Unraveling the Bone–Brain Communication Network
Simple Summary
Abstract
1. Introduction
2. Similarities Between Bone and Brain Cells
2.1. Osteoclasts and Microglia
2.2. Osteocytes and Neurons
2.3. Osteoblasts and Brain Cells
3. Signals and Brain Functions
3.1. Osteocalcin
3.2. Osteopontin
3.3. Sclerostin
3.4. Exosomes
4. Brain and Bone Interactions
4.1. Traumatic Brain Injury
4.2. Alzheimer’s Disease
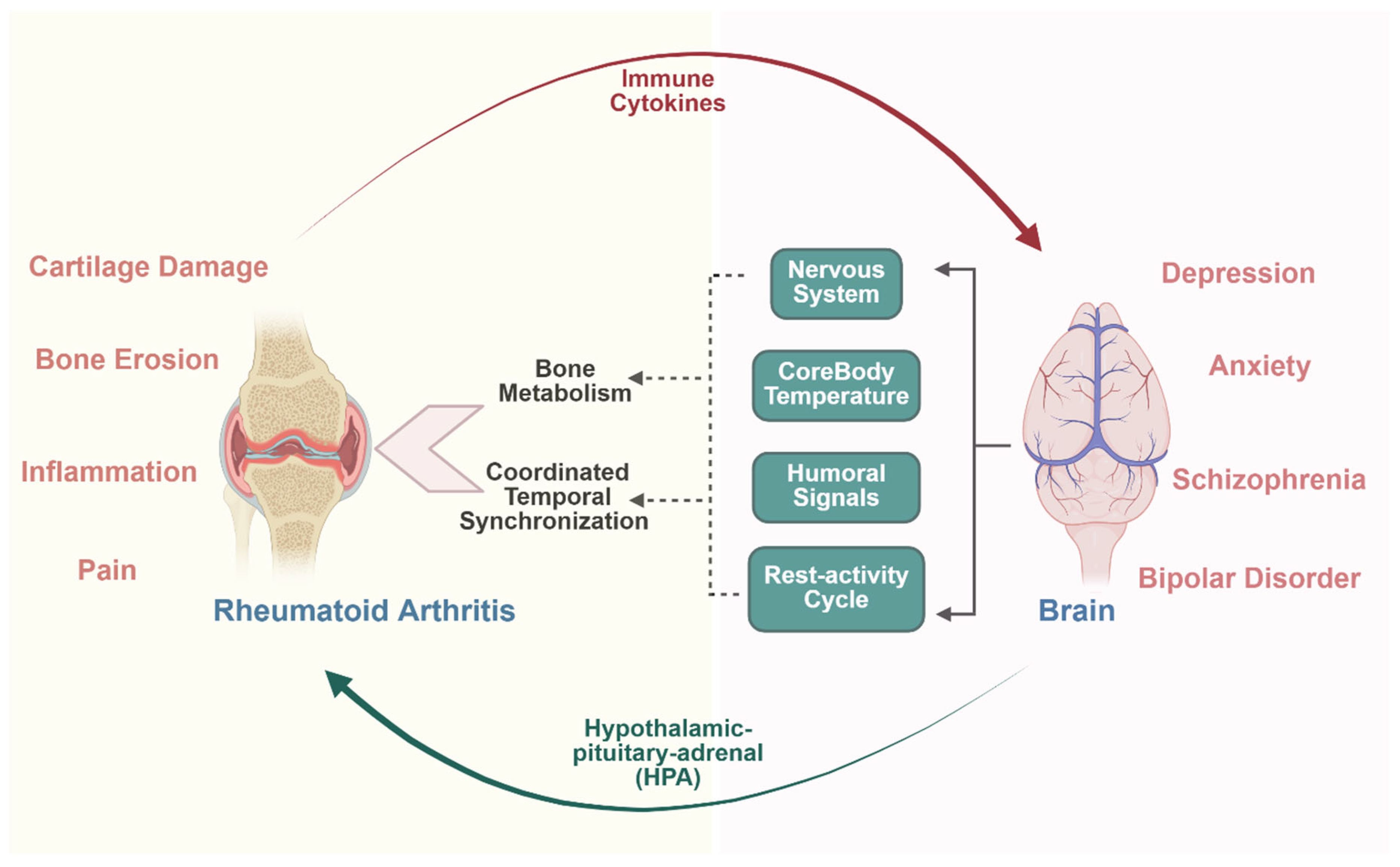
4.3. Depression
4.4. Nasu–Hakola Disease
5. Aging and Bone Diseases
6. Mechanism of Crosstalk
7. Brain Mediators and Cellular Functions
8. Stem Cells and Crosstalk
9. Extracellular Vesicles and Brain Cell Function
10. Metabolic Disorders and Crosstalk
11. Osteoimmunology and Crosstalk
12. Inflammation and Brain Cells
13. Transcriptional Regulators and Brain Cells
14. KLFs and Crosstalk
15. Biomarkers and Crosstalk
16. Signaling Pathways
17. Pre-Clinical and Clinical Studies
18. Conclusions
19. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karsenty, G.; Ferron, M. The contribution of bone to whole-organism physiology. Nature 2012, 481, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Amling, M.; Takeda, S.; Priemel, M.; Schilling, A.F.; Beil, F.T.; Shen, J.; Vinson, C.; Rueger, J.M.; Karsenty, G. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell 2000, 100, 197–207. [Google Scholar] [CrossRef]
- Oury, F.; Khrimian, L.; Denny, C.A.; Gardin, A.; Chamouni, A.; Goeden, N.; Huang, Y.-y.; Lee, H.; Srinivas, P.; Gao, X.-B. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell 2013, 155, 228–241. [Google Scholar] [CrossRef]
- Oury, F.; Sumara, G.; Sumara, O.; Ferron, M.; Chang, H.; Smith, C.E.; Hermo, L.; Suarez, S.; Roth, B.L.; Ducy, P. Endocrine regulation of male fertility by the skeleton. Cell 2011, 144, 796–809. [Google Scholar] [CrossRef]
- Schett, G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Investig. 2011, 41, 1361–1366. [Google Scholar] [CrossRef]
- Perry, V.H.; Cunningham, C.; Holmes, C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007, 7, 161–167. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Hansda, S.; Prateeksha, P.; Das, H. Krüppel-like factor 2 (KLF2), a potential target for neuroregeneration. Neural Regen. Res. 2024, 19, 2327–2328. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Almeida, M. Aging mechanisms in bone. BoneKEy Rep. 2012, 1, 102. [Google Scholar] [CrossRef]
- Li, X.-h.; Chen, C.; Tu, Y.; Sun, H.-t.; Zhao, M.-l.; Cheng, S.-x.; Qu, Y.; Zhang, S. Sirt1 promotes axonogenesis by deacetylation of Akt and inactivation of GSK3. Mol. Neurobiol. 2013, 48, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Z.; Jiang, Y.; Meng, Z.; Lu, M. mTOR signaling: The interface linking cellular metabolism and hepatitis B virus replication. Virol. Sin. 2021, 36, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Schweingruber, C.; Nijssen, J.; Mechtersheimer, J.; Reber, S.; Lebœuf, M.; O’Brien, N.L.; Mei, I.; Hedges, E.; Keuper, M.; Benitez, J.A.; et al. Single-cell RNA-sequencing reveals early mitochondrial dysfunction unique to motor neurons shared across FUS- and TARDBP-ALS. Nat. Commun. 2025, 16, 4633. [Google Scholar] [CrossRef]
- Wang, J.S.; Kamath, T.; Mazur, C.M.; Mirzamohammadi, F.; Rotter, D.; Hojo, H.; Castro, C.D.; Tokavanich, N.; Patel, R.; Govea, N.; et al. Control of osteocyte dendrite formation by Sp7 and its target gene osteocrin. Nat. Commun. 2021, 12, 6271. [Google Scholar] [CrossRef]
- Pulimood, N.S.; Rodrigues, W.D.S.J.; Atkinson, D.A.; Mooney, S.M.; Medina, A.E. The Role of CREB, SRF, and MEF2 in Activity-Dependent Neuronal Plasticity in the Visual Cortex. J. Neurosci. 2017, 37, 6628–6637. [Google Scholar] [CrossRef]
- Tang, K.C.; Pan, W.; Doschak, M.R.; Alexander, R.T. Increased FoxO3a expression prevents osteoblast differentiation and matrix calcification. Bone Rep. 2019, 10, 100206. [Google Scholar] [CrossRef]
- Liu, T.M.; Lee, E.H. Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Eng. Part B Rev. 2013, 19, 254–263. [Google Scholar] [CrossRef]
- Lv, X.; Gao, F.; Cao, X. Skeletal interoception in bone homeostasis and pain. Cell Metab. 2022, 34, 1914–1931. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Zhao, J.; Meyers, C.A.; Lee, S.; Qin, Q.; James, A.W. Interaction between the nervous and skeletal systems. Front. Cell Dev. Biol. 2022, 10, 976736. [Google Scholar] [CrossRef]
- Chen, H.; Hu, B.; Lv, X.; Zhu, S.; Zhen, G.; Wan, M.; Jain, A.; Gao, B.; Chai, Y.; Yang, M.; et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat. Commun. 2019, 10, 181. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Q.; Chen, W.; Li, J.; Qi, C.; Yan, Y.; Qian, C.; Wan, M.; Ficke, J.; Zheng, J.; et al. Brain regulates weight bearing bone through PGE2 skeletal interoception: Implication of ankle osteoarthritis and pain. Bone Res. 2024, 12, 16. [Google Scholar] [CrossRef]
