Comparative Analysis of Perceived Threat Threshold from Various Drivers to Cranes Along Indus Flyway, Punjab, Pakistan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
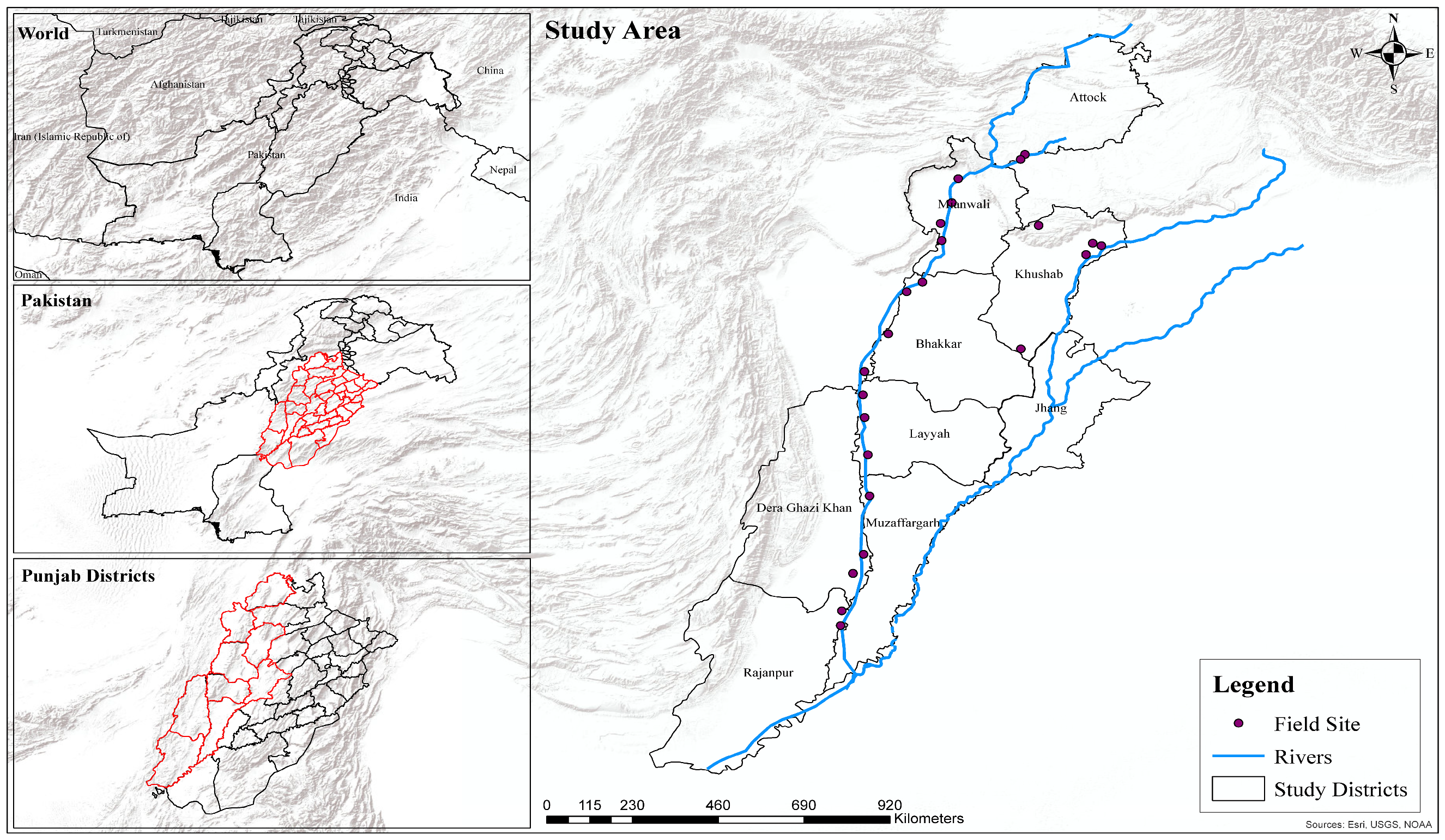
2.1. Study Area
2.2. Study Design
2.3. Sampling Strategy
2.4. Statistical Analysis
3. Results
3.1. Poaching (Illegal Killing) Ranked Highest in Perceived Threat Hierarchy: Frequency and Severity Analysis of Anthropogenic Risks to Crane Populations
3.2. Identification of Regional High-Risk Zones for Prioritized Intervention
3.2.1. Spatial Distribution of Perceived Threats (Location-Based Analysis)
