Imiquimod (R837), a TLR7-Specific Agonist, Regulates Boar Sperm Motility via PI3K/GSK3α/β/Hexokinase Pathway
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Boar Sperm Swim-Up Technique
2.4. Analyses of Sperm Motility
2.5. Analyses of Swim-Up Sperm Count
2.6. Analyses of Sperm Acrosome Integrity and Membrane Integrity
2.7. Analyses of Sperm ATP Content
2.8. Metabolite Analysis
2.9. Analyses of Sperm Mitochondrial Membrane Potential
2.10. Analyses of Sperm Hexokinase (HK), 6-Phosphofructokinase (PFK), and Pyruvate Kinase (PK) Activities
2.11. Flow Cytometric Analysis of TLR7
2.12. Western Blotting Analysis
2.13. Immunofluorescence (IF) of Sperm
2.14. Statistical Analysis
3. Results
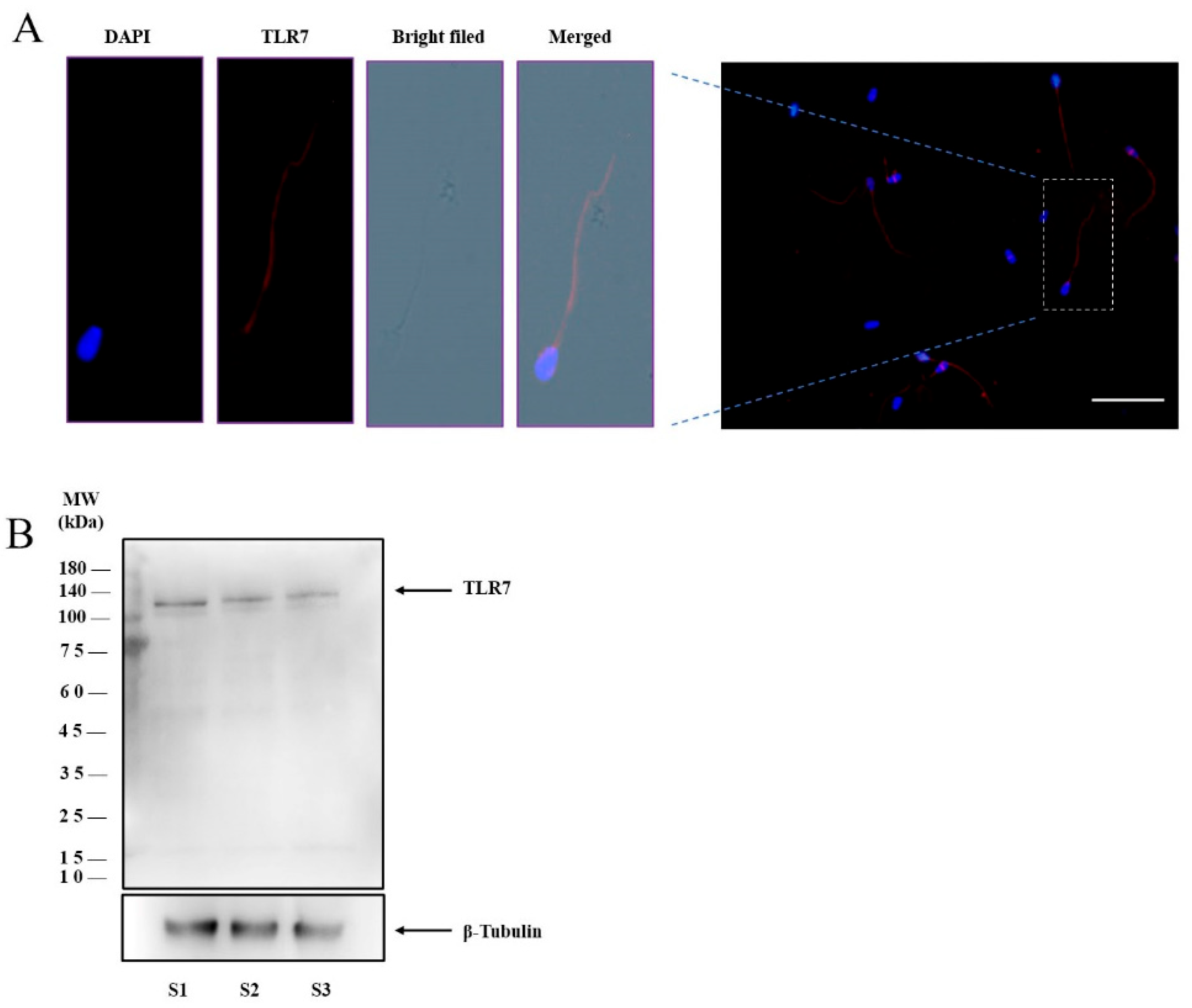
3.1. TLR7 Expression in Boar Sperm
3.2. Sperm Incubated with R837 Exhibited Decreased Motility and Preserved Acrosome and Membrane Integrity
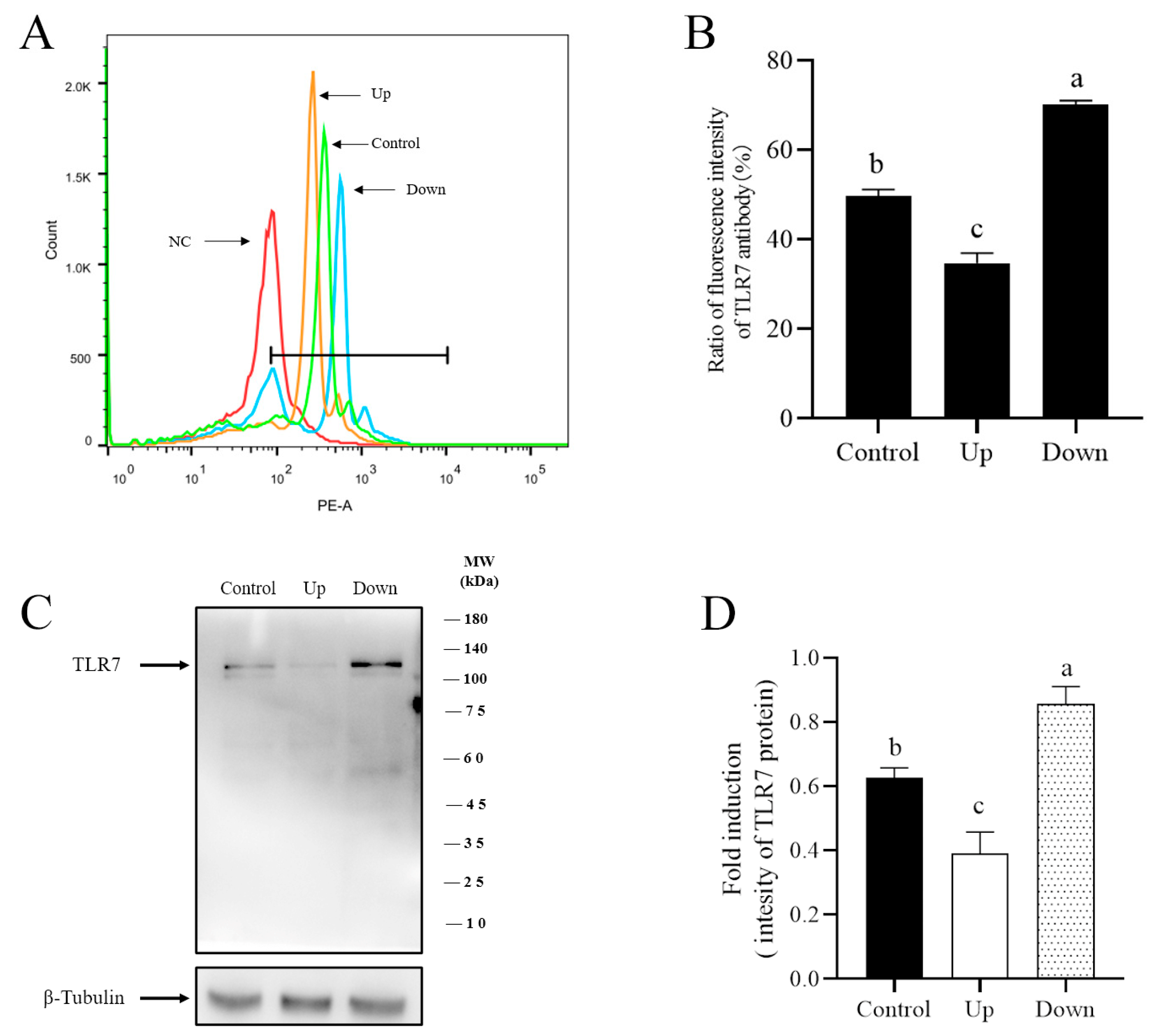
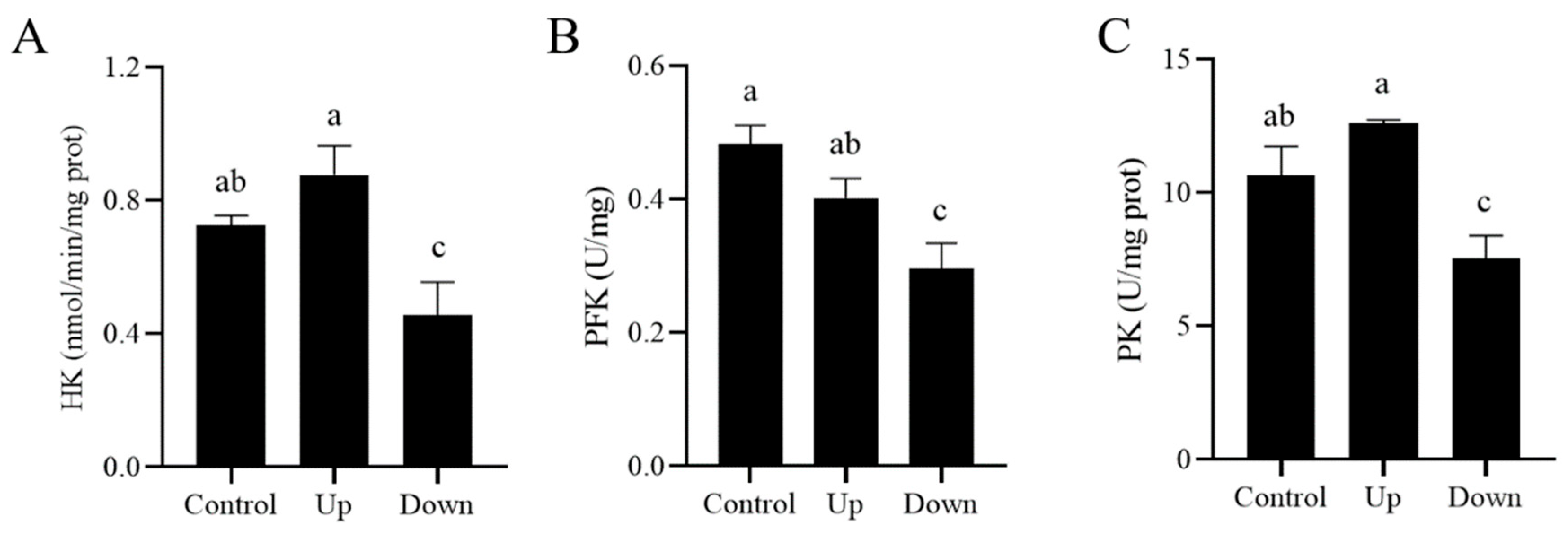
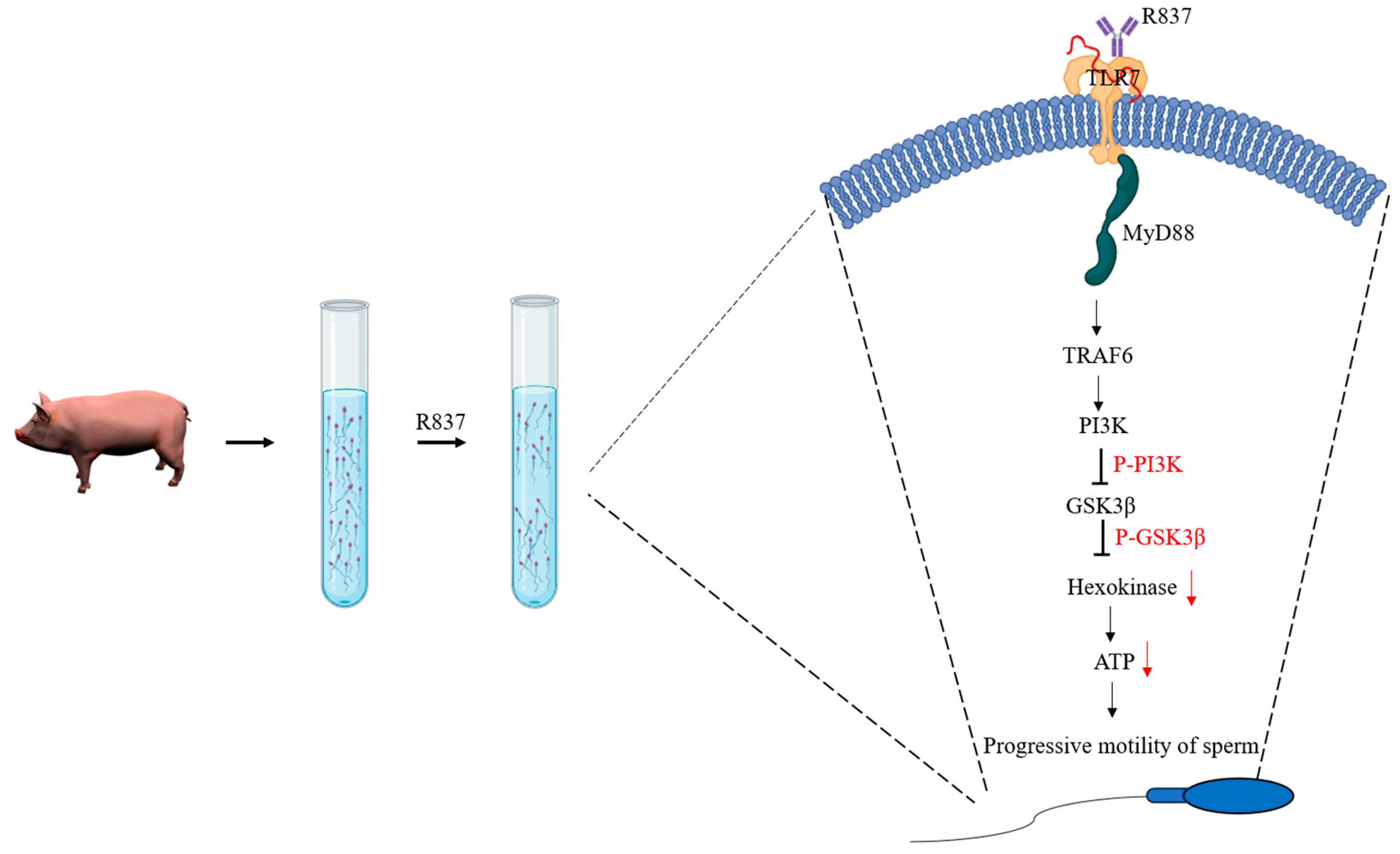
3.3. R837, a TRL7 Agonist, Decreased Sperm Motility Through Mitochondrial Activity and the PI3K/GSK3α/β/Hexokinase Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bortolozzo, F.P.; Zanin, G.P.; Christ, T.S.; Rech, R.D.; da Rosa Ulguim, R.; Mellagi, A.P.G. Artificial insemination and optimization of the use of seminal doses in swine. Anim. Reprod. Sci. 2024, 18, 107501. [Google Scholar] [CrossRef] [PubMed]