- Thi, M.M.; Suadicani, S.O.; Schaffler, M.B.; Weinbaum, S.; Spray, D.C. Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require αVβ3 integrin. Proc. Natl. Acad. Sci. USA 2013, 110, 21012–21017. [Google Scholar] [CrossRef]
- Turner, C.H.; Warden, S.J.; Bellido, T.; Plotkin, L.I.; Kumar, N.; Jasiuk, I.; Danzig, J.; Robling, A.G. Mechanobiology of the skeleton. Sci. Signal. 2009, 2, pt3. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lo, R.Y. Alzheimer’s disease and osteoporosis. Tzu Chi Med. J. 2017, 29, 138–142. [Google Scholar] [PubMed]
- Oughli, H.A.; Chen, G.; Philip Miller, J.; Nicol, G.; Butters, M.A.; Avidan, M.; Stark, S.; Lenze, E.J. Cognitive Improvement in Older Adults in the Year After Hip Fracture: Implications for Brain Resilience in Advanced Aging. Am. J. Geriatr. Psychiatry 2018, 26, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Birkner, D.; Pigorsch, M.; Riedlinger, D.; Möckel, M.; Lindner, T.; Schenk, L.; Deutschbein, J. The vulnerability of hip fracture patients with cognitive impairment: An analysis of health conditions, hospital care, and outcomes. BMC Geriatr. 2025, 25, 99. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Liu, C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Wang, Y. TREM2 variants: New keys to decipher Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2016, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Titus, A.R.; Humphrey, M.B. The TREM2-DAP12 signaling pathway in Nasu-Hakola disease: A molecular genetics perspective. Res. Rep. Biochem. 2015, 5, 89–100. [Google Scholar] [CrossRef]
- Spangenberg, E.E.; Green, K.N. Inflammation in Alzheimer’s disease: Lessons learned from microglia-depletion models. Brain Behav. Immun. 2017, 61, 1–11. [Google Scholar] [CrossRef]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell … and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef]
- Youlten, S.E.; Kemp, J.P.; Logan, J.G.; Ghirardello, E.J.; Sergio, C.M.; Dack, M.R.; Guilfoyle, S.E.; Leitch, V.D.; Butterfield, N.C.; Komla-Ebri, D. Osteocyte transcriptome mapping identifies a molecular landscape controlling skeletal homeostasis and susceptibility to skeletal disease. Nat. Commun. 2021, 12, 2444. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef]
- Dobson, P.F.; Dennis, E.P.; Hipps, D.; Reeve, A.; Laude, A.; Bradshaw, C.; Stamp, C.; Smith, A.; Deehan, D.J.; Turnbull, D.M.; et al. Mitochondrial dysfunction impairs osteogenesis, increases osteoclast activity, and accelerates age related bone loss. Sci. Rep. 2020, 10, 11643. [Google Scholar] [CrossRef]
- Elefteriou, F. Regulation of bone remodeling by the central and peripheral nervous system. Arch. Biochem. Biophys. 2008, 473, 231–236. [Google Scholar] [CrossRef]
- Méndez-Maldonado, K.; Vega-López, G.A.; Aybar, M.J.; Velasco, I. Neurogenesis From Neural Crest Cells: Molecular Mechanisms in the Formation of Cranial Nerves and Ganglia. Front. Cell Dev. Biol. 2020, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of bone development and maintenance by Runx2. Front. Biosci. 2008, 13, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Khrimian, L.; Obri, A.; Ramos-Brossier, M.; Rousseaud, A.; Moriceau, S.; Nicot, A.S.; Mera, P.; Kosmidis, S.; Karnavas, T.; Saudou, F.; et al. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 2017, 214, 2859–2873. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, Q.; Lei, X.; Zhao, H. The emerging roles of GPR158 in the regulation of the endocrine system. Front. Cell Dev. Biol. 2022, 10, 1034348. [Google Scholar] [CrossRef]
- Li, J.; Lou, S.; Bian, X. Osteocalcin and GPR158: Linking bone and brain function. Front. Cell Dev. Biol. 2025, 13, 1564751. [Google Scholar] [CrossRef]
- Diegel, C.R.; Hann, S.; Ayturk, U.M.; Hu, J.C.W.; Lim, K.E.; Droscha, C.J.; Madaj, Z.B.; Foxa, G.E.; Izaguirre, I.; Transgenics Core, V.V.A.; et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet. 2020, 16, e1008361. [Google Scholar] [CrossRef]
- Knapen, M.H.; Eisenwiener, H.G.; Vermeer, C. Osteocalcin detection in aging serum and whole blood: Stability of different osteocalcin fractions. Clin. Chim. Acta 1996, 256, 151–164. [Google Scholar] [CrossRef]
- Terreni, A.; Pezzati, P. Biochemical markers in the follow-up of the osteoporotic patients. Clin. Cases Miner. Bone Metab. 2012, 9, 80–84. [Google Scholar]
