Selenium and Mammalian Uterine Health: A Comprehensive Review
Simple Summary
Abstract
1. Introduction
2. Physiological Functions of Selenium in Animals
2.1. Antioxidant Function
2.2. Anti-Inflammatory Function
2.3. Anti-Apoptotic Function
2.4. Immune-Regulatory Function
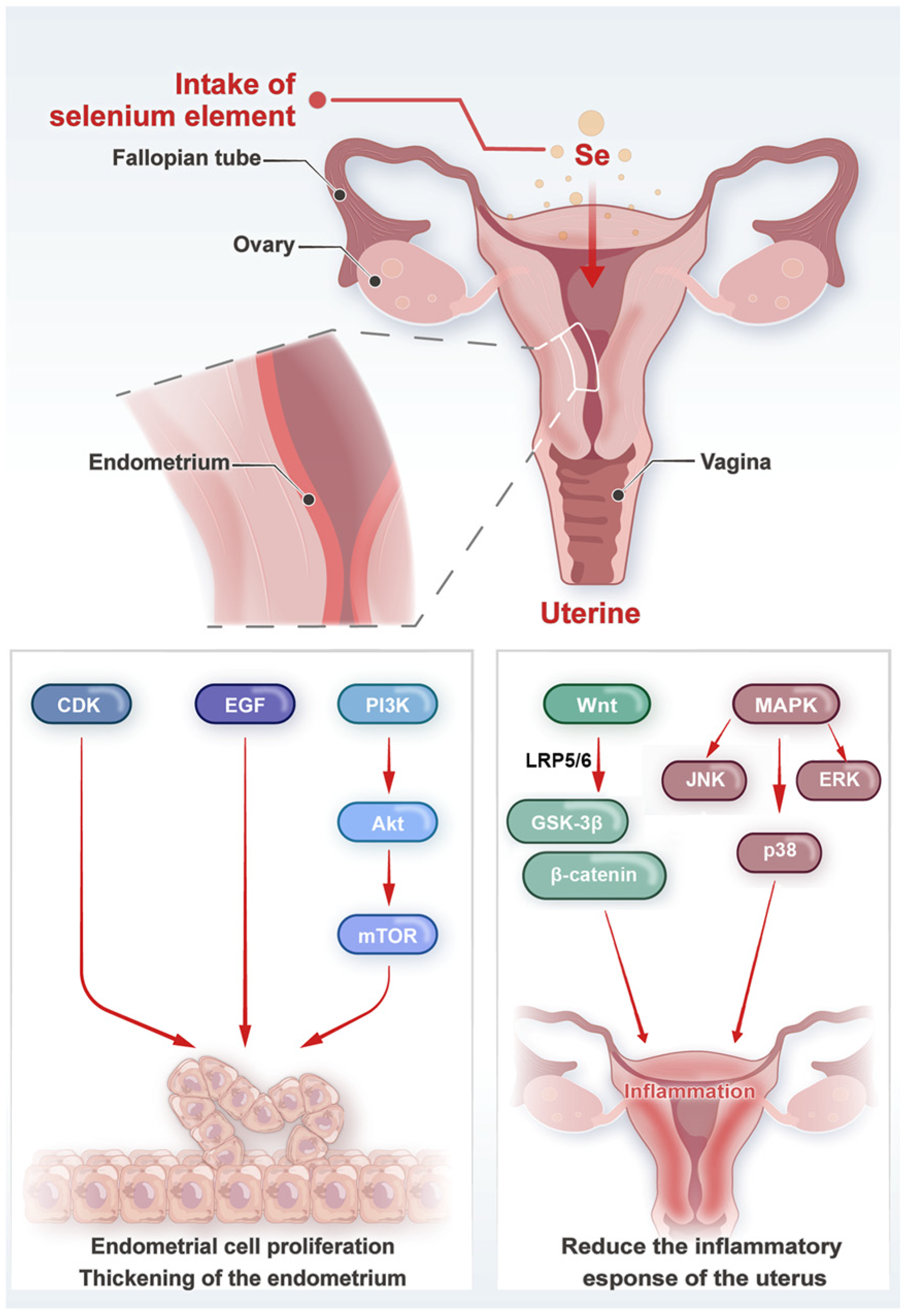
3. Selenium and Uterine Development
3.1. Effects on Uterine Morphology and Structure
3.2. Molecular Mechanisms and Signaling Pathways
| Species | Recommended Dietary Se Intakes [80] | Use the Maximum Dose |
|---|---|---|
| Rats | 0.15 mg/kg Diet | selenite/selenate LOAELlethality 2: 1.2 mg Se/kg bw/day [133] |
| Mice | 0.15 mg/kg Diet | selenocystine LOAELlethality 2: 15 mg Se/kg bw/day [133] |
| Pigs | 0.15–0.3 mg/kg Diet | 4 mg/kg |
| Dogs | 0.35 mg/kg Diet | 5 mg/kg |
| Cats | 0.30 mg/kg Diet | 5 mg/kg |
| Beef cattle | 0.1 mg/kg DMI 1 | 3–8 mg/kg |
| Dairy cows | 0.3 mg/kg DMI 1 | 5 mg/kg |
| Sheep & Goats | 0.1 mg/kg DMI 1 | Not available |
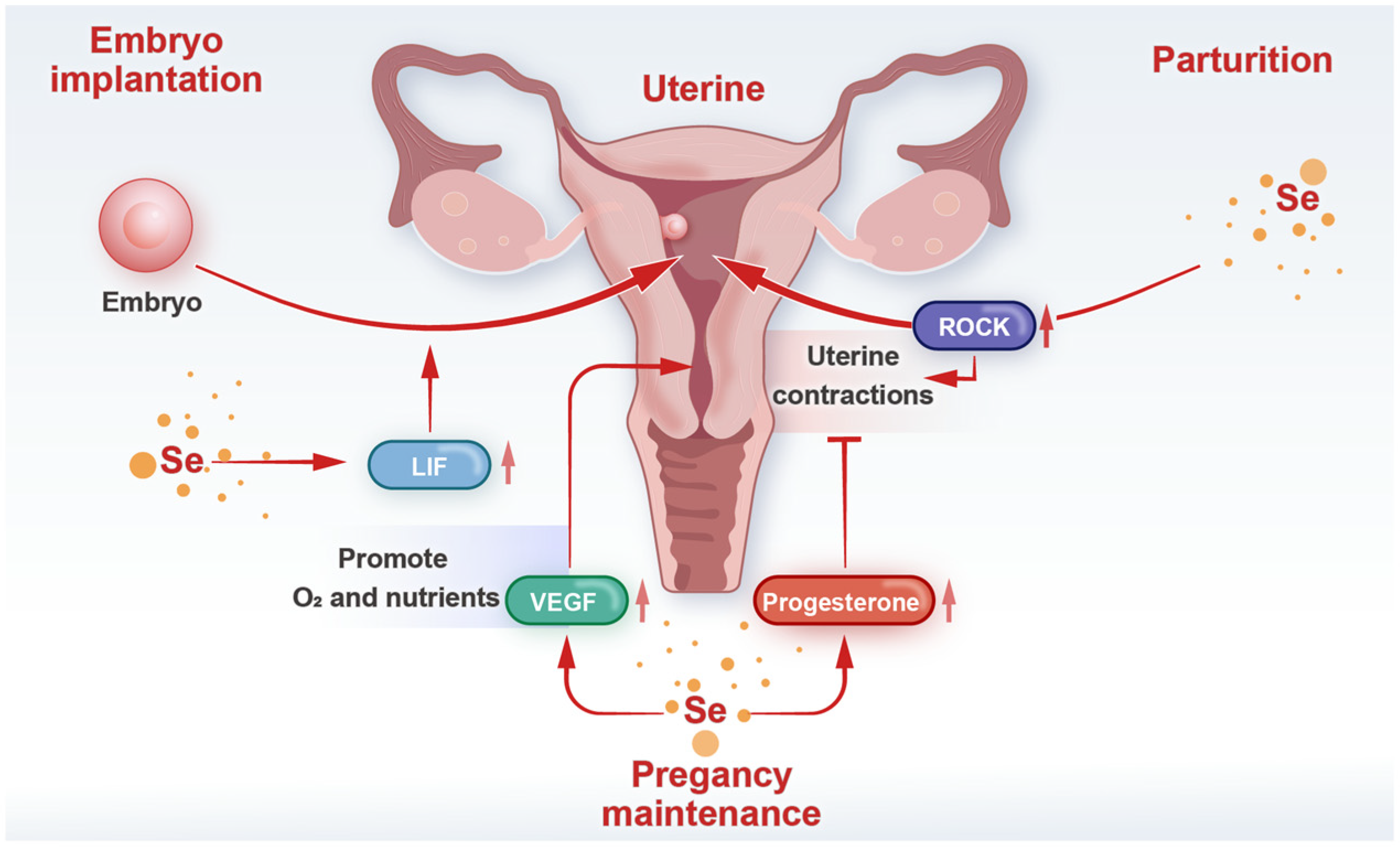
4. Selenium and Uterine Function During Pregnancy
4.1. Embryo Implantation
4.2. Pregnancy Maintenance
4.3. Parturition
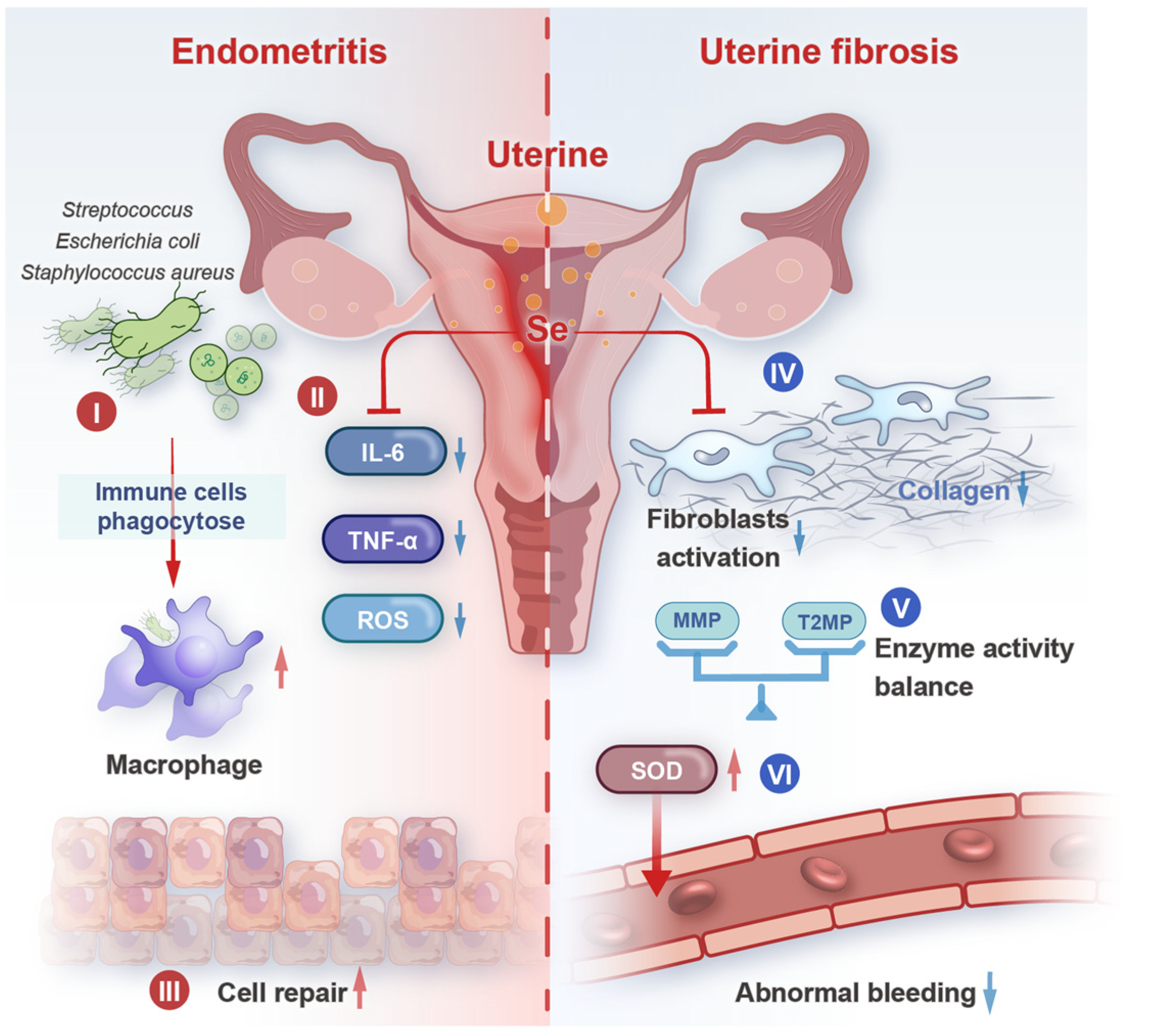
5. Selenium and Uterine Diseases
5.1. Endometritis
5.2. Uterine Fibrosis
5.3. Retained Placenta
5.4. Other Uterine Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedüs, L. Selenium in thyroid disorders—Essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef]
- Ye, R.; Guo, Q.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Eucommia ulmoides polysaccharide modified nano-selenium effectively alleviated DSS-induced colitis through enhancing intestinal mucosal barrier function and antioxidant capacity. J. Nanobiotechnol. 2023, 21, 222. [Google Scholar] [CrossRef]
- Papadomichelakis, G.; Zoidis, E.; Pappas, A.C.; Danezis, G.; Georgiou, C.A.; Fegeros, K. Dietary organic selenium addition and accumulation of toxic and essential trace elements in liver and meat of growing rabbits. Meat Sci. 2018, 145, 383–388. [Google Scholar] [CrossRef]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Van Dael, P.; Davidsson, L.; Muñoz-Box, R.; Fay, L.B.; Barclay, D. Selenium absorption and retention from a selenite- or selenate-fortified milk-based formula in men measured by a stable-isotope technique. Br. J. Nutr. 2001, 85, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Suzuki, N.; Ogra, Y. Bioavailability Comparison of Nine Bioselenocompounds In Vitro and In Vivo. Int. J. Mol. Sci. 2017, 18, 506. [Google Scholar] [CrossRef]
- Sutter, M.E.; Thomas, J.D.; Brown, J.; Morgan, B. Selenium toxicity: A case of selenosis caused by a nutritional supplement. Ann. Intern. Med. 2008, 148, 970–971. [Google Scholar] [CrossRef]
- Zhong, Y.; Jin, Y.; Zhang, Q.; Mao, B.; Tang, X.; Huang, J.; Guo, R.; Zhao, J.; Cui, S.; Chen, W. Comparison of Selenium-Enriched Lactobacillusparacasei, Selenium-Enriched Yeast, and Selenite for the Alleviation of DSS-Induced Colitis in Mice. Nutrients 2022, 14, 2433. [Google Scholar] [CrossRef]
- Flood, D.T.; Hintzen, J.C.J.; Knouse, K.W.; Hill, D.E.; Lu, C.; Cistrone, P.A.; Chen, J.S.; Otomo, T.; Dawson, P.E. Selenomethionine as an expressible handle for bioconjugations. Proc. Natl. Acad. Sci. USA 2021, 118, e2005164118. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Wu, Q.-Y.; Chen, C.; Zheng, R.; Chen, Y.; Ni, J.-Z.; Song, G.-L. Comparison of the effects of selenomethionine and selenium-enriched yeast in the triple-transgenic mouse model of Alzheimer’s disease. Food Funct. 2018, 9, 3965–3973. [Google Scholar] [CrossRef]
- Bierla, K.; Lobinski, R.; Szpunar, J. Determination of Proteinaceous Selenocysteine in Selenized Yeast. Int. J. Mol. Sci. 2018, 19, 543. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Y.; Jiang, M.; Sang, L.; Chang, B. Selenium Yeast Alleviates Dextran Sulfate Sodium-Induced Chronic Colitis in Mice by Reducing Proinflammatory Cytokines and Regulating the Gut Microbiota and Their Metabolites. J. Inflamm. Res. 2024, 17, 2023–2037. [Google Scholar] [CrossRef]
- Wang, H.; Shi, D.; Chen, Y.; Wang, Z.; Yuan, Y.; Yue, T. Dietary supplementation with novel selenium-enriched Pichia kudriavzevii regulates gut microbiota and host metabolism in mice. Food Funct. 2024, 15, 10896–10912. [Google Scholar] [CrossRef]
- Li, C.; Wang, N.; Zheng, G.; Yang, L. Oral Administration of Resveratrol-Selenium-Peptide Nanocomposites Alleviates Alzheimer’s Disease-like Pathogenesis by Inhibiting Aβ Aggregation and Regulating Gut Microbiota. ACS Appl. Mater. Interfaces 2021, 13, 46406–46420. [Google Scholar] [CrossRef]
- Guo, P.; Wang, W.; Xiang, Q.; Pan, C.; Qiu, Y.; Li, T.; Wang, D.; Ouyang, J.; Jia, R.; Shi, M.; et al. Engineered probiotic ameliorates ulcerative colitis by restoring gut microbiota and redox homeostasis. Cell Host Microbe 2024, 32, 1502–1518.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Li, S.; Li, C.; Hu, X.; Li, Z.; Yue, T.; Hu, Z. Effect of the selenized yeast added in feed on selenium-containing proteins of albumins in egg yolk. Food Chem. 2023, 402, 134435. [Google Scholar] [CrossRef] [PubMed]
- Calvo, L.; Segura, J.; Toldrá, F.; Flores, M.; Rodríguez, A.I.; López-Bote, C.J.; Rey, A.I. Meat quality, free fatty acid concentration, and oxidative stability of pork from animals fed diets containing different sources of selenium. Food Sci. Technol. Int. 2017, 23, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Gao, C.-q. New horizons for selenium in animal nutrition and functional foods. Anim. Nutr. 2022, 11, 80–86. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, S.; Tang, J.; Liu, Y.; Jia, G.; Liu, G.; Tian, G.; Chen, X.; Cai, J.; Kang, B.; et al. Hydroxy Selenomethionine Improves Meat Quality through Optimal Skeletal Metabolism and Functions of Selenoproteins of Pigs under Chronic Heat Stress. Antioxidants 2021, 10, 1558. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Dalia, A.M.; Loh, T.C.; Akit, H.; Samsudin, A.A. Effect of organic and inorganic dietary selenium supplementation on gene expression in oviduct tissues and Selenoproteins gene expression in Lohman Brown-classic laying hens. BMC Vet. Res. 2021, 17, 281. [Google Scholar] [CrossRef]