- García-Vázquez, F.A.; Gadea, J.; Matás, C.; Holt, W.V. Importance of sperm morphology during sperm transport and fertilization in mammals. Asian J. Androl. 2016, 18, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Pusch, H.H. The importance of sperm motility for the fertilization of human oocytes in vivo and in vitro. Andrologia 1987, 19, 514–527. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Miki, K. Energy metabolism and sperm function. Soc. Reprod. Fertil. Suppl. 2007, 65, 309–325. [Google Scholar]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm chromatin structure assay: Its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J. Androl. 2002, 23, 25–43. [Google Scholar] [CrossRef]
- Castillo, J.; Amaral, A.; Oliva, R. Sperm nuclear proteome and its epigenetic potential. Andrology 2014, 2, 326–338. [Google Scholar] [CrossRef]
- Freitas, M.J.; Silva, J.V.; Brothag, C.; Regadas-Correia, B.; Fardilha, M.; Vijayaraghavan, S. Isoform-specific GSK3A activity is negatively correlated with human sperm motility. Mol. Hum. Reprod. 2019, 25, 171–183. [Google Scholar] [CrossRef]
- Adetunji, A.O.; Kawai, T.; Shimada, M. Impact of lipopolysaccharide administration on luteinizing hormone/choriogonadotropin receptor (Lhcgr) expression in mouse ovaries. J. Reprod. Immunol. 2020, 142, 103193. [Google Scholar] [CrossRef]
- Nazmi, A.; Mukherjee, S.; Kundu, K.; Dutta, K.; Mahadevan, A.; Shankar, S.K.; Basu, A. TLR7 is a key regulator of innate immunity against Japanese encephalitis virus infection. Neurobiol. Dis. 2014, 69, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zheng, M.; Lv, H.; Guo, K.; Zhang, Y. Tissue expression of Toll-like receptors 2, 3, 4 and 7 in swine in response to the Shimen strain of classical swine fever virus. Mol. Med. Rep. 2018, 17, 7122–7130. [Google Scholar] [CrossRef]