- Kakonen, S.-M.; Hellman, J.; Karp, M.; Laaksonen, P.; Obrant, K.J.; Vaananen, H.K.; Lovgren, T.; Pettersson, K. Development and evaluation of three immunofluorometric assays that measure different forms of osteocalcin in serum. Clin. Chem. 2000, 46, 332–337. [Google Scholar] [CrossRef]
- Obri, A.; Khrimian, L.; Karsenty, G.; Oury, F. Osteocalcin in the brain: From embryonic development to age-related decline in cognition. Nat. Rev. Endocrinol. 2018, 14, 174–182. [Google Scholar] [CrossRef]
- Shan, C.; Ghosh, A.; Guo, X.Z.; Wang, S.M.; Hou, Y.F.; Li, S.T.; Liu, J.M. Roles for osteocalcin in brain signalling: Implications in cognition- and motor-related disorders. Mol. Brain 2019, 12, 23. [Google Scholar] [CrossRef]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef]
- Boggio, E.; Dianzani, C.; Gigliotti, C.L.; Soluri, M.F.; Clemente, N.; Cappellano, G.; Toth, E.; Raineri, D.; Ferrara, B.; Comi, C.; et al. Thrombin Cleavage of Osteopontin Modulates Its Activities in Human Cells In Vitro and Mouse Experimental Autoimmune Encephalomyelitis In Vivo. J. Immunol. Res. 2016, 2016, 9345495. [Google Scholar] [CrossRef] [PubMed]
- Hansda, S.; Das, H. Insights into Cancer-Associated Thrombosis Leading Towards Ischemic Stroke. Biology 2025, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Azizan, Z.; Bazrgar, M.; Bazgir, N.; Moini, S.H.; Ghaseminejad-Kermani, S.; Safa, K.; Eshaghian-Dorcheh, A.; Harirchian, M.H. Osteopontin in Alzheimer’s Disease: A Double-Edged Sword in Neurodegeneration and Neuroprotection-A Systematic Review. CNS Neurosci. Ther. 2025, 31, e70269. [Google Scholar] [CrossRef] [PubMed]
- Castello, L.M.; Raineri, D.; Salmi, L.; Clemente, N.; Vaschetto, R.; Quaglia, M.; Garzaro, M.; Gentilli, S.; Navalesi, P.; Cantaluppi, V.; et al. Osteopontin at the Crossroads of Inflammation and Tumor Progression. Mediat. Inflamm. 2017, 2017, 4049098. [Google Scholar] [CrossRef]
- Chabas, D.; Baranzini, S.E.; Mitchell, D.; Bernard, C.C.; Rittling, S.R.; Denhardt, D.T.; Sobel, R.A.; Lock, C.; Karpuj, M.; Pedotti, R.; et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 2001, 294, 1731–1735. [Google Scholar] [CrossRef]
- Comi, C.; Carecchio, M.; Chiocchetti, A.; Nicola, S.; Galimberti, D.; Fenoglio, C.; Cappellano, G.; Monaco, F.; Scarpini, E.; Dianzani, U. Osteopontin is increased in the cerebrospinal fluid of patients with Alzheimer’s disease and its levels correlate with cognitive decline. J. Alzheimer’s Dis. 2010, 19, 1143–1148. [Google Scholar] [CrossRef]
- Zwamborn, R.A.J.; Snijders, C.; An, N.; Thomson, A.; Rutten, B.P.F.; de Nijs, L. Wnt Signaling in the Hippocampus in Relation to Neurogenesis, Neuroplasticity, Stress and Epigenetics. Prog. Mol. Biol. Transl. Sci. 2018, 158, 129–157. [Google Scholar] [CrossRef]
- Tu, X.; Rhee, Y.; Condon, K.W.; Bivi, N.; Allen, M.R.; Dwyer, D.; Stolina, M.; Turner, C.H.; Robling, A.G.; Plotkin, L.I.; et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 2012, 50, 209–217. [Google Scholar] [CrossRef]
- Fairfield, H.; Rosen, C.J.; Reagan, M.R. Connecting Bone and Fat: The Potential Role for Sclerostin. Curr. Mol. Biol. Rep. 2017, 3, 114–121. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Ahmed, M.; Osaid, Z.; Hamoudi, R.; Harati, R. Insights into Exosome Transport through the Blood-Brain Barrier and the Potential Therapeutical Applications in Brain Diseases. Pharmaceuticals 2023, 16, 571. [Google Scholar] [CrossRef]
- Pulgar, V.M. Transcytosis to cross the blood brain barrier, new advancements and challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Gao, W.; Papadimitriou, J.M.; Zhang, C.; Gao, J.; Zheng, M. Exosomes-the enigmatic regulators of bone homeostasis. Bone Res. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Xiao, Y.; Guo, Q.; Huang, Y.; Luo, X. The Role of Bone-Derived Exosomes in Regulating Skeletal Metabolism and Extraosseous Diseases. Front. Cell Dev. Biol. 2020, 8, 89. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, W.; Zhang, P.; Ling, S.; Li, Y.; Zhao, D.; Peng, J.; Wang, A.; Li, Q.; Song, J.; et al. miR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol. 2015, 12, 343–353. [Google Scholar] [CrossRef]
- Lee, E.C.; Choi, D.; Lee, D.H.; Oh, J.S. Engineering Exosomes for CNS Disorders: Advances, Challenges, and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 3137. [Google Scholar] [CrossRef]
- Sakellariou, V.I.; Grigoriou, E.; Mavrogenis, A.F.; Soucacos, P.N.; Papagelopoulos, P.J. Heterotopic ossification following traumatic brain injury and spinal cord injury: Insight into the etiology and pathophysiology. J. Musculoskelet. Neuronal Interact. 2012, 12, 230–240. [Google Scholar]
- Bajwa, N.M.; Kesavan, C.; Mohan, S. Long-term Consequences of Traumatic Brain Injury in Bone Metabolism. Front. Neurol. 2018, 9, 115. [Google Scholar] [CrossRef]