3.2.2. Poaching Camps and Their Role in Perceived Threat Intensification
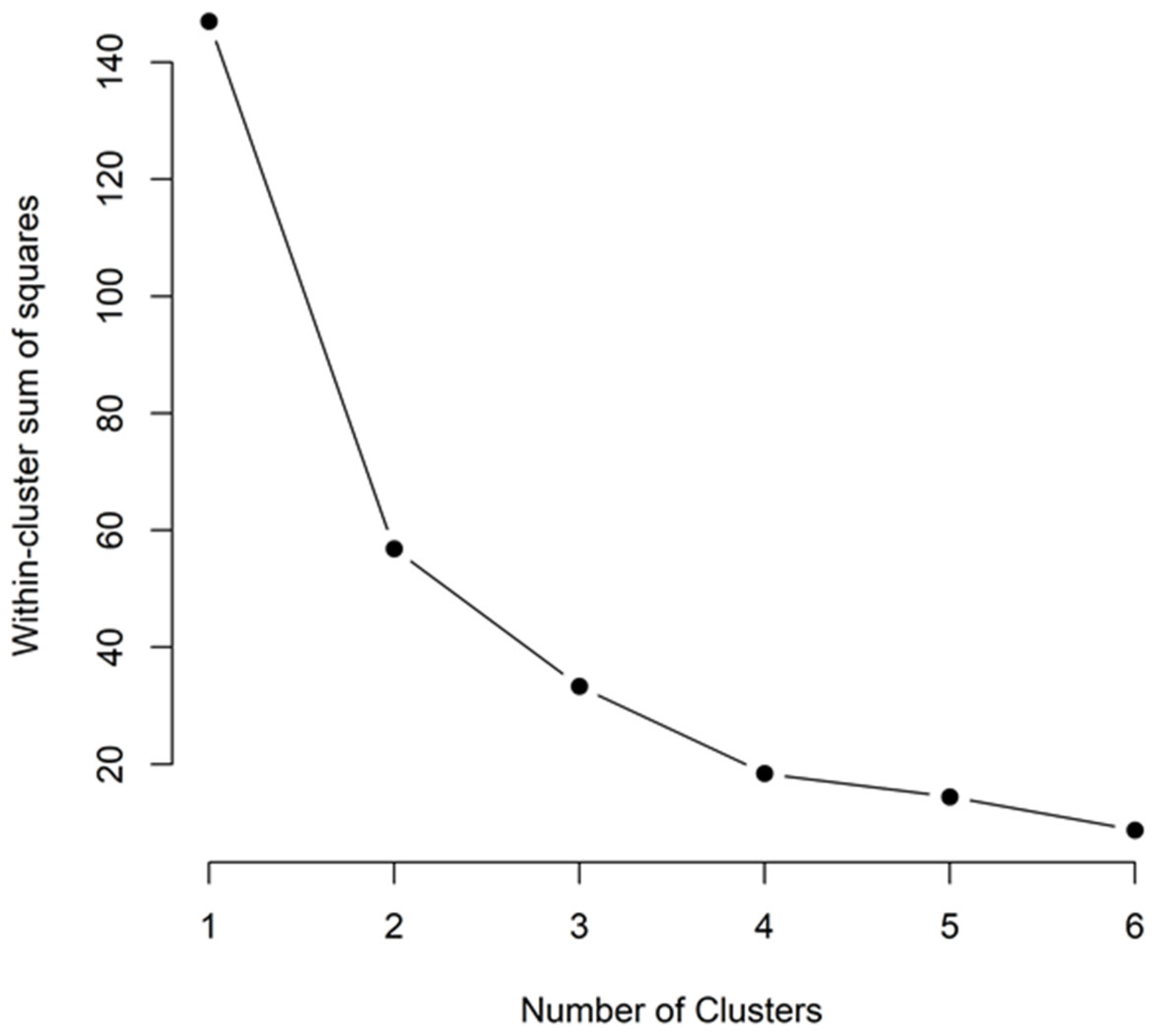
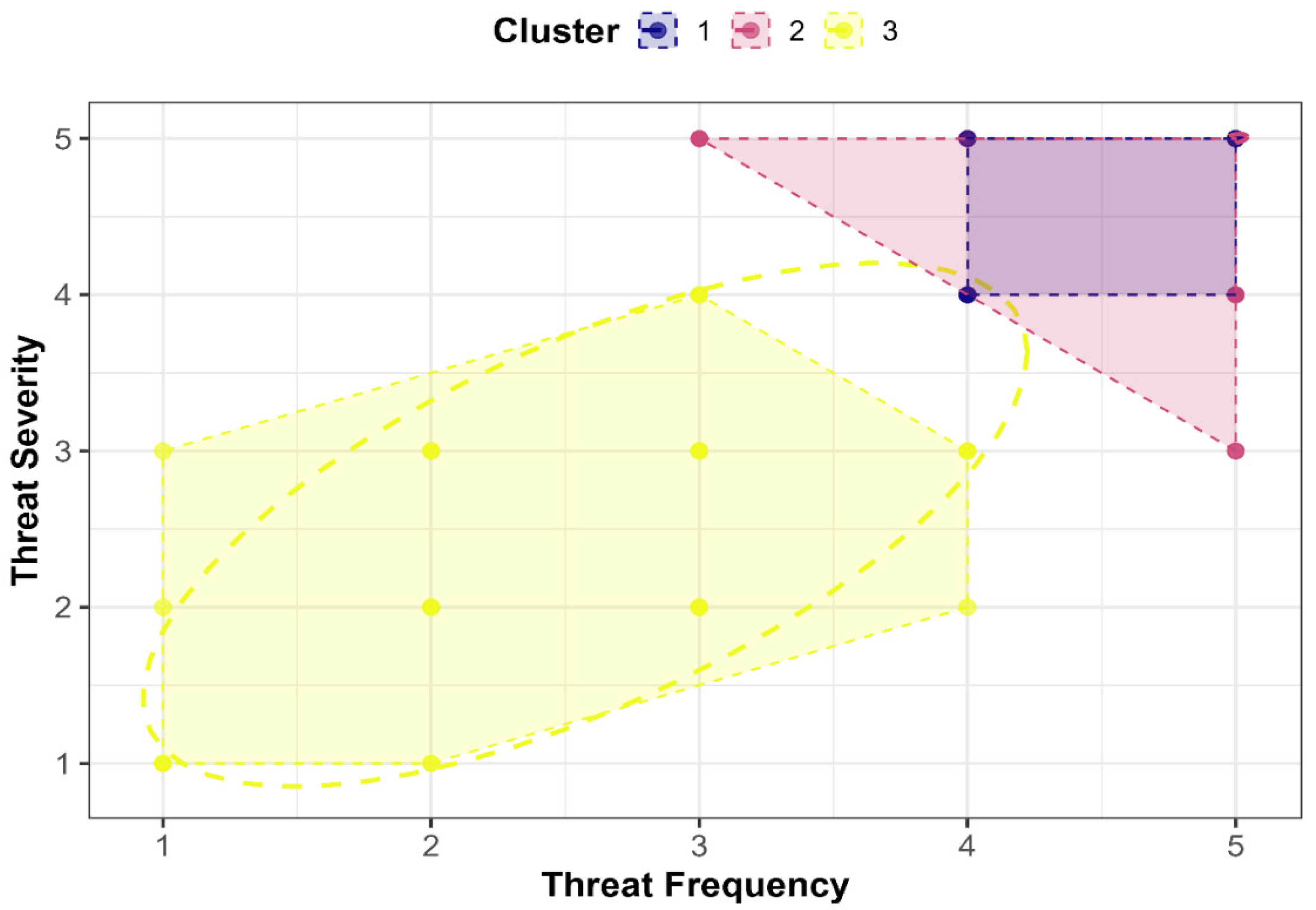
3.2.3. Identification and Characterization of Perceived Threat Categories by Cluster Analysis
3.3. Analysis of Threat Perception Determinants in Crane Conservation
3.3.1. Perceived Frequency of Threats: Significant/Non-Significant Determinants
3.3.2. Perceived Severity of Threats: Significant/Non-Significant Determinants
4. Discussion
4.1. Integrated Threat Hierarchy and Spatial Prioritization
4.2. Illicit Trade and Taming: Underestimated Localized Threats
4.3. Poaching Networks: Scale and Sophistication
4.4. Regional Disparities and Cluster-Based Vulnerability
4.5. Determinants of Threat Perception: Ecological over Demographic
4.6. Conservation Recommendations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
References
- United Nations. World Population Prospects 2022: Summary of Results; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2022; Available online: https://www.un.org/development/desa/pd/content/World-Population-Prospects-2022 (accessed on 20 January 2025).