- Thomalla, M.; Schmid, A.; Hehner, J.; Koehler, S.; Neumann, E.; Muller-Ladner, U.; Schaffler, A.; Karrasch, T. Toll-like Receptor 7 (TLR7) Is Expressed in Adipocytes and the Pharmacological TLR7 Agonist Imiquimod and Adipocyte-Derived Cell-Free Nucleic Acids (cfDNA) Regulate Adipocyte Function. Int. J. Mol. Sci. 2022, 23, 8475. [Google Scholar] [CrossRef]
- Fujita, Y.; Mihara, T.; Okazaki, T.; Shitanaka, M.; Kushino, R.; Ikeda, C.; Negishi, H.; Liu, Z.; Richards, J.S.; Shimada, M. Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum. Reprod. 2011, 26, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Guo, H.; Gong, Y.; Zhao, R. Lipopolysaccharide-induced mitochondrial dysfunction in boar sperm is mediated by activation of oxidative phosphorylation. Theriogenology 2017, 87, 1–8. [Google Scholar] [CrossRef]
- Umehara, T.; Tsujita, N.; Zhu, Z.; Ikedo, M.; Shimada, M. A simple sperm-sexing method that activates TLR7/8 on X sperm for the efficient production of sexed mouse or cattle embryos. Nat. Protoc. 2020, 15, 2645–2667. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Cao, Z.; Li, J.; Long, H.; Wu, Y.; Zhang, Z.; Sun, Y. R848 Is Involved in the Antibacterial Immune Response of Golden Pompano (Trachinotus ovatus) Through TLR7/8-MyD88-NF-kappaB-Signaling Pathway. Front. Immunol. 2020, 11, 617522. [Google Scholar]
- Chosidow, O.; Dummer, R. Imiquimod: Mode of action and therapeutic potential. Acta Derm. Venereol. Suppl. 2003, 214, 8–11. [Google Scholar] [CrossRef]
- Ren, F.; Xi, H.; Ren, Y.; Li, Y.; Wen, F.; Xian, M.; Zhao, M.; Zhu, D.; Wang, L.; Lei, A.; et al. TLR7/8 signalling affects X-sperm motility via the GSK3 alpha/beta-hexokinase pathway for the efficient production of sexed dairy goat embryos. J. Anim. Sci. Biotechnol. 2021, 12, 89. [Google Scholar] [CrossRef]