- Crites, B.R.; Carr, S.N.; Anderson, L.H.; Matthews, J.C.; Bridges, P.J. Form of dietary selenium affects mRNA encoding interferon-stimulated and progesterone-induced genes in the bovine endometrium and conceptus length at maternal recognition of pregnancy. J. Anim. Sci. 2022, 100, skac137. [Google Scholar] [CrossRef]
- Manojlović-Stojanoski, M.; Borković-Mitić, S.; Nestorović, N.; Ristić, N.; Trifunović, S.; Stevanović, M.; Filipović, N.; Stojsavljević, A.; Pavlović, S. The Effects of BSA-Stabilized Selenium Nanoparticles and Sodium Selenite Supplementation on the Structure, Oxidative Stress Parameters and Selenium Redox Biology in Rat Placenta. Int. J. Mol. Sci. 2022, 23, 13068. [Google Scholar] [CrossRef]
- Mou, D.; Ding, D.; Li, S.; Yan, H.; Qin, B.; Li, Z.; Zhao, L.; Che, L.; Fang, Z.; Xu, S.; et al. Effect of maternal organic selenium supplementation during pregnancy on sow reproductive performance and long-term effect on their progeny. J. Anim. Sci. 2020, 98, skaa366. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Lee, S.; Lee, E.-K.; Kang, D.H.; Lee, J.; Hong, S.H.; Jeong, W.; Kang, S.W. Glutathione peroxidase-1 regulates ASK1-dependent apoptosis via interaction with TRAF2 in RIPK3-negative cancer cells. Exp. Mol. Med. 2021, 53, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shao, Y.; Cui, W.; Jia, Z.; Zhang, Q.; Zhao, Q.; Chen, Z.-J.; Yan, J.; Chu, B.; Yuan, J. Excessive Lipid Peroxidation in Uterine Epithelium Causes Implantation Failure and Pregnancy Loss. Adv. Sci. 2024, 11, e2302887. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiang, H.; Lin, H.; Yang, H.; Wang, K. Asiaticoside ameliorates uterine injury induced by zearalenone in mice by reversing endometrial barrier disruption, oxidative stress and apoptosis. Reprod. Biol. Endocrinol. 2024, 22, 118. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, J.; Hou, J.; Chen, C. Free radical scavenging efficiency of Nano-Se in vitro. Free Radic. Biol. Med. 2003, 35, 805–813. [Google Scholar] [CrossRef]
- Huang, H.; Shen, Z.; Chen, B.; Wang, X.; Xia, Q.; Ge, Z.; Wang, Y.; Li, X. Selenium-doped two-photon fluorescent carbon nanodots for in-situ free radical scavenging in mitochondria. J. Colloid Interface Sci. 2020, 567, 402–409. [Google Scholar] [CrossRef]
- LeBlanc, S.J. Review: Postpartum reproductive disease and fertility in dairy cows. Animal 2023, 17 (Suppl. S1), 100781. [Google Scholar] [CrossRef]
- Figueiredo, C.C.; Balzano-Nogueira, L.; Bisinotto, D.Z.; Ruiz, A.R.; Duarte, G.A.; Conesa, A.; Galvão, K.N.; Bisinotto, R.S. Differences in uterine and serum metabolome associated with metritis in dairy cows. J. Dairy Sci. 2023, 106, 3525–3536. [Google Scholar] [CrossRef]
- Harrison, J.H.; Conrad, H.R. Selenium content and glutathione peroxidase activity in tissues of the dairy cow after short-term feeding. J. Dairy Sci. 1984, 67, 2464–2470. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.-F.; Yang, J.; Jing, H.-Y.; Liang, W.; Chen, M.-Y.; Yang, M.; Wang, Y.; Guo, M.-Y. Selenium alleviates lipopolysaccharide-induced endometritis via regulating the recruitment of TLR4 into lipid rafts in mice. Food Funct. 2020, 11, 200–210. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Li, L.; Wang, X.; Wei, X.; Gou, S.; Ding, Z.; Cai, Z.; Ling, Q.; Hoffmann, P.R.; et al. Mannose coated selenium nanoparticles normalize intestinal homeostasis in mice and mitigate colitis by inhibiting NF-κB activation and enhancing glutathione peroxidase expression. J. Nanobiotechnol. 2024, 22, 613. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, Y.; Li, J.; Zhou, D. Nano-Selenium Modulates NF-κB/NLRP3 Pathway and Mitochondrial Dynamics to Attenuate Microplastic-Induced Liver Injury. Nutrients 2024, 16, 3878. [Google Scholar] [CrossRef]
- Afonina, I.S.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting inflammation—The negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Gasparian, A.V.; Yao, Y.J.; Lü, J.; Yemelyanov, A.Y.; Lyakh, L.A.; Slaga, T.J.; Budunova, I.V. Selenium compounds inhibit I kappa B kinase (IKK) and nuclear factor-kappa B (NF-kappa B) in prostate cancer cells. Mol. Cancer Ther. 2002, 1, 1079–1087. [Google Scholar]
- Kanarek, N.; Ben-Neriah, Y. Regulation of NF-κB by ubiquitination and degradation of the IκBs. Immunol. Rev. 2012, 246, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, Q.; Zhang, L.; Lin, J.; Feng, Z.; Wang, Z.; Gu, L.; Tian, X.; Luan, S.; Li, C.; et al. Ebselen improves fungal keratitis through exerting anti-inflammation, anti-oxidative stress, and antifungal effects. Redox Biol. 2024, 73, 103206. [Google Scholar] [CrossRef]
- Hwang, J.-T.; Kim, Y.M.; Surh, Y.-J.; Baik, H.W.; Lee, S.-K.; Ha, J.; Park, O.J. Selenium regulates cyclooxygenase-2 and extracellular signal-regulated kinase signaling pathways by activating AMP-activated protein kinase in colon cancer cells. Cancer Res. 2006, 66, 10057–10063. [Google Scholar] [CrossRef]
- Dhanjal, N.I.; Sharma, S.; Skalny, A.V.; Skalnaya, M.G.; Ajsuvakova, O.P.; Tinkov, A.A.; Zhang, F.; Guo, X.; Prabhu, K.S.; Tejo Prakash, N. Selenium-rich maize modulates the expression of prostaglandin genes in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Funct. 2019, 10, 2839–2846. [Google Scholar] [CrossRef]
- Vunta, H.; Davis, F.; Palempalli, U.D.; Bhat, D.; Arner, R.J.; Thompson, J.T.; Peterson, D.G.; Reddy, C.C.; Prabhu, K.S. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J. Biol. Chem. 2007, 282, 17964–17973. [Google Scholar] [CrossRef]
- Yuan, J.; Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Liu, M.; Zhang, X.; Wang, J.; Zhang, J.; Peng, Z.; Meng, L.; Wang, R.; Guo, L.; Zhang, Q.; et al. A novel selenium analog of HDACi-based twin drug induces apoptosis and cell cycle arrest via CDC25A to improve prostate cancer therapy. Theranostics 2024, 14, 3565–3582. [Google Scholar] [CrossRef]