- Zhou, B.N.; Zhang, Q.; Li, M. Alzheimer’s disease and its associated risk of bone fractures: A narrative review. Front. Endocrinol. 2023, 14, 1190762. [Google Scholar] [CrossRef]
- Yirmiya, R.; Bab, I. Major depression is a risk factor for low bone mineral density: A meta-analysis. Biol. Psychiatry 2009, 66, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Westbroek, I.; van der Plas, A.; de Rooij, K.E.; Klein-Nulend, J.; Nijweide, P.J. Expression of serotonin receptors in bone. J. Biol. Chem. 2001, 276, 28961–28968. [Google Scholar] [CrossRef] [PubMed]
- Paloneva, J.; Kestilä, M.; Wu, J.; Salminen, A.; Böhling, T.; Ruotsalainen, V.; Hakola, P.; Bakker, A.B.; Phillips, J.H.; Pekkarinen, P.; et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 2000, 25, 357–361. [Google Scholar] [CrossRef]
- Jay, T.R.; von Saucken, V.E.; Landreth, G.E. TREM2 in neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 56. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Marcantonio, E.R.; Saczynski, J.S.; Jones, R.N. Cognitive trajectories after postoperative delirium. N. Engl. J. Med. 2012, 367, 1164–1165. [Google Scholar]
- Zhao, Y.; Chen, H.; Qiu, F.; He, J.; Chen, J. Cognitive impairment and risks of osteoporosis: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 106, 104879. [Google Scholar] [CrossRef]
- Zhou, R.; Deng, J.; Zhang, M.; Zhou, H.D.; Wang, Y.J. Association between bone mineral density and the risk of Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 24, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N.; Khosla, S. Cellular senescence in bone. Bone 2019, 121, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Hansda, S.; Ghosh, G.; Ghosh, R. 9-phenyl acridine photosensitizes A375 cells to UVA radiation. Heliyon 2020, 6, e04733. [Google Scholar] [CrossRef]
- Hansda, S.; Ghosh, R. Bystander effect of ultraviolet A radiation protects A375 melanoma cells by induction of antioxidant defense. J. Environ. Sci. Health C Toxicol. Carcinog. 2022, 40, 46–67. [Google Scholar] [CrossRef]
- Ghosh, R.; Hansda, S. Targeted and non-targeted effects of radiation in mammalian cells: An overview. Arch. Biotechnol. Biomed. 2021, 5, 13–19. [Google Scholar] [CrossRef]
- Li, M.C.M.; Chow, S.K.H.; Wong, R.M.Y.; Qin, L.; Cheung, W.H. The role of osteocytes-specific molecular mechanism in regulation of mechanotransduction—A systematic review. J. Orthop. Transl. 2021, 29, 1–9. [Google Scholar] [CrossRef]
- Stewart, S.; Darwood, A.; Masouros, S.; Higgins, C.; Ramasamy, A. Mechanotransduction in osteogenesis. Bone Jt. Res. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Deng, A.F.; Wang, F.X.; Wang, S.C.; Zhang, Y.Z.; Bai, L.; Su, J.C. Bone-organ axes: Bidirectional crosstalk. Mil. Med. Res. 2024, 11, 37. [Google Scholar] [CrossRef]
- Shi, T.; Shen, S.; Shi, Y.; Wang, Q.; Zhang, G.; Lin, J.; Chen, J.; Bai, F.; Zhang, L.; Wang, Y.; et al. Osteocyte-derived sclerostin impairs cognitive function during ageing and Alzheimer’s disease progression. Nat. Metab. 2024, 6, 531–549. [Google Scholar] [CrossRef]
- Rosić, D.; Budišin, V.; Vrabec-Matković, D. Osteoporosis in patients with Parkinson’s disease. Reumatizam 2014, 61, 129. [Google Scholar]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J.; Hassett, S.M.; Bond, J.L.; Rydberg, J.; Grogg, J.D.; Hilles, E.L.; Bogenschutz, E.D.; Smith, H.D.; Fuchs, R.K.; Bliziotes, M.M.; et al. Psychotropic drugs have contrasting skeletal effects that are independent of their effects on physical activity levels. Bone 2010, 46, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Sjögren, K. Effects of the gut microbiota on bone mass. Trends Endocrinol. Metab. 2015, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.M.; Darby, T.M.; Hsu, E.; Yu, M.; Pal, S.; Dar, H.; Li, J.-Y.; Adams, J.; Jones, R.M.; Pacifici, R. The gut microbiota is a transmissible determinant of skeletal maturation. eLife 2021, 10, e64237. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2018, 102, 415–425. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Duffuler, P.; Bhullar, K.S.; Wu, J. Targeting gut microbiota in osteoporosis: Impact of the microbial-based functional food ingredients. Food Sci. Hum. Wellness 2024, 13, 1–15. [Google Scholar] [CrossRef]
- Zhang, M.J.; Pisco, A.O.; Darmanis, S.; Zou, J. Mouse aging cell atlas analysis reveals global and cell type-specific aging signatures. eLife 2021, 10, e62293. [Google Scholar] [CrossRef]
- Cai, D.; Khor, S. “Hypothalamic microinflammation” paradigm in aging and metabolic diseases. Cell Metab. 2019, 30, 19–35. [Google Scholar] [CrossRef]