- Wen, F.; Liu, W.; Li, Y.; Zou, Q.; Xian, M.; Han, S.; Zhang, H.; Liu, S.; Feng, X.; Hu, J. TLR7/8 agonist (R848) inhibit bovine X sperm motility via PI3K/GSK3alpha/beta and PI3K/NFkappaB pathways. Int. J. Biol. Macromol. 2023, 232, 123485. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.; Ismail, N.F.B.; Calvert, S.J.; Pacey, A.A.; Paley, M.N.J. Evidence for Rapid Oxidative Phosphorylation and Lactate Fermentation in Motile Human Sperm by Hyperpolarized (13)C Magnetic Resonance Spectroscopy. Sci. Rep. 2017, 7, 4322. [Google Scholar] [CrossRef]
- Liu, P.; Shi, J.; Sheng, D.; Lu, W.; Guo, J.; Gao, L.; Wang, X.; Wu, S.; Feng, Y.; Dong, D.; et al. Mitopherogenesis, a form of mitochondria-specific ectocytosis, regulates sperm mitochondrial quantity and fertility. Nat. Cell Biol. 2023, 25, 1625–1636. [Google Scholar] [CrossRef]
- Mbizvo, M.T.; Thomas, S.; Fulgham, D.L.; Alexander, N.J. Serum hormone levels affect sperm function. Fertil. Steril. 1990, 54, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Bonato, M.; Cornwallis, C.K.; Malecki, I.A.; Rybnik-Trzaskowska, P.K.; Cloete, S.W. The effect of temperature and pH on the motility and viability of ostrich sperm. Anim. Reprod. Sci. 2012, 133, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Bornhövd, C.; Vogel, F.; Neupert, W.; Reichert, A.S. Mitochondrial membrane potential is dependent on the oligomeric state of F1F0-ATP synthase supracomplexes. J. Biol. Chem. 2006, 19, 13990–13998. [Google Scholar] [CrossRef]