- Chavez Varias, D.; Moon, S.-H.; Shin, S.H.; Ryu, B.-Y. Selenium protects mouse spermatogonia against ivermectin-induced apoptosis by alleviating endoplasmic reticulum stress in vitro. Ecotoxicol. Environ. Saf. 2024, 287, 117307. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, T.; Li, X.; Shi, X.; Wu, H.; Zhang, Z.; Xu, S. Selenoprotein S maintains intestinal homeostasis in ulcerative colitis by inhibiting necroptosis of colonic epithelial cells through modulation of macrophage polarization. Theranostics 2024, 14, 5903–5925. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, B.; Guan, H.; Jiao, X.; Yang, J.; Cai, J.; Liu, Q.; Zhang, Z. Selenium deficiency causes apoptosis through endoplasmic reticulum stress in swine small intestine. Biofactors 2021, 47, 788–800. [Google Scholar] [CrossRef]
- Ren, X.; Wang, S.; Zhang, C.; Hu, X.; Zhou, L.; Li, Y.; Xu, L. Selenium ameliorates cadmium-induced mouse leydig TM3 cell apoptosis via inhibiting the ROS/JNK/c-jun signaling pathway. Ecotoxicol. Environ. Saf. 2020, 192, 110266. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Cui, L.; Liu, K.; Guo, L.; Li, J.; Dong, J. The effect of selenium on the proliferation of bovine endometrial epithelial cells in a lipopolysaccharide-induced damage model. BMC Vet. Res. 2024, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; She, F.; Chen, F.; Li, K.; Qin, S. Selenium-Chitosan Protects Porcine Endometrial Epithelial Cells from Zearalenone-induced Apoptosis via the JNK/SAPK Signaling Pathway. Biol. Trace Elem. Res. 2024, 202, 2075–2084. [Google Scholar] [CrossRef]
- Cui, J.; Qiu, M.; Liu, Y.; Liu, Y.; Tang, Y.; Teng, X.; Li, S. Nano-selenium protects grass carp hepatocytes against 4-tert-butylphenol-induced mitochondrial apoptosis and necroptosis via suppressing ROS-PARP1 axis. Fish Shellfish. Immunol. 2023, 135, 108682. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Reed, J.C. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 2000, 7, 1155–1165. [Google Scholar] [CrossRef]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in cell death, autophagy, and disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, C.; Tang, D.; Yu, Q.; Zhang, L. Effects of dietary supplementation with selenium yeast and jujube powder on mitochondrial oxidative damage and apoptosis of chicken. Poult. Sci. 2022, 101, 102072. [Google Scholar] [CrossRef]
- Liu, N.; Shi, Y.-F.; Diao, H.-Y.; Li, Y.-X.; Cui, Y.; Song, X.-J.; Tian, X.; Li, T.-Y.; Liu, B. MicroRNA-135a Regulates Apoptosis Induced by Hydrogen Peroxide in Rat Cardiomyoblast Cells. Int. J. Biol. Sci. 2017, 13, 13–21. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Ramos-Campo, D.J.; Mielgo-Ayuso, J.; Dalamitros, A.A.; Nikolaidis, P.A.; Hormeño-Holgado, A.; Tornero-Aguilera, J.F. Nutrition in the Actual COVID-19 Pandemic. A Narrative Review. Nutrients 2021, 13, 1924. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Sun, H.; Dong, J.; Fu, Z.; Lu, X.; Chen, X.; Lei, H.; Xiao, X.; Chen, S.; Lu, J.; Su, D.; et al. TSG6-Exo@CS/GP Attenuates Endometrium Fibrosis by Inhibiting Macrophage Activation in a Murine IUA Model. Adv. Mater. 2024, 36, e2308921. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Du, M.-R.; Li, M.; Wang, H. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell. Mol. Immunol. 2018, 15, 1027–1037. [Google Scholar] [CrossRef]
- Valero-Pacheco, N.; Tang, E.K.; Massri, N.; Loia, R.; Chemerinski, A.; Wu, T.; Begum, S.; El-Naccache, D.W.; Gause, W.C.; Arora, R.; et al. Maternal IL-33 critically regulates tissue remodeling and type 2 immune responses in the uterus during early pregnancy in mice. Proc. Natl. Acad. Sci. USA 2022, 119, e2123267119. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, J.; Wu, W.; Wen, Y.; Lu, S.; El-Seedi, H.R.; Zhao, C. Structure-immunomodulatory activity relationships of dietary polysaccharides. Curr. Res. Food Sci. 2022, 5, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Ruterbusch, M.; Pruner, K.B.; Shehata, L.; Pepper, M. In Vivo CD4+ T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu. Rev. Immunol. 2020, 38, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, U.; Xu, R.; He, X.; Pan, L.; Niu, Z.; Wang, D.; Zeng, H.; Chen, J.X.; Clemmer, J.S.; Chen, Y. High selenium diet attenuates pressure overload-induced cardiopulmonary oxidative stress, inflammation, and heart failure. Redox Biol. 2024, 76, 103325. [Google Scholar] [CrossRef]
- Huang, L.-J.; Mao, X.-T.; Li, Y.-Y.; Liu, D.-D.; Fan, K.-Q.; Liu, R.-B.; Wu, T.-T.; Wang, H.-L.; Zhang, Y.; Yang, B.; et al. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn’s disease. Immunity 2021, 54, 1728–1744.e7. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, Z.; Zhang, H.; Chen, C.; Zeng, M.; Yunis, J.; Wei, Y.; Wan, Y.; Wang, N.; Zhou, M.; et al. Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat. Immunol. 2021, 22, 1127–1139. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Bao, J.; Tang, C.; Zhang, Z. Selenium deficiency induced necroptosis, Th1/Th2 imbalance, and inflammatory responses in swine ileum. J. Cell. Physiol. 2021, 236, 222–234. [Google Scholar] [CrossRef]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.-K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- Ammerman, C.B.; Miller, S.M. Selenium in ruminant nutrition: A review. J. Dairy Sci. 1975, 58, 1561–1577. [Google Scholar] [CrossRef]
- Lei, X.G.; Combs, G.F.; Sunde, R.A.; Caton, J.S.; Arthington, J.D.; Vatamaniuk, M.Z. Dietary Selenium Across Species. Annu. Rev. Nutr. 2022, 42, 337–375. [Google Scholar] [CrossRef]
- Mistry, H.D.; Broughton Pipkin, F.; Redman, C.W.G.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, C.R.; Reynolds, L.P.; Caton, J.S. Selenium supplementation and pregnancy outcomes. Front. Nutr. 2022, 9, 1011850. [Google Scholar] [CrossRef] [PubMed]
- Hidiroglou, M. Trace element deficiencies and fertility in ruminants: A review. J. Dairy Sci. 1979, 62, 1195–1206. [Google Scholar] [CrossRef]
- Nogales, F.; Ojeda, M.L.; Fenutría, M.; Murillo, M.L.; Carreras, O. Role of selenium and glutathione peroxidase on development, growth, and oxidative balance in rat offspring. Reproduction 2013, 146, 659–667. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Cao, Y.; Jiang, H.; Wang, T.; Song, X.; Guo, M.; Zhang, N. Selenium Deficiency Facilitates Inflammation Through the Regulation of TLR4 and TLR4-Related Signaling Pathways in the Mice Uterus. Inflammation 2015, 38, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, J.; Guo, J.; Zhang, M.; Li, W.; Dong, J.; Liu, K.; Guo, L.; Li, J.; Wang, H.; et al. Selenium suppressed the LPS-induced inflammation of bovine endometrial epithelial cells through NF-κB and MAPK pathways under high cortisol background. J. Cell. Mol. Med. 2023, 27, 1373–1383. [Google Scholar] [CrossRef]
