Branched Setae or Attached Macroalgae: A Case Study of an Exceptionally Preserved Brachiopod from the Early Cambrian Chengjiang Lagerstätte
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
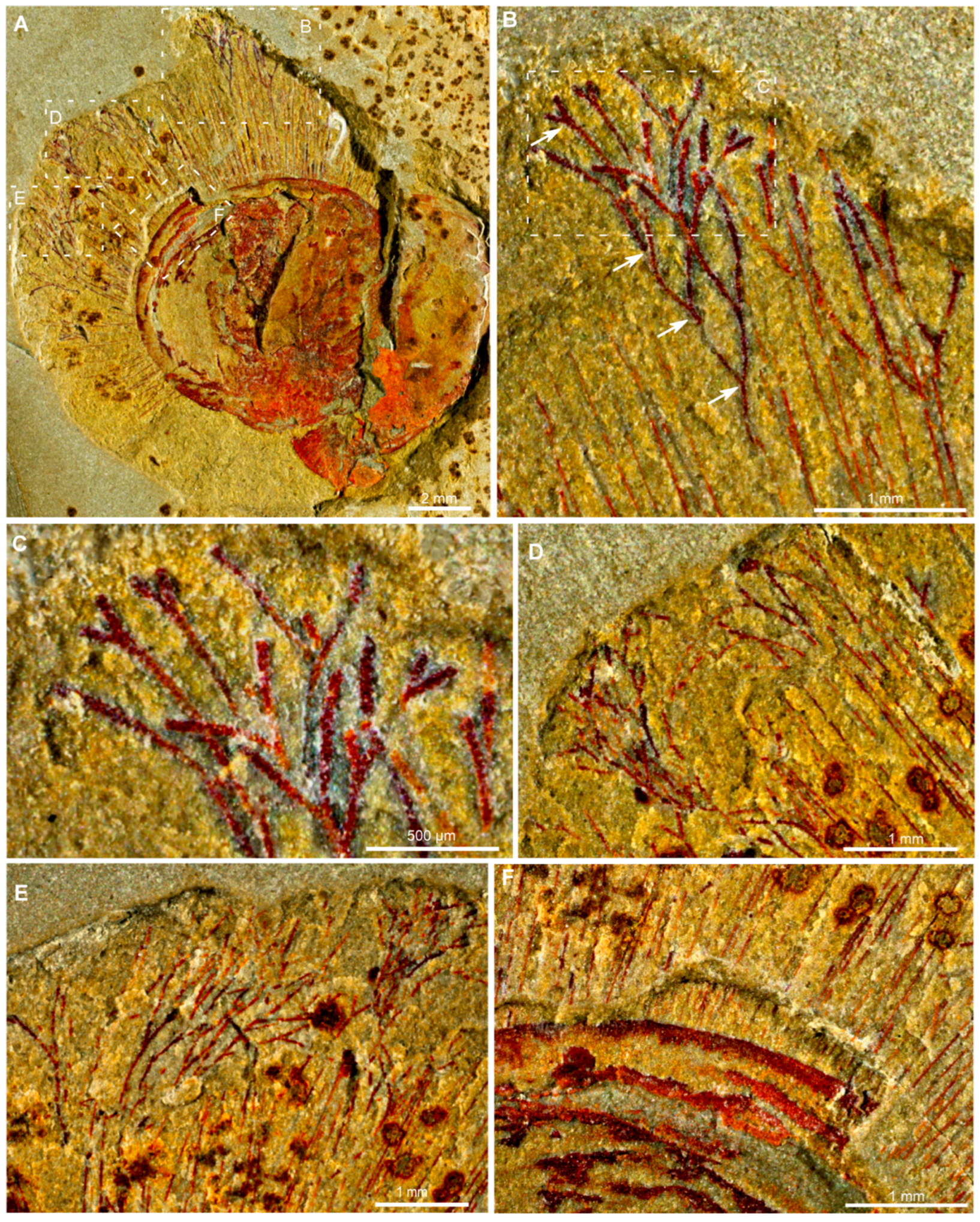
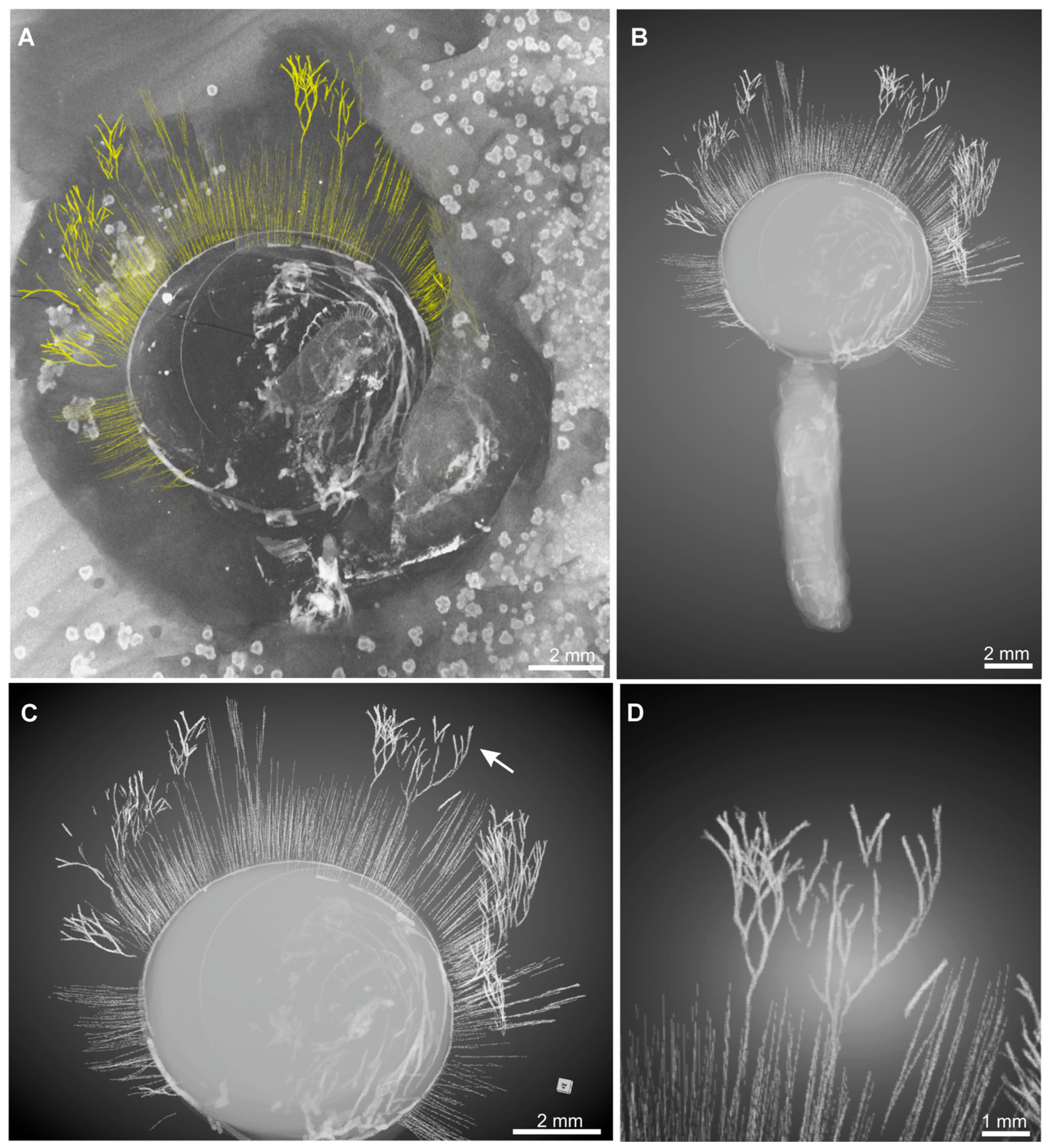
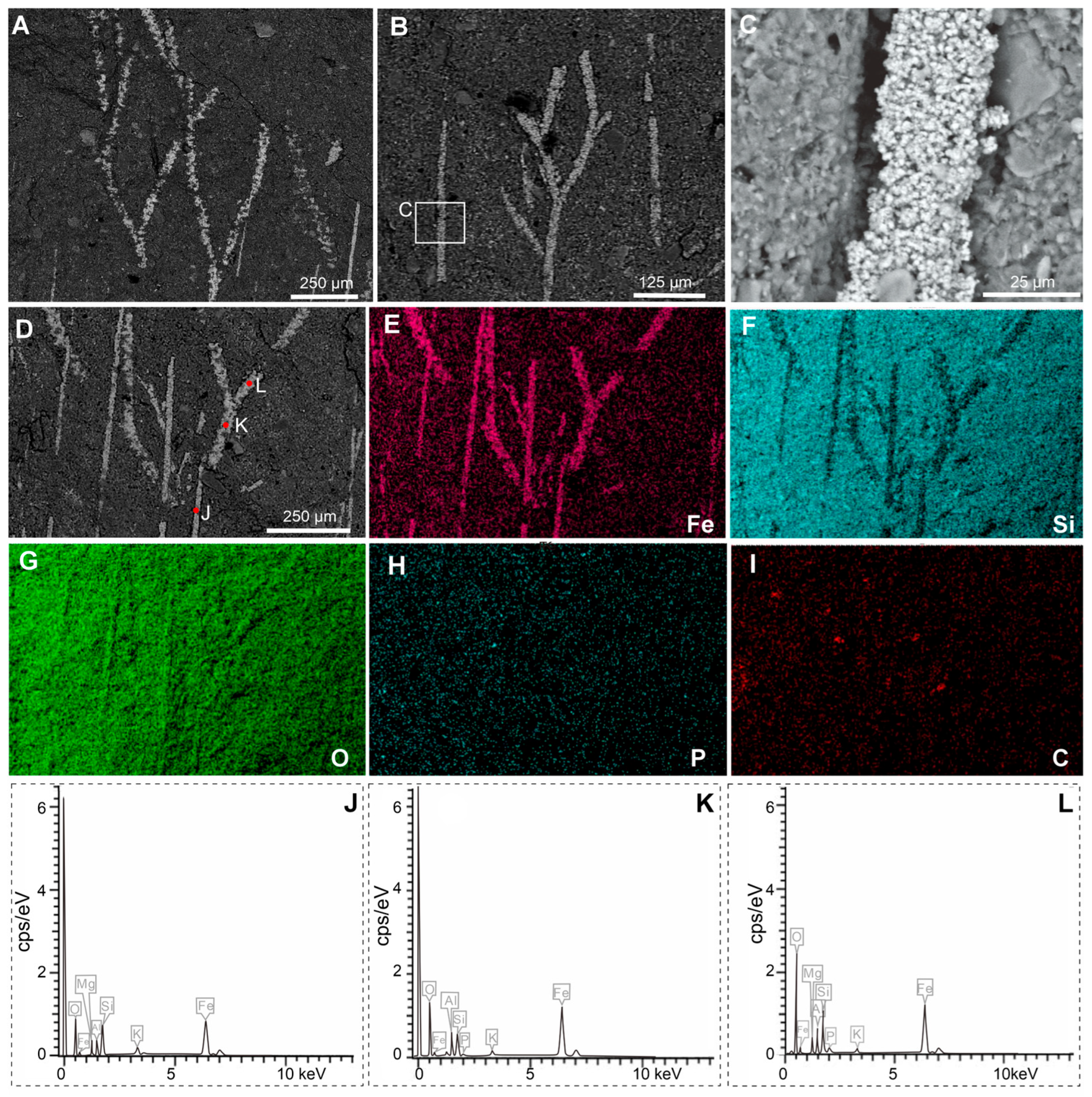
3. Results
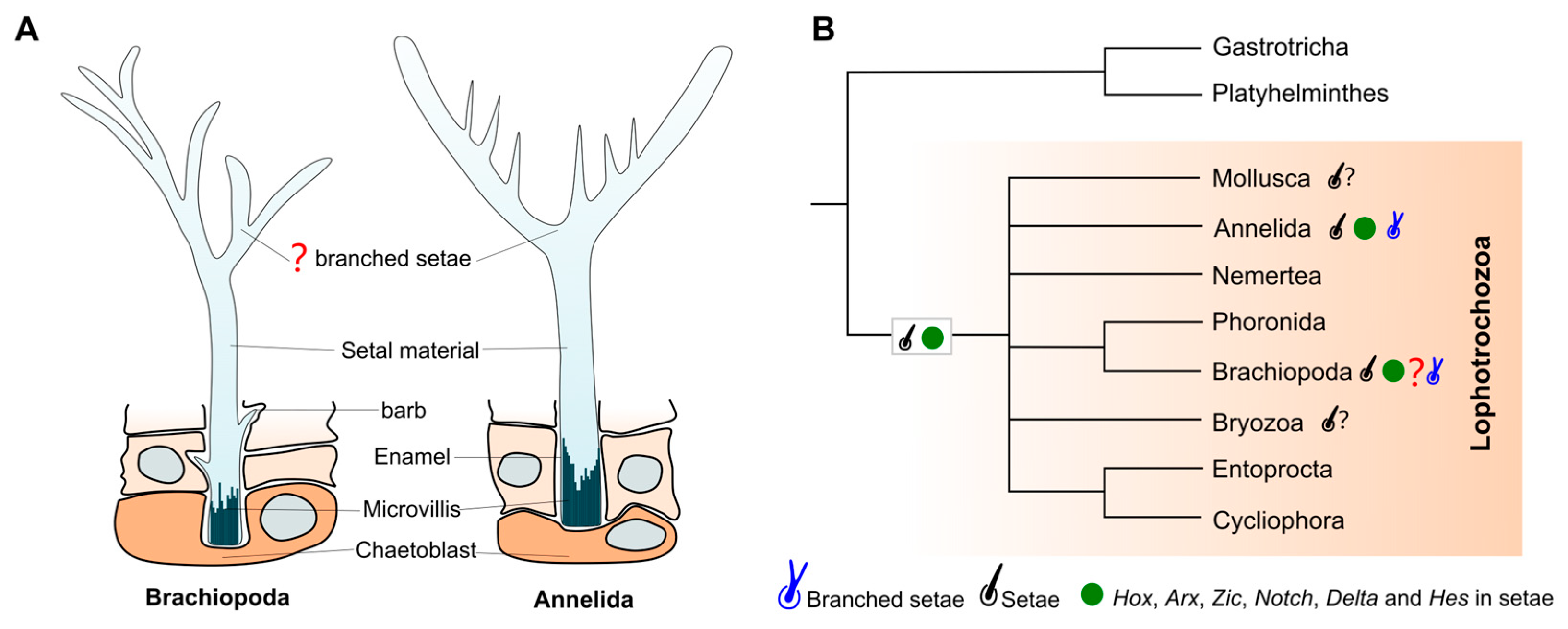
4. Discussion
4.1. Branched Fringes as Elongate Setae and Its Phylogenetical Implications
4.2. Branched Fringes as Attached Macroalgae and Their Ecological Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlson, S.J. The evolution of Brachiopoda. Annu. Rev. Earth Planet. Sci. 2016, 44, 409–438. [Google Scholar] [CrossRef]
- Liang, Y.; Strotz, L.C.; Topper, T.P.; Holmer, L.E.; Budd, G.E.; Chen, Y.; Fang, R.; Hu, Y.; Zhang, Z. Evolutionary contingency in lingulid brachiopods across mass extinctions. Curr. Biol. 2023, 33, 1565–1572. [Google Scholar] [CrossRef]
- Zhang, Z.; Robson, S.P.; Emig, C.; Shu, D. Early Cambrian radiation of brachiopods: A perspective from South China. Gondwana Res. 2008, 14, 241–254. [Google Scholar] [CrossRef]
- Sperling, E.A.; Pisani, D.; Peterson, K.J. Molecular Paleobiological Insights into the Origin of the Brachiopoda. Evol. Dev. 2011, 13, 290–303. [Google Scholar] [CrossRef]
- Luo, Y.; Kanda, M.; Koyanagi, R.; Hisata, K.; Akiyama, T.; Sakamoto, H.; Sakamoto, T.; Satoh, N. Nemertean and Phoronid Genomes Reveal Lophotrochozoan Evolution and the Origin of Bilaterian Heads. Nat. Ecol. Evol. 2017, 2, 141–151. [Google Scholar] [CrossRef]
- Clements, T.; Gabbott, T. Exceptional Preservation of Fossil Soft Tissues. Encycl. Life Sci. 2021, 2, 1–10. [Google Scholar]
- Topper, T.P.; Strotz, L.C.; Holmer, L.E.; Caron, J.B. Survival on a soft seafloor: Life strategies of brachiopods from the Cambrian Burgess Shale. Earth-Sci. Rev. 2015, 151, 266–287. [Google Scholar] [CrossRef]
- Zhang, Z.; Strotz, L.C.; Topper, T.P.; Chen, F.; Chen, Y.; Liang, Y.; Zhang, Z.; Skovsted, C.B.; Brock, G.A. An encrusting kleptoparasite-host interaction from the early Cambrian. Nat. Commun. 2020, 11, 2625. [Google Scholar] [CrossRef] [PubMed]
