hypermutation of Ig Genes
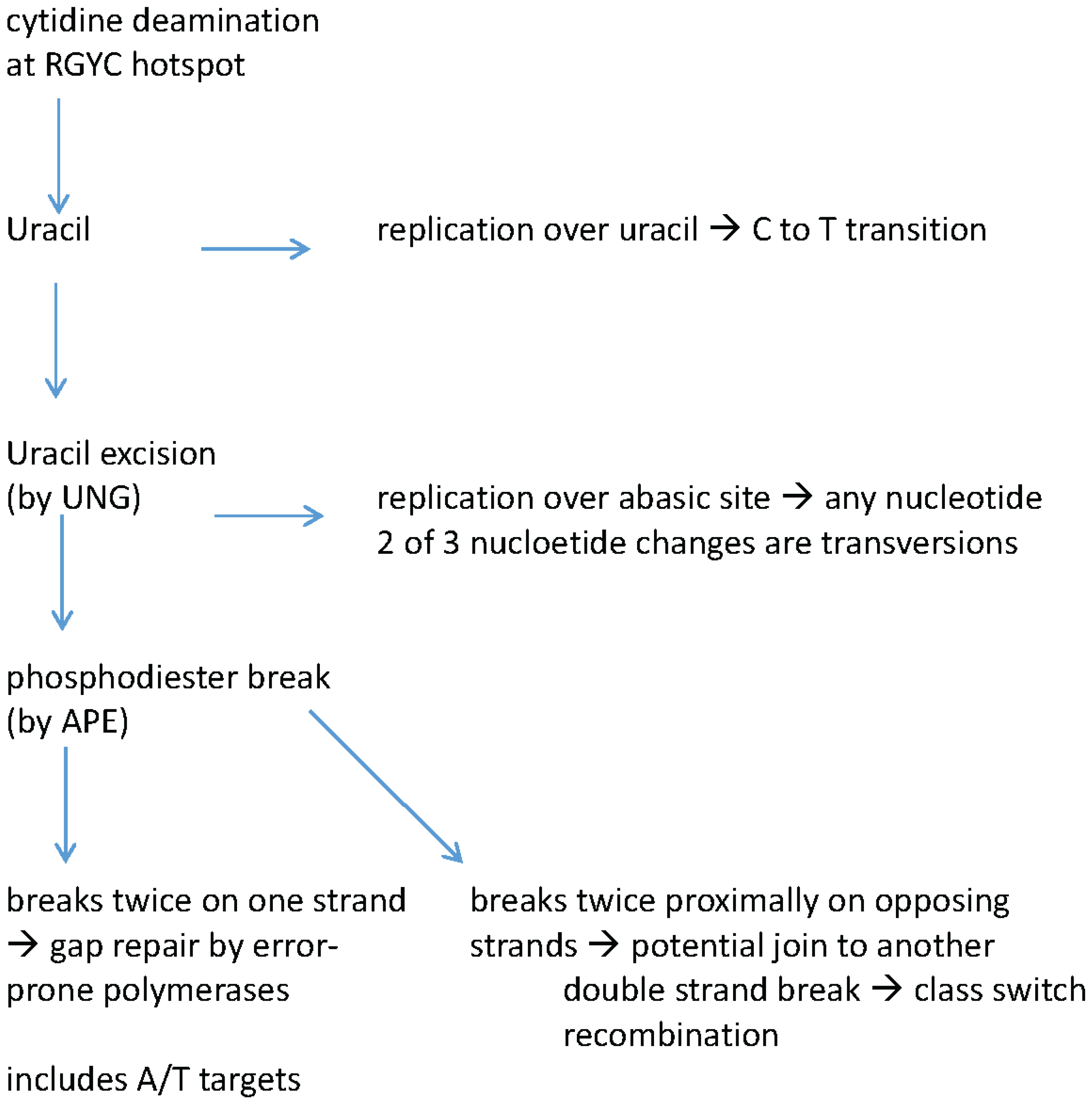
3. Recent Work on Teleost Fish
3.1. Antibody Affinity Measurements
3.2. Fish Have a Fully Functional Ig Mutator Enzyme—AID
3.3. Somatic Hypermutation Patterns in Fish Immunoglobulins
| Species | Gene Analyzed | # & Types of Substitutions | Nucleotide Bias a | % That are Transitions | % in RGYW Hotspot b | Lineage Development | Reference |