- Rutigliano, H.M.; Lima, F.S.; Cerri, R.L.A.; Greco, L.F.; Vilela, J.M.; Magalhães, V.; Silvestre, F.T.; Thatcher, W.W.; Santos, J.E.P. Effects of method of presynchronization and source of selenium on uterine health and reproduction in dairy cows. J. Dairy Sci. 2008, 91, 3323–3336. [Google Scholar] [CrossRef]
- Leiter, O.; Zhuo, Z.; Rust, R.; Wasielewska, J.M.; Grönnert, L.; Kowal, S.; Overall, R.W.; Adusumilli, V.S.; Blackmore, D.G.; Southon, A.; et al. Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging. Cell Metab. 2022, 34, 408–423.e8. [Google Scholar] [CrossRef]
- Zeng, H.; Cao, J.J.; Combs, G.F. Selenium in bone health: Roles in antioxidant protection and cell proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Zu, K.; Bihani, T.; Lin, A.; Park, Y.M.; Mori, K.; Ip, C. Enhanced selenium effect on growth arrest by BiP/GRP78 knockdown in p53-null human prostate cancer cells. Oncogene 2006, 25, 546–554. [Google Scholar] [CrossRef]
- Gangadevi, V.; Thatikonda, S.; Pooladanda, V.; Devabattula, G.; Godugu, C. Selenium nanoparticles produce a beneficial effect in psoriasis by reducing epidermal hyperproliferation and inflammation. J. Nanobiotechnol. 2021, 19, 101. [Google Scholar] [CrossRef]
- Stallaert, W.; Kedziora, K.M.; Taylor, C.D.; Zikry, T.M.; Ranek, J.S.; Sobon, H.K.; Taylor, S.R.; Young, C.L.; Cook, J.G.; Purvis, J.E. The structure of the human cell cycle. Cell Syst. 2022, 13, 230–240.e3. [Google Scholar] [CrossRef]
- Wang, S.; Tian, B.; Feng, X.; Hu, Y.; Zhang, L.; Zhang, Z.; Xu, S.; Hu, Y.; Cui, X.; Li, T.; et al. Selenium promotes broiler myoblast proliferation through the ROS/PTEN/PI3K/AKT signaling axis. Poult. Sci. 2024, 103, 104364. [Google Scholar] [CrossRef]
- Shao, Z.-Q.; Zhang, X.; Fan, H.-H.; Wang, X.-S.; Wu, H.-M.; Zhang, L.; Cheng, W.-H.; Zhu, J.-H. Selenoprotein T Promotes Proliferation and G1-to-S Transition in SK-N-SH Cells: Implications in Parkinson’s Disease. J. Nutr. 2019, 149, 2110–2119. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, K.; Shi, J.; Shi, X.; Qi, X.; Lin, H. The decrease of selenoprotein K induced by selenium deficiency in diet improves apoptosis and cell progression block in chicken liver via the PTEN/PI3K/AKT pathway. Free Radic. Biol. Med. 2022, 189, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Meng, H.; Deng, G.; Lin, H. Sustainable Release Selenium Laden with SiO2 Restoring Peripheral Nerve Injury via Modulating PI3K/AKT Pathway Signaling Pathway. Int. J. Nanomed. 2024, 19, 7851–7870. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Yu, Y.; Chen, R.; Zhu, H.; Fong, H.; Kuang, J.; Jiang, Y.; Chen, Y.; Niu, Y.; Chen, T.; et al. Selenium promotes neural development through the regulation of GPX4 and SEPP1 in an iPSC-derived neuronal model. Biomaterials 2025, 316, 123011. [Google Scholar] [CrossRef]
- Kamal, A.; Nazari, V.M.; Yaseen, M.; Iqbal, M.A.; Ahamed, M.B.K.; Majid, A.S.A.; Bhatti, H.N. Green synthesis of selenium-N-heterocyclic carbene compounds: Evaluation of antimicrobial and anticancer potential. Bioorg. Chem. 2019, 90, 103042. [Google Scholar] [CrossRef] [PubMed]
- Maachi, H.; Fergusson, G.; Ethier, M.; Brill, G.N.; Katz, L.S.; Honig, L.B.; Metukuri, M.R.; Scott, D.K.; Ghislain, J.; Poitout, V. HB-EGF Signaling Is Required for Glucose-Induced Pancreatic β-Cell Proliferation in Rats. Diabetes 2020, 69, 369–380. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, G.; Jiang, P.; Wang, H.; Wang, Z.; Yao, S.; Zhou, Z.; Wang, L.; Liu, D.; Deng, W.; et al. Deciphering the endometrial niche of human thin endometrium at single-cell resolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2115912119. [Google Scholar] [CrossRef]
- Maggio, M.; De Vita, F.; Lauretani, F.; Buttò, V.; Bondi, G.; Cattabiani, C.; Nouvenne, A.; Meschi, T.; Dall’Aglio, E.; Ceda, G.P. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients 2013, 5, 4184–4205. [Google Scholar] [CrossRef] [PubMed]
- Lava Kumar, S.; Kushawaha, B.; Mohanty, A.; Kumari, A.; Kumar, A.; Beniwal, R.; Kiran Kumar, P.; Athar, M.; Krishna Rao, D.; Rao, H.B.D.P. Glutathione peroxidase (GPX1)—Selenocysteine metabolism preserves the follicular fluid’s (FF) redox homeostasis via IGF-1- NMD cascade in follicular ovarian cysts (FOCs). Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167235. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.J.; Ghahary, A. Uterine insulin-like growth factor-1: Regulation of expression and its role in estrogen-induced uterine proliferation. Endocr. Rev. 1990, 11, 443–453. [Google Scholar] [CrossRef]
- Dai, T.; Ma, Z.; Guo, X.; Wei, S.; Ding, B.; Ma, Y.; Dan, X. Study on the Pattern of Postpartum Uterine Involution in Dairy Cows. Animals 2023, 13, 3693. [Google Scholar] [CrossRef]
- Shen, Q.; Zou, S.; Sheng, B.; Zhao, M.; Sun, L.-Z.; Zhu, X. Mifepristone inhibits IGF-1 signaling pathway in the treatment of uterine leiomyomas. Drug Des. Dev. Ther. 2019, 13, 3161–3170. [Google Scholar] [CrossRef]
- Wozney, J.L.; Antonarakis, E.S. Growth factor and signaling pathways and their relevance to prostate cancer therapeutics. Cancer Metastasis Rev. 2014, 33, 581–594. [Google Scholar] [CrossRef][Green Version]
- Klotz, D.M.; Hewitt, S.C.; Ciana, P.; Raviscioni, M.; Lindzey, J.K.; Foley, J.; Maggi, A.; DiAugustine, R.P.; Korach, K.S. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J. Biol. Chem. 2002, 277, 8531–8537. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Xin, Y.; Min, P.; Xu, H.; Zhang, Z.; Zhang, Y.; Zhang, Y. CD26 upregulates proliferation and invasion in keloid fibroblasts through an IGF-1-induced PI3K/AKT/mTOR pathway. Burn. Trauma 2020, 8, tkaa025. [Google Scholar] [CrossRef]
- Jin, N.; Zhu, H.Z.; Liang, X.; Huang, W.; Xie, Q.G.; Xiao, P.; Ni, J.Z.; Liu, Q. Sodium selenate activated Wnt/β-catenin signaling and repressed amyloid-β formation in a triple transgenic mouse model of Alzheimer’s disease. Exp. Neurol. 2017, 297, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.S.; Lin, H.; Li, H.; Zou, B.H.; Xie, B.; Yu, Y.Z.; He, L.Z.; Chen, T.F. Chiral Selenium Nanotherapeutics Regulates Selenoproteins to Attenuate Glucocorticoid-Induced Osteoporosis. Adv. Funct. Mater. 2023, 33, 2212970. [Google Scholar] [CrossRef]