- Halanych, K.M.; Bacheller, J.D.; Aguinaldo, A.M.A.; Liva, S.M.; Hillis, D.M.; Lake, J.A. Evidence from 18S ribosomal DNA that the lophophorates are protostome animals. Science 1995, 267, 1641–1643. [Google Scholar] [CrossRef] [PubMed]
- Halanych, K.M. The new view of animal phylogeny. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 229–256. [Google Scholar] [CrossRef]
- Dunn, C.W.; Hejnol, A.; Matus, D.Q.; Pang, K.; Browne, W.E.; Smith, S.A.; Seaver, E.; Rouse, G.W.; Obst, M.; Edgecombe, G.D.; et al. Broad Phylogenomic Sampling Improves Resolution of the Animal Tree of Life. Nature 2008, 452, 745–749. [Google Scholar] [CrossRef]
- Laumer, C.E.; Bekkouche, N.; Kerbl, A.; Goetz, F.; Neves, R.C.; Sørensen, M.V.; Kristensen, R.M.; Hejnol, A.; Dunn, C.W.; Giribet, G.; et al. Spiralian phylogeny informs the evolution of microscopic lineages. Curr. Biol. 2015, 25, 2000–2006. [Google Scholar] [CrossRef]
- Liang, Y.; Topper, T.P.; Holmer, L.E.; Hu, Y.; Liu, F.; Zhang, Z. Exceptionally preserved setae: A Possible morphological synapomorphy of Cambrian Lophotrochozoans. Evol. Dev. 2025, 27, e70001. [Google Scholar] [CrossRef]
- Schiemann, S.M.; Martin-Duran, J.M.; Børve, A.; Vellutini, B.C.; Passamaneck, Y.J.; Hejnol, A. Clustered brachiopod Hox genes are not expressed collinearly and are associated with lophotrochozoan novelties. Proc. Natl. Acad. Sci. USA 2017, 114, E1913–E1922. [Google Scholar] [CrossRef]
- Eeckhaut, I.; McHugh, D.; Mardulyn, P.; Tiedemann, R.; Monteyne, D.; Jangoux, M.; Milinkovitch, M.C. Myzostomida: A link between Trochozoans and Flatworms. Proc. R. Soc. London Ser. B Biol. Sci. 2000, 267, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Nesnidal, M.P.; Helmkampf, M.; Bruchhaus, I.; Hausdorf, B. Compositional Heterogeneity and Phylogenomic Inference of Metazoan Relationships. Mol. Biol. Evol. 2010, 27, 2095–2104. [Google Scholar] [CrossRef]