- Kim, Y.S.; Joh, T.H. Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006, 38, 333–347. [Google Scholar] [CrossRef]
- Zhou, R.; Guo, Q.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.; Luo, X. Endocrine role of bone in the regulation of energy metabolism. Bone Res. 2021, 9, 25. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kusumbe, A.P.; Ramasamy, S.K.; Itkin, T.; Mäe, M.A.; Langen, U.H.; Betsholtz, C.; Lapidot, T.; Adams, R.H. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 2016, 532, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F. Impact of the autonomic nervous system on the skeleton. Physiol. Rev. 2018, 98, 1083–1112. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Ryu, J.-H.; Suda, N.; Tanaka, K.F.; Gingrich, J.A.; Schütz, G.; Glorieux, F.H.; Chiang, C.Y.; Zajac, J.D.; Insogna, K.L. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 2008, 135, 825–837. [Google Scholar] [CrossRef]
- Lewis, J.W.; Frost, K.; Neag, G.; Wahid, M.; Finlay, M.; Northall, E.H.; Abudu, O.; Kemble, S.; Davis, E.T.; Powell, E. Therapeutic avenues in bone repair: Harnessing an anabolic osteopeptide, PEPITEM, to boost bone growth and prevent bone loss. Cell Rep. Med. 2024, 5, 101574. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; Christiansen, B.A.; Giannone, A.A.; Genetos, D.C. The Role of Nerves in Skeletal Development, Adaptation, and Aging. Front. Endocrinol. 2020, 11, 646. [Google Scholar] [CrossRef]
- Park, E.J.; Truong, V.L.; Jeong, W.S.; Min, W.K. Brain-Derived Neurotrophic Factor (BDNF) Enhances Osteogenesis and May Improve Bone Microarchitecture in an Ovariectomized Rat Model. Cells 2024, 13, 518. [Google Scholar] [CrossRef]
- Howlader, M.S.I.; Prateeksha, P.; Hansda, S.; Naidu, P.; Das, M.; Barthels, D.; Das, H. Secretory products of DPSC mitigate inflammatory effects in microglial cells by targeting MAPK pathway. Biomed. Pharmacother. 2024, 170, 115971. [Google Scholar] [CrossRef] [PubMed]
- Prateeksha, P.; Howlader, M.S.I.; Hansda, S.; Naidu, P.; Das, M.; Abo-Aziza, F.; Das, H. Secretome of dental pulp-derived stem cells reduces inflammation and proliferation of glioblastoma cells by deactivating Mapk-Akt pathway. Dis. Res. 2023, 3, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Grizzle, W.E. Exosomes: A novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am. J. Pathol. 2014, 184, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhang, H.; Feng, L.; Dang, R.; Wang, J.; Cui, C.; Jiang, P. The emerging role of exosomes in communication between the periphery and the central nervous system. MedComm 2023, 4, e410. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, X.; Zhang, M.; Cui, L.; Li, B.; Liu, Y.; Su, R.; Sun, K.; Hu, Y.; Yang, F. Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages. Stem Cell Res. Ther. 2022, 13, 330. [Google Scholar] [CrossRef]
- Jahangard, Y.; Monfared, H.; Moradi, A.; Zare, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Therapeutic effects of transplanted exosomes containing miR-29b to a rat model of Alzheimer’s disease. Front. Neurosci. 2020, 14, 564. [Google Scholar] [CrossRef]
- Arredondo, S.B.; Valenzuela-Bezanilla, D.; Santibanez, S.H.; Varela-Nallar, L. Wnt signaling in the adult hippocampal neurogenic niche. Stem Cells 2022, 40, 630–640. [Google Scholar] [CrossRef]
- Bonilla, C.; Zurita, M. Cell-Based Therapies for Traumatic Brain Injury: Therapeutic Treatments and Clinical Trials. Biomedicines 2021, 9, 669. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Wang, D.; Cao, H.; Hua, W.; Gao, L.; Yuan, Y.; Zhou, X.; Zeng, Z. Mesenchymal stem cell-derived extracellular vesicles for bone defect repair. Membranes 2022, 12, 716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Su, Y.; Wang, J.; Wu, J.Y. Correction: The role of vesicle trafficking genes in osteoblast differentiation and function. Sci. Rep. 2025, 15, 18704. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Wang, Z.X.; Liu, X.X.; Wan, M.D.; Liu, Y.W.; Jiao, B.; Liao, X.X.; Luo, Z.W.; Wang, Y.Y.; Hong, C.G. The protective effects of osteocyte-derived extracellular vesicles against Alzheimer’s disease diminished with aging. Adv. Sci. 2022, 9, 2105316. [Google Scholar] [CrossRef]
- Fang, F.; Yang, J.; Wang, J.; Li, T.; Wang, E.; Zhang, D.; Liu, X.; Zhou, C. The role and applications of extracellular vesicles in osteoporosis. Bone Res. 2024, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- De Felice, F.G. Alzheimer’s disease and insulin resistance: Translating basic science into clinical applications. J. Clin. Investig. 2013, 123, 531–539. [Google Scholar] [CrossRef]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Enriori, P.J.; Evans, A.E.; Sinnayah, P.; Jobst, E.E.; Tonelli-Lemos, L.; Billes, S.K.; Glavas, M.M.; Grayson, B.E.; Perello, M.; Nillni, E.A. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007, 5, 181–194. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Takayanagi, H. New developments in osteoimmunology. Nat. Rev. Rheumatol. 2012, 8, 684–689. [Google Scholar] [CrossRef]