- Liu, S.; Yu, H.B.; Li, P.J.; Wang, C.; Liu, G.H.; Zhang, X.T.; Zhang, C.; Qi, M.; Ji, H. Dietary nano-selenium alleviated intestinal damage of juvenile grass carp (Ctenopharyngodon idella) induced by high-fat diet: Insight from intestinal morphology, tight junction, inflammation, anti-oxidization and intestinal microbiota. Anim. Nutr. 2022, 8, 235–248. [Google Scholar] [CrossRef]
- Xiang, R.Q.; Xiao, X.; Liu, J.X.; Guo, Z.W.; He, H.F.; Wang, X.N.; Wen, X.Y.; Angelo, V.; Han, J. Protective effects of functional Nano-Selenium supplementation on spleen injury through regulation of p38 MAPK and NF-κB protein expression. Int. Immunopharmacol. 2024, 130, 111574. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Liu, H.G.; Zhang, R.X.; Yang, X.S.; Bao, J.; Xing, H.J. Selenomethionine protects against ammonia-induced apoptosis through inhibition of endoplasmic reticulum stress in pig kidneys. Ecotoxicol. Environ. Saf. 2021, 223, 112596. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Perugorria, M.J.; Olaizola, P.; Labiano, I.; Esparza-Baquer, A.; Marzioni, M.; Marin, J.J.G.; Bujanda, L.; Banales, J.M. Wnt-β-catenin signalling in liver development, health and disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 121–136. [Google Scholar] [CrossRef]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT-β-catenin signalling—A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.S.; Wang, Z.; Fei, F.; Jiang, Y.Q.; Jiang, Y.S.; Guo, L.; Liu, K.J.; Cui, L.Y.; Meng, X.; Li, J.J.; et al. Selenium Enhances the Growth of Bovine Endometrial Stromal Cells by PI3K/AKT/GSK-3β and Wnt/β-Catenin Pathways. Vet. Sci. 2024, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Yang, Z.H.; Wang, S.; Wang, X.L.; Mao, J.Y. Targeting MAPK-ERK/JNK pathway: A potential intervention mechanism of myocardial fibrosis in heart failure. Biomed. Pharmacother. 2024, 173, 116413. [Google Scholar] [CrossRef]
- Zhou, F.; Mei, J.T.; Han, X.G.; Li, H.J.; Yang, S.B.; Wang, M.Q.; Chu, L.Y.; Qiao, H.; Tang, T.T. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-κB/MAPK signaling and protecting chondrocytes. Acta Pharm. Sin. B 2019, 9, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.L.; Guo, D.D.; Fang, L.; Sang, T.T.; Wu, J.J.; Guo, C.J.; Wang, Y.J.; Wang, Y.; Chen, C.J.; Chen, J.J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Xing, H.Z.; Bai, X.W.; Pei, X.Y.; Zhang, Y.Q.; Zhang, X.Y.; Chen, S.J.; Li, D.; Lv, B.; Wang, X.Y.; Wu, X.L. Synergistic anti-oxidative/anti-inflammatory treatment for acute lung injury with selenium based chlorogenic acid nanoparticles through modulating Mapk8ip1/MAPK and Itga2b/PI3k-AKT axis. J. Nanobiotechnol. 2025, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.B.; Zhang, H.; Hao, D.Z.; Wang, J.Q.; Zhu, R.X.; Liu, W.A.; Liu, C. Selenium regulates the mitogen-activated protein kinase pathway to protect broilers from hexavalent chromium-induced kidney dysfunction and apoptosis. Ecotoxicol. Environ. Saf. 2022, 239, 113629. [Google Scholar] [CrossRef]
- Yuan, J.M.; Dong, X.D.; Yap, J.J.; Hu, J.C. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, M.; Zheng, F.; Yuan, C.; Wang, Z.; Qiu, S.; Meng, X.; Dong, J.; Liu, K.; Guo, L.; et al. Selenium elicited an enhanced anti-inflammatory effect in primary bovine endometrial stromal cells with high cortisol background. BMC Vet. Res. 2024, 20, 383. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Lim, W.W.; Viswanathan, S.; Chothani, S.; Ben, C.; Dasan, C.M.; Goh, J.W.T.; Lim, R.; Singh, B.K.; Tan, J.; et al. Inhibition of IL-11 signalling extends mammalian healthspan and lifespan. Nature 2024, 632, 157–165. [Google Scholar] [CrossRef]
- Luo, J.; Xu, Y.W.; Zhang, M.F.; Gao, L.; Fang, C.; Zhou, C.Q. Magnolol Inhibits LPS-Induced Inflammatory Response in Uterine Epithelial Cells. Inflammation 2013, 36, 997–1003. [Google Scholar] [CrossRef]
- Cheleuitte, D.; Mizuno, S.; Glowacki, J. In vitro secretion of cytokines by human bone marrow: Effects of age and estrogen status. J. Clin. Endocrinol. Metab. 1998, 83, 2043–2051. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, X.J.; Yao, Y.J.; Zhao, X.; Shi, X.; Cai, Y. Selenium Deficiency Induces Apoptosis and Necroptosis Through ROS/MAPK Signal in Human Uterine Smooth Muscle Cells. Biol. Trace Elem. Res. 2022, 200, 3147–3158. [Google Scholar] [CrossRef] [PubMed]
- Conley, S.M.; Bruhn, R.L.; Morgan, P.V.; Stamer, W.D. Selenium’s effects on MMP-2 and TIMP-1 secretion by human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 473–479. [Google Scholar] [CrossRef]
- Kreindl, C.; Soto-Alarcón, S.A.; Hidalgo, M.; Riveros, A.L.; Añazco, C.; Pulgar, R.; Porras, O. Selenium Compounds Affect Differently the Cytoplasmic Thiol/Disulfide State in Dermic Fibroblasts and Improve Cell Migration by Interacting with the Extracellular Matrix. Antioxidants 2024, 13, 159. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Toxicity of repeated oral intake of organic selenium, inorganic selenium, and selenium nanoparticles: A review. J. Trace Elem. Med. Biol. 2023, 79, 127235. [Google Scholar] [CrossRef]
- Deglincerti, A.; Croft, G.F.; Pietila, L.N.; Zernicka-Goetz, M.; Siggia, E.D.; Brivanlou, A.H. Self-organization of the in vitro attached human embryo. Nature 2016, 533, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Bosl, M.R.; Takaku, K.; Oshima, M.; Nishimura, S.; Taketo, M.M. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp). Proc. Natl. Acad. Sci. USA 1997, 94, 5531–5534. [Google Scholar] [CrossRef]
- Mamon, M.A.C.; Ramos, G.B. Maternal selenium-supplementation at various stages of periconception period: Influence on murine blastocyst morphology and implantation status. J. Anim. Sci. Technol. 2017, 59, 7. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Lin, N. Three Oxidative Stress-Related Genes That Associate Endometrial Immune Cells Are Considered as Potential Biomarkers for the Prediction of Unexplained Recurrent Implantation Failure. Front. Immunol. 2022, 13, 902268. [Google Scholar] [CrossRef]