- Struck, T.H.; Paul, C.; Hill, N.; Hartmann, S.; Hösel, C.; Kube, M.; Lieb, B.; Meyer, A.; Tiedemann, R.; Purschke, G.; et al. Phylogenomic analyses unravel annelid evolution. Nature 2011, 471, 95–98. [Google Scholar] [CrossRef]
- Collin, R.; Venera-Pontón, D.E.; Driskell, A.C.; Macdonald, K.S., III; Boyle, M.J. Planktotrophic Brachiopod Larvae from the Pacific and Caribbean of Panama. Diversity 2018, 11, 2. [Google Scholar] [CrossRef]
- Zakrzewski, A.C.; Suh, A.; Lüter, C. New Insights into the Larval Development of Macandrevia cranium (Müller, 1776) (Brachiopoda: Rhynchonelliformea). Zool. Anz. 2012, 251, 263–269. [Google Scholar] [CrossRef]
- Lüter, C. Ultrastructure of Larval and Adult Setae of Brachiopoda. Zool. Anz. 2000, 239, 75–90. [Google Scholar]
- Lüter, C.; Bartolomaeus, T. The Phylogenetic Position of Brachiopoda—A Comparison of Morphological and Molecular Data. Zool. Scr. 1997, 26, 245–253. [Google Scholar] [CrossRef]
- Giribet, G. Assembling the lophotrochozoan (=spiralian) tree of life. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1513–1522. [Google Scholar] [CrossRef]
- Gustus, R.M.; Cloney, R.A. Ultrastructural Similarities Between Setae of Brachiopods and Polychaetes. Acta Zool. 1972, 53, 229–233. [Google Scholar] [CrossRef]
- Zakrzewski, A.C. Molecular Characterization of Chaetae Formation in Annelida and Other Lophotrochozoa. Ph.D. Thesis, Freie Universität, Berlin, Germany, 2011. [Google Scholar]
- Thomas, R.D.; Runnegar, B.; Matt, K. Pelagiella exigua, an early Cambrian stem gastropod with chaetae: Lophotrochozoan heritage and conchiferan novelty. Palaeontology 2020, 63, 601–627. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Holmer, L.E.; Hu, S.; Wang, X.; Li, G. Peduncular attached secondary tiering acrotretoid brachiopods from the Chengjiang fauna: Implications for the ecological expansion of brachiopods during the Cambrian Explosion. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 323–325, 60–67. [Google Scholar] [CrossRef]