- Saeidi, S.; Shapouri, F.; Amirchaghmaghi, E.; Hoseinifar, H.; Sabbaghian, M.; Sadighi Gilani, M.A.; Pacey, A.A.; Aflatoonian, R. Sperm protection in the male reproductive tract by Toll-like receptors. Andrologia 2014, 46, 784–790. [Google Scholar] [CrossRef]
- Arias, M.E.; Andara, K.; Briones, E.; Felmer, R. Bovine sperm separation by Swim-up and density gradients (Percoll and BoviPure): Effect on sperm quality, function and gene expression. Reprod. Biol. 2017, 17, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Csakai, A.; Jiang, S.; Smith, C.; Tanji, H.; Huang, J.; Jones, T.; Sakaniwa, K.; Broadwell, L.; Shi, C.; et al. Tetrasubstituted imidazoles as incognito Toll-like receptor 8 a(nta)gonists. Nat. Commun. 2021, 16, 4351. [Google Scholar] [CrossRef]
- Beigi, S.A.D.; Khalili, M.A.; Nabi, A.; Hosseini, M.; Sarcheshmeh, A.A.; Sabour, M. Prolonged semen incubation alters the biological characteristics of human spermatozoa. Clin. Exp. Reprod. Med. 2022, 49, 270–276. [Google Scholar] [CrossRef]
- Bozrova, S.V.; Levitskii, V.A.; Nedospasov, S.A.; Drutskaia, M.S. [Imiquimod: The biochemical mechanisms of immunomodulatory and anti-inflammatory activity]. Biomed. Khim 2013, 59, 249–266. [Google Scholar] [CrossRef]
- Sassone-Corsi, P. Transcriptional checkpoints determining the fate of male germ cells. Cell 1997, 88, 163–166. [Google Scholar] [CrossRef]
- Lee, B.L.; Barton, G.M. Trafficking of endosomal Toll-like receptors. Trends Cell Biol. 2014, 24, 360–369. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Li, H.; Wang, H.; Zhang, T.; Hutchinson, M.R.; Yin, H.; Wang, X. Small-Molecule Modulators of Toll-like Receptors. Acc. Chem. Res. 2020, 53, 1046–1055. [Google Scholar] [CrossRef]