|---|---|---|---|---|---|---|---|
| channel catfish | VH | 459 | 58% GC | 60 | 47 | Yes | [37] |
| zebrafish | VL | 93 | 59% GCc | 85 | 49 | Yes | [38] |
| nurse shark | VH | 78 tandem | 56% AT | 36 | 39 | No | [39] |
| nurse shark | VH | 53 singlet | 57% GC | 53 | 39 | No | [39] |
| nurse shark | VL | 338 tandem | No | 38 | 42 | Yes | [40] |
| nurse shark | VL | 293 singlet | No | 58 | 43 | Yes | [40] |
| nurse shark | VL | 245 tandem d | 59% AT | 31 | 46 | ? | [41] |
| nurse shark | VL | 187 singlet d | No | 55 | 46 | ? | [39] |
| nurse shark | VNAR | 231 synonymous | No | 62 | ? | ? | [5] |
| nurse shark | VNAR | 523 | No | 39 | ? | ? | [3,42] |
3.4. A Cellular Context for Generation of Somatic Hypermutations
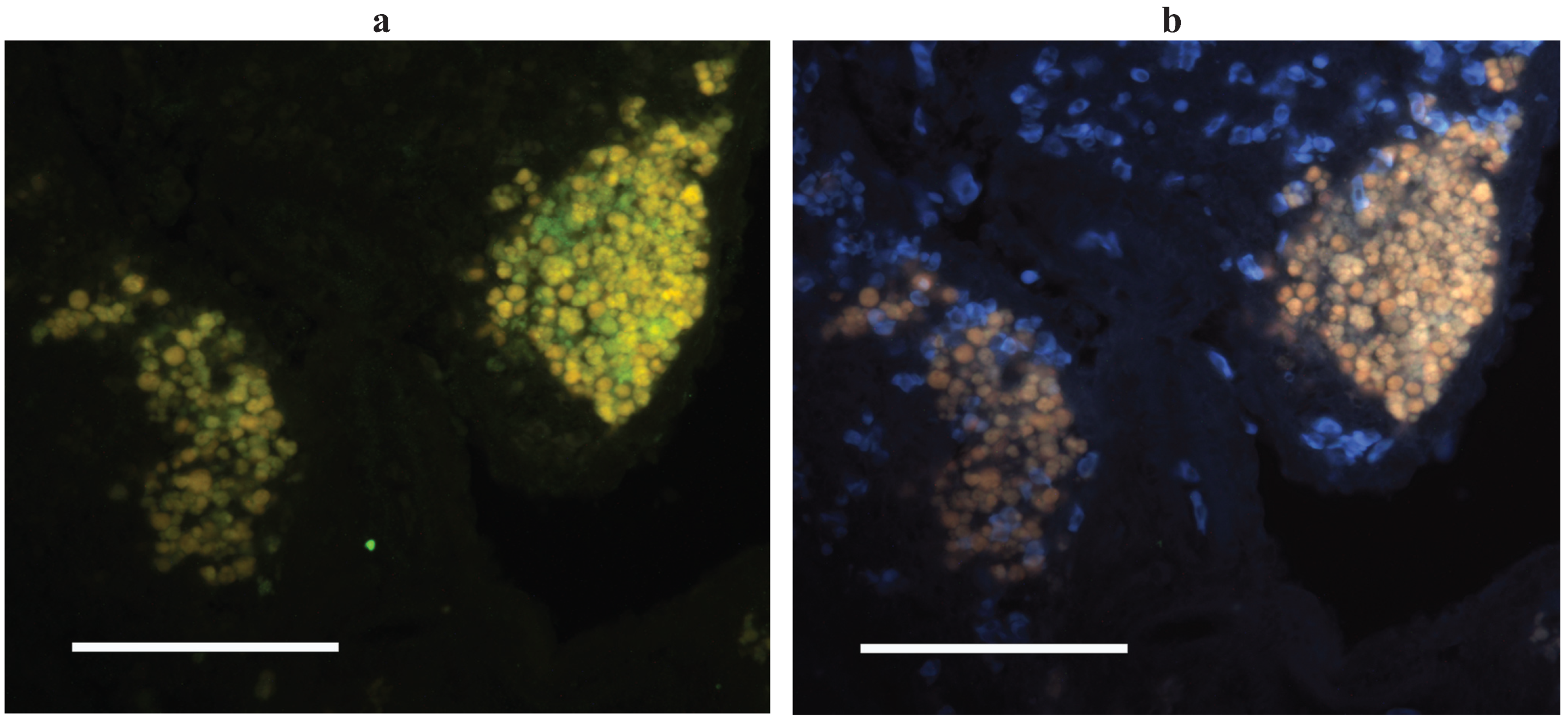
3.5. Why is Affinity Maturation so Poor in Fish?
3.6. Issues to Resolve around Antibody Affinity Maturation in Fishes
4. Conclusions
- 1)
- In both fish and elasmobranches there is accumulation of somatic point mutations in V(D)J exons with preferential targeting of the canonical hotspot motif RGYW.
- 2)
- There is evidence for development of mutation lineages consistent with a hypermutation process occurring in proliferating B-cells.
- 3)
- There are some clear differences in how mutations in the fish and elasmobranches are resolved. Fish have limited mutations in A:T while in sharks there is evidence for gap repair by error prone polymerases (G:C and A:T mutations) as well as a propensity to develop tandem point mutations.
- 4)
- The fish Ig mutator AID is found to be fully functional for SHM and CSR though enzyme kinetics vary with species and temperature. Furthermore all regulatory aspects of fish AID expression and sub-cellular localization studied to date are also functionally conserved with AID from homeotherms.
- 5)
- Recent evidence from sharks indicates that a form of CSR (class switch recombination) developed among the earliest divergent gnathostomes.
- 6)
- In early gnathostomes the actual protein affinities are not raised to the extent seen in homeotherms though this may relate either to differences in affinity maturation kinetics or in the manner by which cells undergo selection. This remains one of the outstanding issues to be resolved.
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Wang, J.H. Generation and repair of AID-initiated DNA lesions in B lymphocytes. Front. Med. 2014, 8, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Hsu, E.; Marcuz, A.; Courtet, M.; du Pasquier, L.; Steinberg, C. What limits affinity maturation of antibodies in Xenopus—The rate of somatic mutation or the ability to select mutants? EMBO J. 1992, 11, 4337–4347. [Google Scholar] [PubMed]
- Greenberg, A.S.; Avila, D.; Hughes, M.; Hughes, A.; McKinney, E.C.; Flajnik, M.F. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature 1995, 374, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Greenberg, A.; Flajnik, M. Somatic hypermutation of the new antigen receptor gene (NAR) in the nurse shark does not generate the repertoire: Possible role in antigen-driven reactions in the absence of germinal centers. Proc. Natl. Acad. Sci. USA 1998, 95, 14343–14348. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Velez, J.; Singh, M.; Cerny, J.; Flajnik, M. Mutational pattern of the nurse shark antigen receptor gene (NAR) is similar to that of mammalian Ig genes and to spontaneous mutations in evolution: The translesion synthesis model of somatic hypermutation. Int. Immunol. 1999, 11, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, O.; Litman, G.W. Lack of heterogeneity in antihapten antibodies of a phylogenetically primitive shark. Nature 1980, 287, 639–640. [Google Scholar] [CrossRef] [PubMed]