- Fisher, R.A. The Genetical Theory of Natural Selection; Oxford University Press: London, UK, 1930; pp. 1–302. [Google Scholar]
- Hanlon, R.T.; Conroy, L.A.; Forsythe, J.W. Mimicry and foraging behaviour of two tropical sand-flat octopus species off North Sulawesi, Indonesia. Biol. J. Linn. Soc. 2008, 93, 23–38. [Google Scholar] [CrossRef]
- Huang, B.; Rong, J. Ancient seabed checkerboard: How setae shaped spatial distributions of Silurian brachiopods. Proc. Natl. Acad. Sci. USA 2024, 122, e2509354122. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, J. A new linguliform brachiopod from the Chengjiang Lagerstätte. J. Northwest Univ. (Nat. Sci. Ed.) 2004, 34, 449–452. [Google Scholar]
- Zhang, Z.; Shu, D.; Han, J.; Liu, J. New data on the rare Chengjiang (Lower Cambrian, South China) linguloid brachiopod Xianshanella haikouensis. J. Paleontol. 2006, 80, 203–211. [Google Scholar] [CrossRef]
- Butterfield, N.J. Secular distribution of Burgess-Shale-type preservation. Lethaia 1995, 28, 1–13. [Google Scholar] [CrossRef]
- Gabbott, S.E.; Hou, X.; Norry, M.J.; Siveter, D.J. Preservation of Early Cambrian animals of the Chengjiang biota. Geology 2004, 32, 901–904. [Google Scholar] [CrossRef]
- Hou, X.; Siveter, D.J.; Siveter, D.J.; Aldridge, R.J.; Cong, P.; Gabbott, S.E.; Ma, X.; Purnell, M.A.; Williams, M. The Cambrian Fossils of Chengjiang, China: The Flowering of Early Animal Life, 2nd ed.; Wiley-Blackwell: New York, NY, USA, 2017; pp. 1–316. [Google Scholar]
- Gaines, R.R. Burgess Shale-Type Preservation and Its Distribution in Space and Time. Paleontol. Soc. Pap. 2014, 20, 123–146. [Google Scholar] [CrossRef]
- Gaines, R.R.; Hammarlund, E.U.; Hou, X.; Qi, C.; Gabbott, S.E.; Zhao, Y.; Peng, J.; Canfield, D.E. Mechanism for Burgess Shale-Type Preservation. Proc. Natl. Acad. Sci. USA 2012, 109, 5180–5184. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.A.; Butterfield, N.J. Sediment effects on the preservation of Burgess Shale–type compression fossils. Palaios 2014, 29, 145–154. [Google Scholar] [CrossRef]