- Deluao, J.C.; Winstanley, Y.; Robker, R.L.; Pacella-Ince, L.; Gonzalez, M.B.; McPherson, N.O. OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: Reactive oxygen species in the mammalian pre-implantation embryo. Reproduction 2022, 164, F95–F108. [Google Scholar] [CrossRef]
- Wróblewski, M.; Wróblewska, W.; Sobiesiak, M. The Role of Selected Elements in Oxidative Stress Protection: Key to Healthy Fertility and Reproduction. Int. J. Mol. Sci. 2024, 25, 9409. [Google Scholar] [CrossRef]
- Avery, J.C.; Yamazaki, Y.; Hoffmann, F.W.; Folgelgren, B.; Hoffmann, P.R. Selenoprotein I is essential for murine embryogenesis. Arch. Biochem. Biophys. 2020, 689, 108444. [Google Scholar] [CrossRef]
- Moffett, A.; Chazara, O.; Colucci, F. Maternal allo-recognition of the fetus. Fertil. Steril. 2017, 107, 1269–1272. [Google Scholar] [CrossRef]
- Martinez, C.A.; Alvarez-Rodriguez, M.; Rodriguez-Martinez, H. A decreased expression of interferon stimulated genes in peri-implantation endometrium of embryo transfer recipient sows could contribute to embryo death. Animal 2022, 16, 100590. [Google Scholar] [CrossRef]
- Cai, X.; Jiang, Y.; Cao, Z.; Zhang, M.; Kong, N.; Yu, L.; Tang, Y.; Kong, S.; Deng, W.; Wang, H.; et al. Mst1-mediated phosphorylation of Nur77 improves the endometrial receptivity in human and mice. EBioMedicine 2023, 88, 104433. [Google Scholar] [CrossRef]
- Jiang, W.; Fu, Y.; Yang, F.; Yang, Y.; Liu, T.; Zheng, W.; Zeng, L.; Chen, T. Gracilaria lemaneiformis polysaccharide as integrin-targeting surface decorator of selenium nanoparticles to achieve enhanced anticancer efficacy. ACS Appl. Mater. Interfaces 2014, 6, 13738–13748. [Google Scholar] [CrossRef]
- Paiva, P.; Menkhorst, E.; Salamonsen, L.; Dimitriadis, E. Leukemia inhibitory factor and interleukin-11: Critical regulators in the establishment of pregnancy. Cytokine Growth Factor Rev. 2009, 20, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Qi, Q.; Yan, F.; Wang, C.; Hou, F.; Ren, W.; Zhang, L.; Hou, J. Enhancing the developmental competence of prepubertal lamb oocytes by supplementing the in vitro maturation medium with sericin and the fibroblast growth factor 2—Leukemia inhibitory factor—Insulin-like growth factor 1 combination. Theriogenology 2021, 159, 13–19. [Google Scholar] [CrossRef]
- Hofstee, P.; Bartho, L.A.; McKeating, D.R.; Radenkovic, F.; McEnroe, G.; Fisher, J.J.; Holland, O.J.; Vanderlelie, J.J.; Perkins, A.; Cuffe, J.S.M. Maternal selenium deficiency during pregnancy in mice increases thyroid hormone concentrations, alters placental function and reduces fetal growth. J. Physiol. 2019, 597, 5597–5617. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.; Vanderlelie, J.J.; Holland, O.; Perkins, A.V. Overexpression of Endogenous Anti-Oxidants with Selenium Supplementation Protects Trophoblast Cells from Reactive Oxygen Species-Induced Apoptosis in a Bcl-2-Dependent Manner. Biol. Trace Elem. Res. 2017, 177, 394–403. [Google Scholar] [CrossRef]
- Carr, S.; Jia, Y.; Crites, B.; Hamilton, C.; Burris, W.; Edwards, J.L.; Matthews, J.; Bridges, P.J. Form of Supplemental Selenium in Vitamin-Mineral Premixes Differentially Affects Early Luteal and Gestational Concentrations of Progesterone, and Postpartum Concentrations of Prolactin in Beef Cows. Animals 2020, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Pop, V.; Krabbe, J.; Maret, W.; Rayman, M. Plasma mineral (selenium, zinc or copper) concentrations in the general pregnant population, adjusted for supplement intake, in relation to thyroid function. Br. J. Nutr. 2021, 125, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo Alvarez, S.; Castañón, S.G.; Ruata, M.L.C.; Aragüés, E.F.; Terraz, P.B.; Irazabal, Y.G.; González, E.G.; Rodríguez, B.G. Updating of normal levels of copper, zinc and selenium in serum of pregnant women. J. Trace Elem. Med. Biol. 2007, 21 (Suppl. S1), 49–52. [Google Scholar] [CrossRef]
- Iqbal, S.; Ali, I.; Rust, P.; Kundi, M.; Ekmekcioglu, C. Selenium, Zinc, and Manganese Status in Pregnant Women and Its Relation to Maternal and Child Complications. Nutrients 2020, 12, 725. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, D.Y.; Zhang, Q.; Yin, T.; Jiang, T.T.; He, S.T.; Li, M.Z.; Yue, X.Y.; Luo, G.Y.; Tao, F.B.; et al. Serum Cu, Zn and IL-1β Levels May Predict Fetal Miscarriage Risk After IVF Cycles: A Nested Case-Control Study. Biol. Trace Elem. Res. 2023, 201, 5561–5574. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhang, L.; Sa, P.; Luo, J.; Li, M. Transcriptomic analysis reveals the effects of maternal selenium deficiency on placental transport, hormone synthesis, and immune response in mice. Metallomics 2022, 14, mfac062. [Google Scholar] [CrossRef] [PubMed]
- Cindrova-Davies, T.; Sanders, D.A.; Burton, G.J.; Charnock-Jones, D.S. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc. Res. 2011, 89, 671–679. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Liu, T.; Yang, J.; Sun, X.; Gao, X.J. The mechanism of selenium regulating the permeability of vascular endothelial cells through selenoprotein O. Redox Biol. 2024, 70, 103063. [Google Scholar] [CrossRef]
- Jamilian, M.; Samimi, M.; Ebrahimi, F.A.; Aghadavod, E.; Mohammadbeigi, R.; Rahimi, M.; Asemi, Z. Effects of Selenium Supplementation on Gene Expression Levels of Inflammatory Cytokines and Vascular Endothelial Growth Factor in Patients with Gestational Diabetes (Publication with Expression of Concern). Biol. Trace Elem. Res. 2018, 181, 199–206. [Google Scholar] [CrossRef]
- Wu, S.; Chen, N.; Tong, X.; Xu, X.; Chen, Q.; Wang, F. Selenium attenuates the cadmium-induced placenta glucocorticoid barrier damage by up-regulating the expression of specificity protein 1. J. Biochem. Mol. Toxicol. 2022, 36, e23056. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Gu, G.; Wang, Q.; Guo, M. Selenoprotein N Was Required for the Regulation of Selenium on the Uterine Smooth Muscle Contraction in Mice. Biol. Trace Elem. Res. 2018, 183, 138–146. [Google Scholar] [CrossRef]