- Hoffmann, M.; Hilton-Taylor, C.; Angulo, A.; Böhm, M.; Brooks, T.M.; Butchart, S.H.; Carpenter, K.E.; Chanson, J.; Collen, B.; Cox, N.A.; et al. The impact of conservation on the status of the world’s vertebrates. Science 2010, 330, 1503–1509. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef]
- Hogue, A.S.; Breon, K. The greatest threats to species. Conserv. Sci. Pract. 2022, 4, e12670. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Aronson, M.F.J.; Lepczyk, C.A.; Horton, K.G. Assessing the Combined Threats of Artificial Light at Night and Air Pollution for the World’s Nocturnally Migrating Birds. Glob. Ecol. Biogeogr. 2022, 31, 912–924. [Google Scholar] [CrossRef]
- Nemes, C.E.; Cabrera-Cruz, S.A.; Anderson, M.J.; DeGroote, L.W.; DeSimone, J.G.; Massa, M.L.; Cohen, E.B. More Than Mortality: Consequences of Human Activity on Migrating Birds Extend Beyond Direct Mortality. Ornithol. Appl. 2023, 125, duad020. [Google Scholar] [CrossRef]
- Khan, S.R.; Pervaiz, A.N. The Integration of Economic Measures into the National Biodiversity Strategy and Action Plan of Pakistan; IUCN Pakistan: Islamabad, Pakistan, 2001; Volume 7. [Google Scholar]
- Grimmett, R.; Roberts, T.J.; Inskipp, T. Birds of Pakistan; Yale University Press: New Haven, CT, USA, 2008. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species, Version 2021-1; IUCN: Geneva, Switzerland, 2021. [Google Scholar]
- Ullah, I.; Sun, X.Y.; Wu, Q.M.; Deng, W.Y.; Rajpar, M.N.; Majeed, A.; Ditta, A. Determining the Relative Abundance of, Habitat Preferences of and Occurrences of Gastrointestinal Parasites in Common Crane and Demoiselle Crane Inhabiting Three Distinct Habitats. Appl. Ecol. Environ. Res. 2023, 21, 451–465. [Google Scholar] [CrossRef]
- Suliman, M.; Rehman, F.U.; Khan, M.A.; Khan, H.; Khan, M.; Wu, Q. Assessing the Biological Status of Demoiselle Cranes (Anthropoidesvirgo) and Eurasian Crane (Grus grus) with Respect to Illegal Hunting and Captivity in District LakkiMarwat and Bannu, Khyber-Pakhtunkhwa Province, Pakistan. Appl. Ecol. Environ. Res. 2023, 21, 1–15. [Google Scholar] [CrossRef]
- Harris, J.; Mirande, C. A global overview of cranes: Status, threats and conservation priorities. Avian Res. 2013, 4, 189–209. [Google Scholar] [CrossRef]
- Umar, M.; Hussain, M.; Murtaza, G.; Shaheen, F.A.; Zafar, F. Ecological concerns of migratory birds in Pakistan: A review. Punjab Univ. J. Zool. 2018, 33, 69–76. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Ullah, U.; Khan, M.Z.; Ghalib, S.A.; Zehra, A.; Khan, A.R.; Beg, M.N. Recent observation on the status and distribution of threatened and near threatened birds of Sindh, Pakistan. Int. J. Biol. Biotechnol. 2023, 20, 277–292. [Google Scholar]
- Batool, A.; Parveen, A.; Nawaz, M.; Razzaq, D.; Mukhtar, M.; Mustafavi, N. Wetlands of Plains of Pakistan. In Wetlands of Tropical and Subtropical Asia and Africa: Biodiversity, Livelihoods and Conservation; John Wiley & Sons: Hoboken, NJ, USA, 2025; pp. 67–83. [Google Scholar]
- Rasool, G.; Aihetasham, A.; Ali, Z.; Ahmad, R. Avian Richness, Assemblages and Migration Connectivity of Geese Species with Habitat Suitability in Wetlands of the Punjab Pakistan. Pakistan J. Zool. 2023, 56, 2401. [Google Scholar] [CrossRef]
- Tariq, M.; Aziz, R. Threats and Hunting Methods of Crane Species in District Karak of Khyber Pakhtunkhwa, Pakistan. J. Environ. Earth Sci. 2015, 5, 11–15. [Google Scholar]
- Perveen, F.; Khan, H.U. Pressure from Hunting on Crane Species in Southern Districts of Northern Pakistan. Avian Res. 2010, 1, 244–250. [Google Scholar] [CrossRef]