- Yahara, Y.; Barrientos, T.; Tang, Y.J.; Puviindran, V.; Nadesan, P.; Zhang, H.; Gibson, J.R.; Gregory, S.G.; Diao, Y.; Xiang, Y. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat. Cell Biol. 2020, 22, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, T.; Ibáñez, L.; Boucoiran, A.; Birgy-Barelli, E.; Pène, J.; Abou-Ezzi, G.; Arab, N.; Rouleau, M.; Hébuterne, X.; Yssel, H. Bone marrow Th17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut 2015, 64, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Chen, H.; Shang, D.; Wen, Y.; Liang, C. Bone-derived modulators that regulate brain function: Emerging therapeutic targets for neurological disorders. Front. Cell Dev. Biol. 2021, 9, 683457. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gong, C. NLRP3 inflammasome in Alzheimer’s disease: Molecular mechanisms and emerging therapies. Front. Immunol. 2025, 16, 1583886. [Google Scholar] [CrossRef]
- Novack, D.V. Role of NF-κB in the skeleton. Cell Res. 2011, 21, 169–182. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, B.; Huang, J.; Chen, R.; Sun, W.; Ye, Q.; Yang, L.; Zhu, X.; Li, X.; Zhang, R. Neuropeptides as regulators of bone metabolism: From molecular mechanisms to traditional Chinese medicine intervention strategies. Front. Pharmacol. 2025, 16, 1516038. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.-S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Ambrogini, E.; Almeida, M.; Martin-Millan, M.; Paik, J.H.; Depinho, R.A.; Han, L.; Goellner, J.; Weinstein, R.S.; Jilka, R.L.; O’Brien, C.A.; et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010, 11, 136–146. [Google Scholar] [CrossRef]
- Long, F.; Schipani, E.; Asahara, H.; Kronenberg, H.; Montminy, M. The CREB family of activators is required for endochondral bone development. Development 2001, 128, 541–550. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Varela-Nallar, L. Wnt signaling in the nervous system and in Alzheimer’s disease. J. Mol. Cell Biol. 2014, 6, 64–74. [Google Scholar] [CrossRef]
- Otto, E.; Knapstein, P.-R.; Jahn, D.; Appelt, J.; Frosch, K.-H.; Tsitsilonis, S.; Keller, J. Crosstalk of brain and bone—Clinical observations and their molecular bases. Int. J. Mol. Sci. 2020, 21, 4946. [Google Scholar] [CrossRef]
- Das, H.; Kumar, A.; Lin, Z.; Patino, W.D.; Hwang, P.M.; Feinberg, M.W.; Majumder, P.K.; Jain, M.K. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 6653–6658. [Google Scholar] [CrossRef]
- Fisch, S.; Gray, S.; Heymans, S.; Haldar, S.M.; Wang, B.; Pfister, O.; Cui, L.; Kumar, A.; Lin, Z.; Sen-Banerjee, S. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 2007, 104, 7074–7079. [Google Scholar] [CrossRef] [PubMed]
- Sen-Banerjee, S.; Mir, S.; Lin, Z.; Hamik, A.; Atkins, G.B.; Das, H.; Banerjee, P.; Kumar, A.; Jain, M.K. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 2005, 112, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Das, H. KLF2 in regulation of NF-κB-mediated immune cell function and inflammation. Int. J. Mol. Sci. 2017, 18, 2383. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Deb, M.; Das, H. KLF2 (kruppel-like factor 2 [lung]) regulates osteoclastogenesis by modulating autophagy. Autophagy 2019, 15, 2063–2075. [Google Scholar] [CrossRef]
- Maity, J.; Deb, M.; Greene, C.; Das, H. KLF2 regulates dental pulp-derived stem cell differentiation through the induction of mitophagy and altering mitochondrial metabolism. Redox Biol. 2020, 36, 101622. [Google Scholar] [CrossRef]
- Rolph, D.; Das, H. Transcriptional regulation of osteoclastogenesis: The emerging role of KLF2. Front. Immunol. 2020, 11, 937. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like factor 4 (KLF4): What we currently know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Sheng, B.; Zhang, F.; Wu, C.; Zhang, R.; Zhu, J.; Xu, K.; Kuang, Y.; Jameson, S.C.; Lin, Z.; et al. Kruppel-like factor 2 protects against ischemic stroke by regulating endothelial blood brain barrier function. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H796–H805. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-R.; Sun, Y.-C.; Gao, Y.; Guan, X.-M.; Yan, H.; Cui, X.-D.; Zhang, X.-Y.; Li, X.; Li, H.; Cheng, M. Function of Krüppel-like factor 2 in the shear stress-induced cell differentiation of endothelial progenitor cells to endothelial cells. Mol. Med. Rep. 2019, 19, 1739–1746. [Google Scholar] [PubMed]