- You, J.S.; Kim, Y.; Lee, S.; Bashir, R.; Chen, J. RhoA/ROCK signalling activated by ARHGEF3 promotes muscle weakness via autophagy in dystrophic mdx mice. J. Cachexia Sarcopenia Muscle 2023, 14, 1880–1893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, X.R.; Riddick, N.; Bryden, M.; Baur, W.; Zhang, X.; Surks, H.K. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ. Res. 2009, 104, 531–540. [Google Scholar] [CrossRef]
- Chen, C.J.; Xiao, P.; Chen, Y.; Fang, R. Selenium Deficiency Affects Uterine Smooth Muscle Contraction Through Regulation of the RhoA/ROCK Signalling Pathway in Mice. Biol. Trace Elem. Res. 2019, 192, 277–286. [Google Scholar] [CrossRef]
- Qian, F.; Misra, S.; Prabhu, K.S. Selenium and selenoproteins in prostanoid metabolism and immunity. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 484–516. [Google Scholar] [CrossRef]
- Kalansuriya, D.M.; Lim, R.; Lappas, M. In vitroselenium supplementation suppresses key mediators involved in myometrial activation and rupture of fetal membranes. Metallomics 2020, 12, 935–951. [Google Scholar] [CrossRef]
- Lappas, M.; Permezel, M. The anti-inflammatory and antioxidative effects of nicotinamide, a vitamin B3 derivative, are elicited by FoxO3 in human gestational tissues: Implications for preterm birth. J. Nutr. Biochem. 2011, 22, 1195–1201. [Google Scholar] [CrossRef]
- Sato, T.; Michizu, H.; Hashizume, K.; Ito, A. Hormonal regulation of PGE2 and COX-2 production in rabbit uterine cervical fibroblasts. J. Appl. Physiol. 2001, 90, 1227–1231. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Wang, J.; Zhao, W. The emerging role of neutrophil extracellular traps in endometritis. Front. Immunol. 2023, 14, 1153851. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, H.; Zhang, M.; Wang, Z.; Dong, J.; Cui, L.; Guo, L.; Liu, K.; Li, J.; Wang, H. Selenium Yeast Alleviates Escherichia coli-Induced Endometritis in Goats Under High Cortisol Background. Animals 2025, 15, 693. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, C.; Li, W.; Mu, W.; Li, C.; Guo, M. Selenium Plays a Protective Role in Staphylococcus aureus-Induced Endometritis in the Uterine Tissue of Rats. Biol. Trace Elem. Res. 2016, 173, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Cerri, R.L.A.; Rutigliano, H.M.; Lima, F.S.; Araújo, D.B.; Santos, J.E.P. Effect of source of supplemental selenium on uterine health and embryo quality in high-producing dairy cows. Theriogenology 2009, 71, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Lin, T.; Yue, X.; Zhang, S.; Liu, X.; Chen, F.; Zhang, S.; Guan, W. Maternal Selenium-Enriched Yeast Supplementation in Sows Enhances Offspring Growth and Antioxidant Status through the Nrf2/Keap1 Pathway. Antioxidants 2023, 12, 2064. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Q.; Wang, Y.; Gan, Z.; Wu, M.; Shang, L.; Duan, P. Multiresponsive Microcapsules for Prevention of Intrauterine Adhesion. ACS Nano 2025, 19, 6499–6510. [Google Scholar] [CrossRef]
- Li, H.; Dong, J.; Cui, L.; Liu, K.; Guo, L.; Li, J.; Wang, H. The effect and mechanism of selenium supplementation on the proliferation capacity of bovine endometrial epithelial cells exposed to lipopolysaccharide in vitro under high cortisol background. J. Anim. Sci. 2024, 102, skae021. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Huang, K.; Zhu, M.; Liu, Q.; Liu, G.; Chen, F.; Zhang, H.; Qin, S. Selenium enriched Bacillus subtilis yb-1114246 activated the TLR2-NF-κB1 signaling pathway to regulate chicken intestinal β-defensin 1 expression. Food Funct. 2021, 12, 5913–5926. [Google Scholar] [CrossRef]
- Lv, H.; Sun, H.; Wang, L.; Yao, S.; Liu, D.; Zhang, X.; Pei, Z.; Zhou, J.; Wang, H.; Dai, J.; et al. Targeting CD301(+) macrophages inhibits endometrial fibrosis and improves pregnancy outcome. EMBO Mol. Med. 2023, 15, e17601. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Nguyen, H.N.; Brenner, M.B. Fibroblast pathology in inflammatory diseases. J. Clin. Investig. 2021, 131, e149538. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Lin, W.; Tang, Y.; Li, T.; Chen, Q.; Zhang, W.; Zhou, X.; Ma, J.; Liu, B.; Yu, Z.; et al. Selenium supplementation elevated SELENBP1 to inhibit fibroblast activation in pulmonary arterial hypertension. iScience 2024, 27, 111036. [Google Scholar] [CrossRef]
- Lin, J.-H.; Liu, C.-C.; Liu, C.-Y.; Hsu, T.-W.; Yeh, Y.-C.; How, C.-K.; Hsu, H.-S.; Hung, S.-C. Selenite selectively kills lung fibroblasts to treat bleomycin-induced pulmonary fibrosis. Redox Biol. 2024, 72, 103148. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Peng, Y.; Tang, Q.; Liu, K.; Peng, C. Human Serum Albumin/Selenium Complex Nanoparticles Protect the Skin from Photoaging Injury. Int. J. Nanomed. 2024, 19, 9161–9174. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tian, X.; Zhou, Q.; Lv, J.; Li, Y.; Jin, C.; Liu, H.; Yang, H.; Xu, Y.; He, F.; et al. Selenium nanoparticles ameliorate lumbar disc degeneration by restoring GPX1-mediated redox homeostasis and mitochondrial function of nucleus pulposus cells. J. Nanobiotechnol. 2024, 22, 634. [Google Scholar] [CrossRef]
- Casaril, A.M.; Ignasiak, M.T.; Chuang, C.Y.; Vieira, B.; Padilha, N.B.; Carroll, L.; Lenardão, E.J.; Savegnago, L.; Davies, M.J. Selenium-containing indolyl compounds: Kinetics of reaction with inflammation-associated oxidants and protective effect against oxidation of extracellular matrix proteins. Free. Radic. Biol. Med. 2017, 113, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.D.; Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004, 16, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Rudra, D.S.; Pal, U.; Chowdhury, N.; Maiti, N.C.; Bagchi, A.; Swarnakar, S. Omeprazole prevents stress induced gastric ulcer by direct inhibition of MMP-2/TIMP-3 interactions. Free. Radic. Biol. Med. 2022, 181, 221–234. [Google Scholar] [CrossRef]
- Chen, J.; Ren, Z.; Zhu, M.; Khalil, R.A. Decreased homodimerization and increased TIMP-1 complexation of uteroplacental and uterine arterial matrix metalloproteinase-9 during hypertension-in-pregnancy. Biochem. Pharmacol. 2017, 138, 81–95. [Google Scholar] [CrossRef]