- Teixeira, H.S.; Zhao, J.; Kazmierski, E.; Kinane, D.F.; Benakanakere, M.R. TLR3-Dependent Activation of TLR2 Endogenous Ligands via the MyD88 Signaling Pathway Augments the Innate Immune Response. Cells 2020, 9, 1910. [Google Scholar] [CrossRef]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Cisse, O.; Quraishi, M.; Gulluni, F.; Guffanti, F.; Mavrommati, I.; Suthanthirakumaran, M.; Oh, L.C.R.; Schlatter, J.N.; Sarvananthan, A.; Broggini, M.; et al. Downregulation of class II phosphoinositide 3-kinase PI3K-C2beta delays cell division and potentiates the effect of docetaxel on cancer cell growth. J. Exp. Clin. Cancer Res. 2019, 38, 472. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, Y.; Fan, W.; Jing, J.; Xue, K.; Zhang, X.; Ye, B.; Ji, Y.; Liu, Y.; Ding, Z. RNASET2 impairs the sperm motility via PKA/PI3K/calcium signal pathways. Reproduction 2018, 155, 383–392. [Google Scholar] [CrossRef]
- Harwood, A.J.; Plyte, S.E.; Woodgett, J.; Strutt, H.; Kay, R.R. Glycogen synthase kinase 3 regulates cell fate in Dictyostelium. Cell 1995, 80, 139–148. [Google Scholar] [CrossRef]
- Harwood, A.J. Regulation of GSK-3: A cellular multiprocessor. Cell 2001, 105, 821–824. [Google Scholar] [CrossRef]
- Aytenfisu, T.Y.; Campbell, H.M.; Chakrabarti, M.; Amzel, L.M.; Gabelli, S.B. Class I PI3K Biology. Curr. Top. Microbiol. Immunol. 2022, 436, 43–49. [Google Scholar]
- Risso, G.; Blaustein, M.; Pozzi, B.; Mammi, P.; Srebrow, A. Akt/PKB: One kinase, many modifications. Biochem. J. 2015, 468, 203–214. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Yang, W.; Mo, J.; Hong, L.; Zhang, X.; Huang, S. Expression of toll-like Receptors7/8 in boar reproductive system and evaluation of effect of their ligands on boar X/Ysperm sorting. Acta Vet. Zootech. Sin. 2023, 54, 1490–1499. [Google Scholar]