- Barthels, D.; Prateeksha, P.; Nozohouri, S.; Villalba, H.; Zhang, Y.; Sharma, S.; Anderson, S.; Howlader, M.S.I.; Nambiar, A.; Abbruscato, T.J. Dental pulp-derived stem cells preserve astrocyte health during induced gliosis by modulating mitochondrial activity and functions. Cell. Mol. Neurobiol. 2023, 43, 2105–2127. [Google Scholar] [CrossRef]
- Laha, D.; Sarkar, J.; Maity, J.; Pramanik, A.; Howlader, M.S.I.; Barthels, D.; Das, H. Polyphenolic compounds inhibit osteoclast differentiation while reducing autophagy through limiting ROS and the mitochondrial membrane potential. Biomolecules 2022, 12, 1220. [Google Scholar] [CrossRef]
- Hansda, S.; Das, S.; Howlader, M.S.I.; Das, M.; Naidu, P.; Das, B.C.; Das, H. Small Pharmacological Compound BT881 Inhibits Osteoclastic Differentiation by Limiting ROS and Modulating Mitochondrial Dysfunctions (Abstract ID: 158683). J. Pharmacol. Exp. Ther. 2025, 392, 100657. [Google Scholar] [CrossRef]
- Naidu, P.; Das, M.; Hansda, S.; Prateeksha, P.; Howlader, M.S.I.; Siraj, M.A.; Das, H. Mechanisms of Ellagic Acid (EA)-Mediated Osteogenic Differentiation of Human Dental Pulp-Derived Stem Cells. ACS Omega 2025, 10, 15229–15242. [Google Scholar] [CrossRef]
- Parmar, K.M. Kruppel-like Factor 2 as a Central Link Between Blood Flow and Vascular Endothelial Function; Harvard University: Cambridge, MA, USA, 2008. [Google Scholar]
- Cheng, Z.; Zou, X.; Jin, Y.; Gao, S.; Lv, J.; Li, B.; Cui, R. The Role of KLF4 in Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 325. [Google Scholar] [CrossRef]
- Wang, H.; Han, J.; Dmitrii, G.; Ning, K.; Zhang, X.A. KLF transcription factors in bone diseases. J. Cell. Mol. Med. 2024, 28, e18278. [Google Scholar] [CrossRef]
- Kinisu, M.; Choi, Y.J.; Cattoglio, C.; Liu, K.; de Bezieux, H.R.; Valbuena, R.; Pum, N.; Dudoit, S.; Huang, H.; Xuan, Z. Klf5 establishes bi-potential cell fate by dual regulation of ICM and TE specification genes. Cell Rep. 2021, 37, 109982. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Q.; Qi, C.; Wan, M.; Ficke, J.; Zheng, J.; Cao, X. Mechanical loading-induced change of bone homeostasis is mediated by PGE2-driven hypothalamic interoception. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Chiu, S.H.; Wu, W.T.; Yao, T.K.; Peng, C.H.; Yeh, K.T. Sclerostin and Cardiovascular Risk: Evaluating the Cardiovascular Safety of Romosozumab in Osteoporosis Treatment. Biomedicines 2024, 12, 2880. [Google Scholar] [CrossRef] [PubMed]
- Fabre, S.; Funck-Brentano, T.; Cohen-Solal, M. Anti-sclerostin antibodies in osteoporosis and other bone diseases. J. Clin. Med. 2020, 9, 3439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, Z.; Wang, J. MicroRNA-124: A key player in microglia-mediated inflammation in neurological diseases. Front. Cell. Neurosci. 2021, 15, 771898. [Google Scholar] [CrossRef]
- Takasugi, M.; Nonaka, Y.; Takemura, K.; Yoshida, Y.; Stein, F.; Schwarz, J.J.; Adachi, J.; Satoh, J.; Ito, S.; Tombline, G. An atlas of the aging mouse proteome reveals the features of age-related post-transcriptional dysregulation. Nat. Commun. 2024, 15, 8520. [Google Scholar] [CrossRef]
- Nakamura, M.; Imaoka, M.; Takeda, M. Interaction of bone and brain: Osteocalcin and cognition. Int. J. Neurosci. 2021, 131, 1115–1123. [Google Scholar] [CrossRef]
- Schurman, C.A.; Burton, J.B.; Rose, J.; Ellerby, L.M.; Alliston, T.; Schilling, B. Molecular and cellular crosstalk between bone and brain: Accessing bidirectional neural and musculoskeletal signaling during aging and disease. J. Bone Metab. 2023, 30, 1. [Google Scholar] [CrossRef]
- Elefteriou, F.; Ahn, J.D.; Takeda, S.; Starbuck, M.; Yang, X.; Liu, X.; Kondo, H.; Richards, W.G.; Bannon, T.W.; Noda, M. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005, 434, 514–520. [Google Scholar] [CrossRef]
- Zaidi, M.; Kim, S.-M.; Mathew, M.; Korkmaz, F.; Sultana, F.; Miyashita, S.; Gumerova, A.A.; Frolinger, T.; Moldavski, O.; Barak, O. Bone circuitry and interorgan skeletal crosstalk. eLife 2023, 12, e83142. [Google Scholar] [CrossRef]
- Abeynayake, N.; Arthur, A.; Gronthos, S. Crosstalk between skeletal and neural tissues is critical for skeletal health. Bone 2021, 142, 115645. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.-P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.-H. The regulation of TGFβ signal transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef] [PubMed]
- Itasaki, N.; Hoppler, S. Crosstalk between Wnt and bone morphogenic protein signaling: A turbulent relationship. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2010, 239, 16–33. [Google Scholar] [CrossRef]