- Zhu, M.; Babcock, L.E.; Steiner, M. Fossilization modes in the Chengjiang Lagerstätte (Cambrian of China): Testing the roles of organic preservation and diagenetic alteration in exceptional preservation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 220, 31–46. [Google Scholar] [CrossRef]
- Hausam, B.; Bartolomaeus, T. Ultrastructure and development of forked and capillary setae in the polychaetes Orbinia bioreti and Orbinia latreillii (Annelida: Orbiniidae). Invertebr. Biol. 2001, 120, 13–28. [Google Scholar] [CrossRef]
- Tilic, E.; Bartolomaeus, T.; Rouse, G.W. Chaetal type diversity increases during evolution of Eunicida (Annelida). Org. Divers. Evol. 2016, 16, 105–119. [Google Scholar] [CrossRef]
- Rudwick, M.J.S. Living and Fossil Brachiopods; Hutchinson and Co.: London, UK, 1970; pp. 1–199. [Google Scholar]
- Dornbos, S.Q.; Bottjer, D.J.; Chen, J.Y. Paleoecology of benthic metazoans in the Early Cambrian Maotianshan Shale biota and the Middle Cambrian Burgess Shale biota: Evidence for the Cambrian substrate revolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 220, 47–67. [Google Scholar] [CrossRef]
- Zhao, F.; Zhu, M.; Hu, S. Community structure and composition of the Cambrian Chengjiang biota. Sci. China Earth Sci. 2010, 53, 1784–1799. [Google Scholar] [CrossRef]
- Yang, X.; Kimmig, J.; Zhai, D.; Liu, Y.; Kimmig, S.R.; Peng, S. A juvenile-rich palaeocommunity of the lower Cambrian Chengjiang biota sheds light on palaeo-boom or palaeo-bust environments. Nat. Ecol. Evol. 2021, 5, 1082–1090. [Google Scholar] [CrossRef]
- Zhao, F.; Hu, S.; Caron, J.B.; Zhu, M.; Yin, Z.; Lu, M. Spatial variation in the diversity and composition of the Lower Cambrian (Series 2, Stage 3) Chengjiang biota, Southwest China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 346, 54–65. [Google Scholar] [CrossRef]
- Yang, A.; Luo, C.; Han, J.; Zhuravlev, A.Y.; Reitner, J.; Sun, H.; Zeng, H.; Zhao, F.; Hu, S. Niche Expansion of Archaeocyaths during Their Palaeogeographic Migration: Evidence from the Chengjiang Biota. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 653, 112419. [Google Scholar] [CrossRef]