| Layer | Sperm Parameter | 0 μM | 0.05 μM | 0.1 μM | 0.2 μM | 0.4 μM | 0.8 μM |
|---|---|---|---|---|---|---|---|
| Upper layer | TM (%) | 86.9 ± 3.0 b | 92.3 ± 1.3 a | 89.5 ± 2.5 ab | 89.9 ± 1.0 ab | 91.3 ± 1.6 a | 81.6 ± 2.8 c |
| PM (%) | 68.2 ± 3.3 c | 67.3 ± 2.5 c | 71.2 ± 1.1 b | 81.1 ± 1.0 a | 67.2 ± 3.7 c | 58.2 ± 3.0 d | |
| VCL (μm/s) | 70.5 ± 5.5 b | 73.2 ± 2.5 ab | 72.2 ± 1.7 ab | 75.3 ± 3.0 a | 73.3 ± 5.6 ab | 66.8 ± 3.4 c | |
| VSL (μm/s) | 30.0 ± 1.0 | 28.3 ± 1.2 | 30.1 ± 0.7 | 32.8 ± 0.6 | 27.7 ± 1.1 | 27.0 ± 0.6 | |
| VAP (μm/s) | 45.1 ± 2.7 | 42.1 ± 1.8 | 44.3 ± 1.0 | 49.3 ± 1.5 | 43.5 ± 3.5 | 40.3 ± 1.9 | |
| LIN (μm/s) | 43.0 ± 2.6 | 38.9 ± 1.2 | 42.0 ± 1.2 | 43.8 ± 2.3 | 38.2 ± 2.0 | 40.7 ± 2.2 | |
| STR (%) | 66.9 ± 3.1 | 67.4 ± 2.6 | 68.5 ± 1.2 | 66.7 ± 2.7 | 64.5 ± 3.1 | 67.3 ± 3.2 | |
| WOB (%) | 64.2 ± 1.2 | 57.8 ± 1.8 | 61.3 ± 0.8 | 65.6 ± 0.7 | 59.3 ± 1.9 | 60.4 ± 0.5 | |
| ALH (μm) | 4.5 ± 0.3 | 4.7 ± 0.3 | 4.2 ± 0.1 | 3.9 ± 0.1 | 4.6 ± 0.3 | 4.1 ± 0.1 | |
| BCF (Hz) | 5.1 ± 0.1 | 5.0 ± 0.2 | 5.8 ± 0.1 | 6.3 ± 0.3 | 5.6 ± 0.5 | 5.9 ± 0.4 | |
| Lower layer | TM (%) | 85.2 ± 0.4 b | 90.9 ± 3.7 a | 89.0 ± 0.4 a | 87.8 ± 0.8 ab | 85.1 ± 1.7 b | 80.7 ± 0.3 c |
| PM (%) | 76.1 ± 0.7 a | 74.0 ± 2.8 a | 54.7 ± 1.8 c | 53.5 ± 0.5 c | 61.9 ± 1.9 b | 57.7 ± 0.4 b | |
| VCL (μm/s) | 72.2 ± 2.0 a | 72.2 ± 0.8 a | 58.2 ± 1.6 c | 58.2 ± 2.4 c | 64.8 ± 2.5 b | 63.3 ± 0.1 b | |
| VSL (μm/s) | 29.4 ± 0.5 a | 28.5 ± 0.4 a | 24.0 ± 0.5 b | 23.8 ± 0.7 c | 25.1 ± 2.5 b | 24.2 ± 0.2 b | |