- Clem, L.W.; Leslie, G.A. Production of 19S IgM antibodies with restricted heterogeneity from sharks. Proc. Natl. Acad. Sci. USA 1971, 68, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Lamers, C.H.; de Haas, M.J. Antigen localization in the lymphoid organs of carp (Cyprinus carpio). Cell Tissue Res. 1985, 242, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A. Antigen-trapping in the spleen and kidney of the plaice Pleuronectes platessa L. J. Fish Dis. 1980, 3, 413–426. [Google Scholar] [CrossRef]
- Fulop, G.M.; McMillan, D.B. Phagocytosis in the spleen of the sunfish Lepomis spp. J. Morphol. 1984, 179, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Press, C.; Evensen, Ø.; Reitan, L.; Landsverk, T. Retention of furunculosis vaccine components in Atlantic salmon, Salmo salar L., following different routes of vaccine administration. J. Fish Dis. 1996, 19, 215–224. [Google Scholar] [CrossRef]
- Falk, K.; Dannevig, B. Demonstration of a protective immune response in infectious salmon anaemia (ISA)-infected Atlantic salmon Salmo salar. Dis. Aquat. Org. 1995, 21, 1–5. [Google Scholar] [CrossRef]
- Fournier-Betz, V.; Quentel, C.; Lamour, F.; LeVen, A. Immunocytochemical detection of Ig-positive cells in blood, lymphoid organs and the gut associated lymphoid tissue of the turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2000, 10, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Cain, K.; Jones, D.; Raison, R. Antibody-antigen kinetics following immunization of rainbow trout (Oncorhynchus mykiss) with a T-cell dependent antigen. Dev. Comp. Immunol. 2002, 26, 181–190. [Google Scholar] [CrossRef]
- Kaattari, S.; Zhang, H.; Khor, I.; Kaattari, I.; Shapiro, D. Affinity maturation in trout: Clonal dominance of high affinity antibodies late in the immune response. Dev. Comp. Immunol. 2002, 26, 191–200. [Google Scholar] [CrossRef]
- Ye, J.; Bromage, E.S.; Kaattari, S.L. The strength of B cell interaction with antigen determines the degree of IgM polymerization. J. Immunol. 2010, 184, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Bromage, E.S.; Ye, J.; Kaattari, S.L. Antibody structural variation in rainbow trout fluids. Comp. Biochem. Physiol. Part B 2006, 143, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Sankaranand, V.S.; Anant, S.; Sugai, M.; Kinoshita, K.; Davidson, N.O.; Honjo, T. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J. Biol Chem. 1999, 274, 18470–18476. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Rada, C.; Neuberger, M. The in vivo pattern of AID targeting to immunoglobulin switch regions deduced from mutation spectra in msh2−/− ung−/− mice. J. Exp. Med. 2006, 203, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Di Noia, J.M.; Williams, G.T.; Chan, D.T.Y.; Buerstedde, J.M.; Baldwin, G.S.; Neuberger, M.S. Dependence of antibody gene diversification on uracil excision. J. Exp. Med. 2007, 204, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Di Noia, J.; Neuberger, M. Molecular mechanisms of antibody somatic hypermutation. Annu. Rev. Biochem. 