- Mazzitelli, J.A.; Pulous, F.E.; Smyth, L.C.D.; Kaya, Z.; Rustenhoven, J.; Moskowitz, M.A.; Kipnis, J.; Nahrendorf, M. Skull bone marrow channels as immune gateways to the central nervous system. Nat. Neurosci. 2023, 26, 2052–2062. [Google Scholar] [CrossRef]
- Kiialainen, A.; Hovanes, K.; Paloneva, J.; Kopra, O.; Peltonen, L. Dap12 and Trem2, molecules involved in innate immunity and neurodegeneration, are co-expressed in the CNS. Neurobiol. Dis. 2005, 18, 314–322. [Google Scholar] [CrossRef]
- Turnbull, I.R.; Gilfillan, S.; Cella, M.; Aoshi, T.; Miller, M.; Piccio, L.; Hernandez, M.; Colonna, M. Cutting edge: TREM-2 attenuates macrophage activation. J. Immunol. 2006, 177, 3520–3524. [Google Scholar] [CrossRef]
- Bakalenko, N.; Kuznetsova, E.; Malashicheva, A. The complex interplay of TGF-β and notch signaling in the pathogenesis of fibrosis. Int. J. Mol. Sci. 2024, 25, 10803. [Google Scholar] [CrossRef]
- Wu, M.Y.; Hill, C.S. TGF-β superfamily signaling in embryonic development and homeostasis. Dev. Cell 2009, 16, 329–343. [Google Scholar] [CrossRef]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.T. Modulation of Klotho Affects Dendritic Spine Remodeling and Neuronal Network Activity. Ph.D. Thesis, The University of Alabama at Birmingham, Birmingham, AL, USA, 2019. [Google Scholar]
- Maenhaut, C.; Christophe, D.; Vassart, G.; Dumont, J.; Roger, P.; Opitz, R. Ontogeny, anatomy, metabolism and physiology of the thyroid. Endotext [Internet] 2015. [Google Scholar]
- Invernizzi, M.; Carda, S.; Viscontini, G.S.; Cisari, C. Osteoporosis in Parkinson’s disease. Park. Relat. Disord. 2009, 15, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chung, C.-M.; Leu, H.-B.; Lin, L.-Y.; Chiu, C.-C.; Hsu, C.-Y.; Chiang, C.-H.; Huang, P.-H.; Chen, T.-J.; Lin, S.-J. Diabetes mellitus and the risk of Alzheimer’s disease: A nationwide population-based study. PLoS ONE 2014, 9, e87095. [Google Scholar] [CrossRef]
- Hsu, C.C.; Wahlqvist, M.L.; Lee, M.S.; Tsai, H.N. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimer’s Dis. 2011, 24, 485–493. [Google Scholar] [CrossRef]
- Su, L.; Liao, Y.; Liu, X.; Xie, X.; Li, Y. Increased risk of dementia among people with a history of fractures: A systematic review and meta-analysis of population-based studies. Front. Neurol. 2023, 14, 1185721. [Google Scholar] [CrossRef]
- Ioannidis, I.; Mohammad Ismail, A.; Forssten, M.P.; Ahl, R.; Cao, Y.; Borg, T.; Mohseni, S. The mortality burden in patients with hip fractures and dementia. Eur. J. Trauma Emerg. Surg. 2022, 48, 2919–2925. [Google Scholar] [CrossRef]
- Santos, A.; Bakker, A.D.; Klein-Nulend, J. The role of osteocytes in bone mechanotransduction. Osteoporos. Int. 2009, 20, 1027–1031. [Google Scholar] [CrossRef]
- Hiri, O.T.P.; Lawongsa, K.; Kengpanich, S.; Srisuwan, P. Osteoporosis as a Potential Modifiable Risk Factor for Dementia in Thailand: A Cross-Sectional Analysis. Cureus 2024, 16, e63511. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Zhou, G.; Mo, D.; Lin, X.; Wang, P.; Zeng, Y.; Luo, M. Estimated bone mineral density and white matter hyperintensities: A bidirectional Mendelian randomization study. Bone 2024, 187, 117138. [Google Scholar] [CrossRef]
- Ilias, I.; Milionis, C.; Zoumakis, E. An overview of glucocorticoid-induced osteoporosis. Endotext [Internet] 2022. [Google Scholar]

| Predominant Mediator Effect | Types of Secretory Factors | Regulatory Mediators |
|---|---|---|
| Brain-derived | Neurohormones | GH, Melatonin, TSH, OH, FSH, AVP, PRL |
| Neuropeptides | AgRP, CART, POMC, VIP, NPY | |
| Neurotransmitters | Ach, DA, GLU, Serotonin | |
| Sensory Innervation | CGPR, Semaphorin 3A | |
| Bone-derived | Hormones | OCN, LCN2 |
| Peptide | Sclerostin | |
| Adipocyte-derived | Hormones | Adiponectin, Leptin |
| Locally Synthesized Mediators | Peptide | DKK1, OPN, RANKL, Irisin |
| Growth Factors | BDNF, IGF-1, BMPs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansda, S.; Das, H. Unraveling the Bone–Brain Communication Network. Biology 2025, 14, 1279. https://doi.org/10.3390/biology14091279
Hansda S, Das H. Unraveling the Bone–Brain Communication Network. Biology. 2025; 14(9):1279. https://doi.org/10.3390/biology14091279
Chicago/Turabian StyleHansda, Surajit, and Hiranmoy Das. 2025. "Unraveling the Bone–Brain Communication Network" Biology 14, no. 9: 1279. https://doi.org/10.3390/biology14091279
APA StyleHansda, S., & Das, H. (2025). Unraveling the Bone–Brain Communication Network. Biology, 14(9), 1279. https://doi.org/10.3390/biology14091279