| VAP (μm/s) | 43.2 ± 1.3 a | 40.8 ± 0.5 a | 32.4 ± 1.1 b | 33.0 ± 1.7 b | 35.5 ± 1.1 ab | 33.5 ± 0.2 b | |
| LIN (μm/s) | 40.8 ± 0.6 | 39.5 ± 0.8 | 41.3 ± 0.3 | 40.9 ± 0.6 | 38.9 ± 1.4 | 38.2 ± 0.2 | |
| STR (%) | 68.2 ± 1.1 | 70.0 ± 1.6 | 74.2 ± 0.9 | 72.4 ± 1.8 | 70.9 ± 1.7 | 72.0 ± 0.1 | |
| WOB (%) | 59.8 ± 0.2 | 56.4 ± 0.3 | 55.6 ± 0.3 | 56.5 ± 0.7 | 54.8 ± 0.8 | 53.0 ± 0.3 | |
| ALH (μm) | 4.2 ± 0.1 | 4.2 ± 0.2 | 3.6 ± 0.1 | 3.6 ± 0.1 | 3.8 ± 0.1 | 3.9 ± 0.1 | |
| BCF (Hz) | 6.1 ± 0.1 | 6.4 ± 0.8 | 6.6 ± 0.1 | 6.5 ± 0.2 | 7.5 ± 0.3 | 5.8 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Adetunji, A.O.; Zeng, W.; Bastawy, E.M.; Min, L.; Ding, N.; Zhu, Z. Imiquimod (R837), a TLR7-Specific Agonist, Regulates Boar Sperm Motility via PI3K/GSK3α/β/Hexokinase Pathway. Biology 2025, 14, 1182. https://doi.org/10.3390/biology14091182
Zhang W, Adetunji AO, Zeng W, Bastawy EM, Min L, Ding N, Zhu Z. Imiquimod (R837), a TLR7-Specific Agonist, Regulates Boar Sperm Motility via PI3K/GSK3α/β/Hexokinase Pathway. Biology. 2025; 14(9):1182. https://doi.org/10.3390/biology14091182
Chicago/Turabian StyleZhang, Weijing, Adedeji O. Adetunji, Wenxian Zeng, Eslam M. Bastawy, Lingjiang Min, Nengshui Ding, and Zhendong Zhu. 2025. "Imiquimod (R837), a TLR7-Specific Agonist, Regulates Boar Sperm Motility via PI3K/GSK3α/β/Hexokinase Pathway" Biology 14, no. 9: 1182. https://doi.org/10.3390/biology14091182
APA StyleZhang, W., Adetunji, A. O., Zeng, W., Bastawy, E. M., Min, L., Ding, N., & Zhu, Z. (2025). Imiquimod (R837), a TLR7-Specific Agonist, Regulates Boar Sperm Motility via PI3K/GSK3α/β/Hexokinase Pathway. Biology, 14(9), 1182. https://doi.org/10.3390/biology14091182