2007, 76, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; du Pasquier, L.; Hsu, E. Shark IgW C region diversification through RNA processing and isotype switching. J. Immunol. 2013, 191, 3410–3418. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lee, V.; Finn, A.; Senger, K.; Zarrin, A.A.; du Pasquier, L.; Hsu, E. Origin of Immunoglobulin Isotype Switching. Curr. Biol. 2012, 22, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Saunders, H.L.; Magor, B.G. Cloning and expression of the AID gene in the channel catfish. Dev. Comp. Immunol. 2004, 28, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pan-Hammarström, Q.; Zhao, Z.; Hammarström, L. Identification of the activation-induced cytidine deaminase gene from zebrafish: An evolutionary analysis. Dev. Comp. Immunol. 2005, 29, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Barreto, V.M.; Magor, B.G. Activation-induced cytidine deaminase structure and functions: A species comparative view. Dev. Comp. Immunol. 2011, 35, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Barreto, V.M.; Pan-Hammarstrom, Q.; Zhao, Y.; Hammarstrom, L.; Misulovin, Z.; Nussenzweig, M.C. AID from bony fish catalyzes class switch recombination. J. Exp. Med. 2005, 202, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Wakae, K.; Magor, B.G.; Saunders, H.; Nagaoka, H.; Kawamura, A.; Kinoshita, K.; Honjo, T.; Muramatsu, M. Evolution of class switch recombination function in fish activation-induced cytidine deaminase, AID. Int. Immunol. 2006, 18, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dancyger, A.M.; King, J.J.; Quinlan, M.J.; Fifield, H.; Tucker, S.; Saunders, H.L.; Berru, M.; Magor, B.G.; Martin, A.; Larijani, M. Differences in the enzymatic efficiency of human and bony fish AID are mediated by a single residue in the C terminus modulating single-stranded DNA binding. FASEB J. 2012, 26, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Saunders, H.L.; Oko, A.L.; Scott, A.N.; Fan, C.W.; Magor, B.G. The cellular context of AID expressing cells in fish lymphoid tissues. Dev. Comp. Immunol. 2010, 34, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Villota-Herdoiza, D.; Pila, E.A.; Quiniou, S.; Waldbieser, G.C.; Magor, B.G. Transcriptional regulation of teleost Aicda genes. Part 1—Suppressors of promiscuous promoters. Fish Shellfish Immunol. 2013, 35, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Pila, E.A. Transcriptional regulation of Zebrafish “Aicda”. M.Sc. Thesis, University of Alberta, Edmonton, AB, Canada, 2012. [Google Scholar]
- Basu, U.; Wang, Y.; Alt, F. Evolution of phosphorylation-dependent regulation of activation-induced cytidine deaminase. Mol. Cell 2008, 32, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Geisberger, R.; Rada, C.; Neuberger, M.S. The stability of AID and its function in class-switching are critically sensitive to the identity of its nuclear-export sequence. Proc. Natl. Acad. Sci. USA 2009, 106, 6736–6741. [Google Scholar] [CrossRef] [PubMed]