- Rehman, J.U.; Alam, S.; Khalil, S.; Hussain, M.; Iqbal, M.; Khan, K.A.; Habiba, U. Major threats and habitat use status of Demoiselle crane (Anthropoidesvirgo), in district Bannu, Pakistan. Braz. J. Biol. 2021, 82, e242636. [Google Scholar]
- Khan, T.U.; Ullah, I.; Hu, Y.; Liang, J.; Ahmad, S.; Omifolaji, J.K.; Hu, H. Assessment of Suitable Habitat of the Demoiselle Crane (Anthropoidesvirgo) in the Wake of Climate Change: A Study of Its Wintering Refugees in Pakistan. Animals 2024, 14, 1453. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, S. (Ed.) The Trade in Wildlife: Regulation for Conservation; Routledge: Abingdon, UK, 2003. [Google Scholar]
- Sadam, A.; Khan, R.U.; Gabol, K.; Awais, M.; Ismail, M.; Mahmood, S.; Aslam, M. Hunting and illegal trade of White-Breasted Waterhen (Amaurornisphoenicurus): Evidence from District Mardan, Khyber Pakhtunkhwa, Pakistan. Pak. J. Zool. 2022, 55, 2247–2256. [Google Scholar] [CrossRef]
- Sarwar, M.; Hamid, A.; Hussain, I. Hunting pressure on migratory Demoiselle Cranes in Pakistan. Pak. J. Zool. 2022, 54, 10–17582. [Google Scholar] [CrossRef]
- Ullah, I.; Xue-Ying, S.; Qing-Ming, W.; Wen-You, D.; Khan, T.U.; Rajpar, M.N.; Suliman, M. Evaluation of water birds population trends, threats, and conservation status in selected wetlands of Pakistan. Pak. J. Zool. 2024, 56, 1–12. [Google Scholar] [CrossRef]
- Şekercioğlu, Ç.H. Promoting community-based bird monitoring in the tropics: Conservation, research, environmental education, capacity-building, and local incomes. Biol. Conserv. 2012, 151, 69–73. [Google Scholar] [CrossRef]
- Smith, B.P.; Waudby, H.P.; Dickman, C.R.; Soennichsen, K.; Mills, C.; Howe, A.; MacDonald, A.J. Observing wildlife and its signs. Wildl. Res. Aust. Pract. Appl. Methods 2022, 1, 42–74. [Google Scholar]
- Silvertown, J. A new dawn for citizen science. Trends Ecol. Evol. 2009, 24, 467–471. [Google Scholar] [CrossRef]
- Fritz, S.; See, L.; Carlson, T.; Haklay, M.; Oliver, J.L.; Fraisl, D.; West, S. Citizen science and the United Nations Sustainable Development Goals. Nat. Sustain. 2019, 2, 922–930. [Google Scholar] [CrossRef]
- Latif, M.; Shireen, H.; Adnan, S.; Ahmed, R.; Hannachi, A. Drought variability in Pakistan: Navigating historical patterns in a changing climate with global teleconnections. Theor. Appl. Climatol. 2024, 155, 8379–8400. [Google Scholar] [CrossRef]
- Qasim, M.; Ali, S.; Aqeel, M. Geographic diversity and landscape in transition: Analyzing the physical features of Gilgit Baltistan region. J. Soc. Sci. Dev. 2024, 3, 154–169. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Shah, M.H.; Khan, M.A. Wild Edible Vegetables of Lesser Himalayas; Springer International Publisher: Berlin/Heidelberg, Germany, 2015; pp. 141–167. [Google Scholar]
- Government of Pakistan (GoP). Fourth National Report; Convention on Biological Diversity, Ministry of Environment: Islamabad, Pakistan, 2017. [Google Scholar]
- Niederman, E.A.; Porinchu, D.F.; Kotlia, B.S. Hydroclimate change in the Garhwal Himalaya, India, at 4200 yr BP coincident with the contraction of the Indus Civilization. Sci. Rep. 2021, 11, 23082. [Google Scholar] [CrossRef]
- Batista, M.I.; Abril, C.; Veríssimo, A.; Vasconcelos, R.P.; Pais, M.P.; Henriques, S. Mapping elasmobranch occurrences and overlap with human activities using local knowledge and non-invasive sampling to identify areas of potential conflict. Front. Mar. Sci. 2024, 11, 1321620. [Google Scholar] [CrossRef]
- Roux, M.J.; Pedreschi, D. ICES Framework for Ecosystem-Informed Science and Advice (FEISA); ICES: Copenhagen, Denmark, 2024. [Google Scholar]