- Lei, Q.; Han, J.; Ou, Q.; Wan, X. Sedentary habits of anthozoa-like animals in the Chengjiang Lagerstätte: Adaptive strategies for Phanerozoic-style soft substrates. Gondwana Res. 2014, 25, 966–974. [Google Scholar] [CrossRef]
- Ma, X.; Hou, X.; Baines, D. Phylogeny and evolutionary significance of vermiform animals from the Early Cambrian Chengjiang Lagerstätte. Sci. China Earth Sci. 2010, 53, 1774–1783. [Google Scholar] [CrossRef]
- Vannier, J. Early Cambrian origin of complex marine ecosystems. In Deep-Time Perspectives on Climate Change: Marrying the Signal from Computer Models and Biological Proxies; The Geological Society of London on behalf of The Micropalaeontological Society: London, UK, 2007; Volume 2, pp. 81–100. [Google Scholar]
- Zhao, F.; Caron, J.B.; Bottjer, D.J.; Hu, S.; Yin, Z.; Zhu, M. Diversity and species abundance patterns of the early Cambrian (Series 2, Stage 3) Chengjiang Biota from China. Paleobiology 2014, 40, 50–69. [Google Scholar] [CrossRef]
- Hou, X.; Siveter, D.J.; Aldridge, R.J.; Siveter, D.J. A new arthropod in chain-like associations from the Chengjiang Lagerstätte (lower Cambrian), Yunnan, China. Palaeontology 2009, 52, 951–961. [Google Scholar]
- Wang, D.; Vannier, J.; Aria, C.; Sun, J.; Han, J. Tube-dwelling in early animals exemplified by Cambrian calidophoran worms. BMC Biol. 2021, 19, 234. [Google Scholar] [CrossRef]
- Buatois, L.A.; Mángano, M.G.; Minter, N.J.; Zhou, K.; Wisshak, M.; Wilson, M.A.; Olea, R.A. Quantifying ecospace utilization and ecosystem engineering during the early Phanerozoic—The role of bioturbation and bioerosion. Sci. Adv. 2020, 6, eabb0618. [Google Scholar] [CrossRef]
- Harper, D.A.T.; Cascales-Miñana, B.; Servais, T. Early Palaeozoic diversifications and extinctions in the marine biosphere: A continuum of change. Geol. Mag. 2020, 157, 5–21. [Google Scholar] [CrossRef]