- Alpoim-Moreira, J.; Fernandes, C.; Pimenta, J.; Bliebernicht, M.; Rebordão, M.R.; Castelo-Branco, P.; Szóstek-Mioduchowska, A.; Skarzynski, D.J.; Ferreira-Dias, G. Metallopeptidades 2 and 9 genes epigenetically modulate equine endometrial fibrosis. Front. Vet. Sci. 2022, 9, 970003. [Google Scholar] [CrossRef]
- Centeno, L.A.M.; Bastos, H.B.A.; Bueno, V.L.C.; Trentin, J.M.; Fiorenza, M.; Panziera, W.; Winter, G.H.Z.; Kretzmann, N.A.; Fiala-Rechsteiner, S.; Mattos, R.C.; et al. Collagen and collagenases in mare’s endometrium with endometrosis. Theriogenology 2024, 230, 28–36. [Google Scholar] [CrossRef]
- Laven, R.A.; Peters, A.R. Bovine retained placenta: Aetiology, pathogenesis and economic loss. Vet. Rec. 1996, 139, 465–471. [Google Scholar] [CrossRef]
- Julien, W.E.; Conrad, H.R.; Jones, J.E.; Moxon, A.L. Selenium and vitamin E and incidence of retained placenta in parturient dairy cows. J. Dairy Sci. 1976, 59, 1954–1959. [Google Scholar] [CrossRef]
- Bourne, N.; Laven, R.; Wathes, D.C.; Martinez, T.; McGowan, M. A meta-analysis of the effects of Vitamin E supplementation on the incidence of retained foetal membranes in dairy cows. Theriogenology 2007, 67, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, R.G. The influences of dietary intakes and supplementation with selenium and vitamin E on reproduction diseases and reproductive efficiency in cattle and sheep. Vet. Res. Commun. 2003, 27, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Kommisrud, E.; Osterås, O.; Vatn, T. Blood selenium associated with health and fertility in Norwegian dairy herds. Acta Vet. Scand. 2005, 46, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cheng, W.; Zhang, L. Maternal selenium deficiency suppresses proliferation, induces autophagy dysfunction and apoptosis in the placenta of mice. Metallomics 2021, 13, mfab058. [Google Scholar] [CrossRef]
- Brzezinska-Slebodzinska, E.; Miller, J.K.; Quigley, J.D.; Moore, J.R.; Madsen, F.C. Antioxidant Status of Dairy Cows Supplemented Prepartum with Vitamin E and Selenium. J. Dairy Sci. 1994, 77, 3087–3095. [Google Scholar] [CrossRef]
- Middelkoop, M.-A.; Don, E.E.; Hehenkamp, W.J.K.; Polman, N.J.; Griffioen, A.W.; Huirne, J.A.F. Angiogenesis in abnormal uterine bleeding: A narrative review. Hum. Reprod. Updat. 2023, 29, 457–485. [Google Scholar] [CrossRef]
- Valero, P.; Fuentes, G.; Cornejo, M.; Vega, S.; Grismaldo, A.; Pardo, F.; García-Rivas, G.; Hillebrands, J.-L.; Faas, M.M.; Casanello, P.; et al. Exposome and foetoplacental vascular dysfunction in gestational diabetes mellitus. Mol. Asp. Med. 2022, 87, 101019. [Google Scholar] [CrossRef]
- Lopes Junior, E.; Leite, H.P.; Konstantyner, T. Selenium and selenoproteins: From endothelial cytoprotection to clinical outcomes. Transl. Res. 2019, 208, 85–104. [Google Scholar] [CrossRef]
- Panda, S.K.; Peng, V.; Sudan, R.; Ulezko Antonova, A.; Di Luccia, B.; Ohara, T.E.; Fachi, J.L.; Grajales-Reyes, G.E.; Jaeger, N.; Trsan, T.; et al. Repression of the aryl-hydrocarbon receptor prevents oxidative stress and ferroptosis of intestinal intraepithelial lymphocytes. Immunity 2023, 56, 797–812.e794. [Google Scholar] [CrossRef]
- Wesolowski, L.T.; Semanchik, P.L.; White-Springer, S.H. Beyond antioxidants: Selenium and skeletal muscle mitochondria. Front. Vet. Sci. 2022, 9, 1011159. [Google Scholar] [CrossRef]
- Bodnár, D.; Ruzsnavszky, O.; Oláh, T.; Dienes, B.; Balatoni, I.; Ungvári, É.; Benkő, I.; Babka, B.; Prokisch, J.; Csernoch, L.; et al. Dietary selenium augments sarcoplasmic calcium release and mechanical performance in mice. Nutr. Metab. 2016, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, A.M.; Noble, K.; Wray, S. Extracellular acidification increases uterine contraction in pregnant mouse by increasing intracellular calcium. Acta Physiol. 2024, 240, e14147. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, W.; Wang, J.; Chen, T. Theranostic applications of selenium nanomedicines against lung cancer. J. Nanobiotechnol. 2023, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhang, X.; Pi, S.; Chang, J.; Dou, X.; Yan, S.; Song, X.; Chen, Y.; Zeng, X.; Zhu, L.; et al. Dietary supplementation with biogenic selenium nanoparticles alleviate oxidative stress-induced intestinal barrier dysfunction. NPJ Sci. Food 2022, 6, 30. [Google Scholar] [CrossRef] [PubMed]

| Species | Organic Selenium | Inorganic Selenium |
|---|---|---|
| Hens [26] | Bacterial organic selenium supplementation promotes eggshell mineralization and increases blood selenium concentrations. | Supplementation with inorganic selenium also increased antioxidant enzyme levels, but GPX3/GPX4 levels were much lower than supplementation with organic selenium. |
| Cows [27] | Conceptus length was increased in heifers supplemented with MIX (both ISe and Ose) (25.96 ± 3.95 cm) compared with ISe (17.45 ± 3.08 cm). | Glucose in amniotic fluid of ISe-supplemented ewes was less than in the MIX group. |
| Rats [28] | Selenium concentration in placenta samples from the selenium nanoparticle group was 2-fold higher than that in placenta samples from the sodium selenate-treated group. | Body weights of pregnant rats in the SeNPs group were lower than those in the NaSe group at day 21. |
| Pigs [29] | Increase in litter weight of piglets in HMSeBA-supplemented dams during pregnancy compared to the Na2SeO3 group. | Reduced elevation of maternal antioxidant enzyme activities in the Na2SeO3 supplemented group compared to HMSeBA supplemented during pregnancy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Li, X.; Li, Z.; Luo, J.; Zhang, Z.; Guo, M. Selenium and Mammalian Uterine Health: A Comprehensive Review. Biology 2025, 14, 1295. https://doi.org/10.3390/biology14091295
Wu R, Li X, Li Z, Luo J, Zhang Z, Guo M. Selenium and Mammalian Uterine Health: A Comprehensive Review. Biology. 2025; 14(9):1295. https://doi.org/10.3390/biology14091295
Chicago/Turabian StyleWu, Ruoning, Xiaohan Li, Zhaoming Li, Jilong Luo, Ziwei Zhang, and Mengyao Guo. 2025. "Selenium and Mammalian Uterine Health: A Comprehensive Review" Biology 14, no. 9: 1295. https://doi.org/10.3390/biology14091295
APA StyleWu, R., Li, X., Li, Z., Luo, J., Zhang, Z., & Guo, M. (2025). Selenium and Mammalian Uterine Health: A Comprehensive Review. Biology, 14(9), 1295. https://doi.org/10.3390/biology14091295