- Methot, S.P.; Litzler, L.C.; Trajtenberg, F.; Zahn, A.; Robert, F.; Pelletier, J.; Buschiazzo, A.; Magor, B.G.; di Noia, J.M. Consecutive interactions with HSP90 and eEF1A underlie a functional maturation and storage pathway of AID in the cytoplasm. J. Exp. Med. 2015, 212, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Kuppers, R.; Zhao, M.; Hansmann, M.L.; Rajewsky, K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993, 12, 4955–4967. [Google Scholar] [PubMed]
- Yang, F.; Waldbieser, G.; Lobb, C. The nucleotide targets of somatic mutation and the role of selection in immunoglobulin heavy chains of a teleost fish. J. Immunol. 2006, 176, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Marianes, A.E.; Zimmerman, A.M. Targets of somatic hypermutation within immunoglobulin light chain genes in zebrafish. Immunology 2010, 132, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Malecek, K.; Brandman, J.; Brodsky, J.; Ohta, Y.; Flajnik, M.; Hsu, E. Somatic hypermutation and junctional diversification at Ig heavy chain loci in the nurse shark. J. Immunol. 2005, 175, 8105–8115. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Tranchina, D.; Ohta, Y.; Flajnik, M.; Hsu, E. Hypermutation in shark immunoglobulin light chain genes results in contiguous substitutions. Immunity 2002, 16, 571–582. [Google Scholar] [CrossRef]
- Zhu, C.; Hsu, E. Error-prone DNA repair activity during somatic hypermutation in shark B lymphocytes. J. Immunol. 2010, 185, 5336–5347. [Google Scholar] [CrossRef] [PubMed]
- Du Pasquier, L.; Wilson, M.; Greenberg, A.S.; Flajnik, M.F. Somatic mutation in ectothermic vertebrates: Musings on selection and origins. Curr. Top. Microbiol. Immunol. 1998, 229, 199–216. [Google Scholar] [PubMed]
- Weller, S.; Mamani-Matsuda, M.; Picard, C.; Cordier, C.; Lecoeuche, D.; Gauthier, F.; Weill, J.C.; Reynaud, C.A. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J. Exp. Med. 2008, 205, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Mehr, R.; Edelman, H.; Sehgal, D.; Mage, R. Analysis of mutational lineage trees from sites of primary and secondary Ig gene diversification in rabbits and chickens. J. Immunol. 2004, 172, 4790–4796. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Kennedy, L.J.; McCullagh, P.; Reynolds, J.D. A new model of sheep Ig diversification: shifting the emphasis toward combinatorial mechanisms and away from hypermutation. J. Immunol. 2003, 170, 3739–3750. [Google Scholar] [CrossRef] [PubMed]
- Liljavirta, J.; Ekman, A.; Knight, J.S.; Pernthaner, A.; Iivanainen, A.; Niku, M. Activation-induced cytidine deaminase (AID) is strongly expressed in the fetal bovine ileal Peyer’s patch and spleen and is associated with expansion of the primary antibody repertoire in the absence of exogenous antigens. Mucosal Immunol. 2013, 6, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kshirsagar, S.; Jensen, I.; Lau, K.; Covarrubias, R.; Schluter, S.F.; Marchalonis, J.J. Characterization of arrangement and expression of the T cell receptor gamma locus in the sandbar shark. Proc. Natl. Acad. Sci. USA 2009, 106, 8591–8596. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bernstein, H.; Ranganathan, P.; Schluter, S.F. Somatic hypermutation of TCR γ V genes in the sandbar shark. Dev. Comp. Immunol. 2012, 37, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Weinstein, J.A.; Penland, L.; White, R.A.; Fisher, D.S.; Quake, S.R. Determinism and stochasticity during maturation of the zebrafish antibody repertoire. Proc. Natl. Acad. Sci. USA 2011, 108, 5348–5353. [Google Scholar] [CrossRef] [PubMed]