- Li, Y.; Williams, M.; Harvey, T.H.; Wei, F.; Zhao, Y.; Guo, J.; Gabbott, S.; Fletcher, T.; Hou, X.; Cong, P. Symbiotic fouling of Vetulicola, an early Cambrian nektonic animal. Commun. Biol. 2020, 3, 517. [Google Scholar] [CrossRef]
- Skovsted, C.B.; Peel, J.S. Hyolithellus in life position from the lower Cambrian of North Greenland. J. Paleontol. 2011, 85, 37–47. [Google Scholar] [CrossRef]
- Goñi, I.; Monnet, C.; De Baets, K.; Topper, T.P.; Régnier, S.; Schröer, L.; Cnudde, V.; Jell, P.A.; Clausen, S. Symbiotic interactions on middle Cambrian echinoderms reveal the oldest parasitism on deuterostomes. Sci. Rep. 2025, 15, 14257. [Google Scholar] [CrossRef] [PubMed]
- Vinn, O. Early symbiotic interactions in the Cambrian. Palaios 2017, 32, 231–237. [Google Scholar]
- Zhang, G.; Parry, L.A.; Vinther, J.; Ma, X. Exceptional soft tissue preservation reveals a cnidarian affinity for a Cambrian phosphatic tubicolous enigma. Proc. R. Soc. B 2022, 289, 20221623. [Google Scholar] [CrossRef]
- Peel, J.S. Failed predation, commensalism and parasitism on lower Cambrian linguliformean brachiopods. Alcheringa Australas. J. Palaeontol. 2015, 39, 149–163. [Google Scholar] [CrossRef]
- Vinn, O.; Wilson, M.A. Symbiotic interactions in the Silurian of Baltica. Lethaia 2016, 49, 413–420. [Google Scholar] [CrossRef]
- Paczesna, J. Ichnological record of the activity of Anthozoa in the early Cambrian succession of the Upper Silesian Block (southern Poland). Acta Geol. Pol. 2010, 60, 93–103. [Google Scholar]
- García-Bellido, D. The demosponge Leptomitus cf. L. lineatus, first occurrence from the middle Cambrian of Spain (Murero Formation, Western Iberian Chain). Geol. Acta 2003, 1, 113–119. [Google Scholar]
- Gaines, R.R.; Droser, M.L. Paleoecology of the familiar trilobite Elrathia kingii: An early exaerobic zone inhabitant. Geology 2003, 31, 941–944. [Google Scholar] [CrossRef]
- Bengtson, S.; Urbanek, A. Rhabdotubus, a Middle Cambrian rhabdopleurid hemichordate. Lethaia 1986, 19, 293–308. [Google Scholar] [CrossRef]
- Li, G.; Wei, F.; Guo, J.; Cong, P. Macroalgae from the early Cambrian Chengjiang biota. Pap. Palaeontol. 2024, 10, e1585. [Google Scholar] [CrossRef]
- Xiao, S.; Yuan, X.; Steiner, M.; Knoll, A.H. Macroscopic carbonaceous compressions in a terminal Proterozoic shale: A systematic reassessment of the Miaohe biota, South China. J. Paleontol. 2002, 76, 347–376. [Google Scholar] [CrossRef]
- Ye, Q.; Tong, J.; An, Z.; Hu, J.; Tian, L.; Guan, K.; Xiao, S. A systematic description of new macrofossil material from the upper Ediacaran Miaohe Member in South China. J. Syst. Palaeontol. 2019, 17, 183–238. [Google Scholar] [CrossRef]
- Steiner, M. Die Neoproterozoischen Megaalgen Südchinas; Selbstverlag Fachbereich Geowissenschaften: Berlin, Germany, 1994; Volume 15, pp. 1–146. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Topper, T.P.; Song, B.; Zhang, C.; Agbaje, O.B.A.; Holmer, L.E.; Zhang, Z. Branched Setae or Attached Macroalgae: A Case Study of an Exceptionally Preserved Brachiopod from the Early Cambrian Chengjiang Lagerstätte. Biology 2025, 14, 1287. https://doi.org/10.3390/biology14091287
Liang Y, Topper TP, Song B, Zhang C, Agbaje OBA, Holmer LE, Zhang Z. Branched Setae or Attached Macroalgae: A Case Study of an Exceptionally Preserved Brachiopod from the Early Cambrian Chengjiang Lagerstätte. Biology. 2025; 14(9):1287. https://doi.org/10.3390/biology14091287
Chicago/Turabian StyleLiang, Yue, Timothy P. Topper, Baopeng Song, Caibin Zhang, Oluwatoosin B. A. Agbaje, Lars E. Holmer, and Zhifei Zhang. 2025. "Branched Setae or Attached Macroalgae: A Case Study of an Exceptionally Preserved Brachiopod from the Early Cambrian Chengjiang Lagerstätte" Biology 14, no. 9: 1287. https://doi.org/10.3390/biology14091287
APA StyleLiang, Y., Topper, T. P., Song, B., Zhang, C., Agbaje, O. B. A., Holmer, L. E., & Zhang, Z. (2025). Branched Setae or Attached Macroalgae: A Case Study of an Exceptionally Preserved Brachiopod from the Early Cambrian Chengjiang Lagerstätte. Biology, 14(9), 1287. https://doi.org/10.3390/biology14091287