- Dooley, H.; Stanfield, R.L.; Brady, R.A.; Flajnik, M.F. First molecular and biochemical analysis of in vivo affinity maturation in an ectothermic vertebrate. Proc. Natl. Acad. Sci. USA 2006, 103, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Schroder, A.; Greiner, A.; Seyfert, C.; Berek, C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 1996, 93, 221–225. [Google Scholar] [CrossRef] [PubMed]
- William, J.; Euler, C.; Christensen, S.; Shlomchik, M. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 2002, 297, 2066–2070. [Google Scholar] [CrossRef] [PubMed]
- Da, R.R.; Qin, Y.; Baeten, D.; Zhang, Y. B cell clonal expansion and somatic hypermutation of Ig variable heavy chain genes in the synovial membrane of patients with osteoarthritis. J. Immunol. 2007, 178, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.; Manning, M.; Ellis, A. Localization of immune-complexes and heat-aggregated immunoglobulin in the carp cyprinus-carpio L. Immunology 1982, 47, 101–105. [Google Scholar] [PubMed]
- Herraez, M.; Zapata, A. Structural characterization of the melano-macrophage centres (MMC) of goldfish Carassius auratus. Eur. J. Morphol. 1991, 29, 89–102. [Google Scholar] [PubMed]
- Press, C.; Dannevig, B.; Landsverk, T. Immune and enzyme-histochemical phenotypes of lymphoid and nonlymphoid cells within the spleen and head kidney of Atlantic salmon (Salmo-Salar L.). Fish Shellfish Immunol. 1994, 4, 79–93. [Google Scholar] [CrossRef]
- Diaz-Satizabal, L.; Magor, B.G. Isolation and cytochemical characterization of melanomacrophages and melanomacrophage clusters from goldfish (Carassius auratus, L.). Dev. Comp. Immunol. 2015, 48, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Jarjour, M.; Jorquera, A.; Mondor, I.; Wienert, S.; Narang, P.; Coles, M.C.; Klauschen, F.; Bajénoff, M. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J. Exp. Med. 2014, 211, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Marr, S.; Morales, H.; Bottaro, A.; Cooper, M.; Flajnik, M.; Robert, J. Localization and differential expression of activation-induced cytidine deaminase in the amphibian Xenopus upon antigen stimulation and during early development. J. Immunol. 2007, 179, 6783–6789. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magor, B.G. Antibody Affinity Maturation in Fishes—Our Current Understanding. Biology 2015, 4, 512-524. https://doi.org/10.3390/biology4030512
Magor BG. Antibody Affinity Maturation in Fishes—Our Current Understanding. Biology. 2015; 4(3):512-524. https://doi.org/10.3390/biology4030512
Chicago/Turabian StyleMagor, Brad G. 2015. "Antibody Affinity Maturation in Fishes—Our Current Understanding" Biology 4, no. 3: 512-524. https://doi.org/10.3390/biology4030512
APA StyleMagor, B. G. (2015). Antibody Affinity Maturation in Fishes—Our Current Understanding. Biology, 4(3), 512-524. https://doi.org/10.3390/biology4030512