- Treves, A.; Wallace, R.B.; White, S. Participatory planning of interventions to mitigate human–wildlife conflicts. Conserv. Biol. 2009, 23, 1577–1587. [Google Scholar] [CrossRef]
- Bennett, N.J.; Roth, R.; Klain, S.C.; Chan, K.; Christie, P.; Clark, D.A.; Cullman, G.; Curran, D.; Durbin, T.J.; Epstein, G.; et al. Conservation social science: Understanding and integrating human dimensions to improve conservation. Biol. Conserv. 2017, 205, 93–108. [Google Scholar] [CrossRef]
- Sheppard, D.J.; Stark, D.J.; Muturi, S.W.; Munene, P.H. Benefits of traditional and local ecological knowledge for species recovery when scientific inference is limited. Front. Conserv. Sci. 2024, 5, 1383611. [Google Scholar] [CrossRef]
- McKinley, D.C.; Miller-Rushing, A.J.; Ballard, H.L.; Bonney, R.; Brown, H.; Cook-Patton, S.C.; Soukup, M.A.; Evans, D.M.; French, R.A.; Parrish, J.K.; et al. Citizen science can improve conservation science, natural resource management, and environmental protection. Biol. Conserv. 2017, 208, 15–28. [Google Scholar] [CrossRef]
- Brittain, S.M.; Rowcliffe, M. Integrating Local Knowledge into Wildlife Population Monitoring. Ph.D. Thesis, University of Oxford, Oxford, UK, 2019. [Google Scholar]
- Kishore, S.M.; Lingaraj, P.; Yamini, M.; Sandeep, K.S.; Patil, C.; Sowmya, K. Empowering biodiversity conservation: The role of citizen science in monitoring invasive species and insect populations. Insect Environ. 2025, 28, 45–52. [Google Scholar] [CrossRef]
- Inskip, C.; Zimmermann, A. Human–felid conflict: A review of patterns and priorities worldwide. Oryx 2009, 43, 18–34. [Google Scholar] [CrossRef]
- Buys, A. Strategic Management of Conservation Areas: A Systems Thinking Approach to Sustaining Complex Multi-Stakeholder Organisations; University of South Africa: Pretoria, South Africa, 2020. [Google Scholar]
- Lu, Y. The Role of Local Knowledge in Yancheng National Nature Reserve Management. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2016. [Google Scholar]
- Patterson-Abrolat, C.; Ilyashenko, E.; Morrison, K.L. Strategies to manage the crane-agriculture interface using partnerships, ecotourism and educational opportunities. In Cranes and Agriculture: A Global Guide for Sharing the Landscape; International Crane Foundation: Baraboo, WI, USA, 2018; pp. 157–179. [Google Scholar]
- Wutich, A.; Beresford, M.; Bernard, H.R. Sample sizes for 10 types of qualitative data analysis: An integrative review, empirical guidance, and next steps. Int. J. Qual. Methods 2024, 23, 16094069241296206. [Google Scholar] [CrossRef]
- Wardropper, C.B.; Dayer, A.A.; Goebel, M.S.; Martin, V.Y. Conducting conservation social science surveys online. Conserv. Biol. 2021, 35, 1650–1658. [Google Scholar] [CrossRef]
- Westgate, M.J.; Barton, P.S.; Lane, P.W.; Lindenmayer, D.B. Global meta-analysis reveals low consistency of biodiversity congruence relationships. Nat. Commun. 2014, 5, 3899. [Google Scholar] [CrossRef]
- Widlok, T.; Aufgebauer, A.; Bradtmöller, M.; Dikau, R.; Hoffmann, T.; Kretschmer, I.; Zimmermann, A. Towards a theoretical framework for analyzing integrated socio-environmental systems. Quat. Int. 2012, 274, 259–272. [Google Scholar] [CrossRef]
- Mace, G.M.; Collar, N.J.; Gaston, K.J.; Hilton-Taylor, C.R.A.I.G.; Akçakaya, H.R.; Leader-Williams, N.I.G.E.L.; Stuart, S.N. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv. Biol. 2008, 22, 1424–1442. [Google Scholar] [CrossRef]
- Biber, E. The challenge of collecting and using environmental monitoring data. Ecol. Soc. 2013, 18, 14. [Google Scholar] [CrossRef]
- Bélisle, A.C.; Asselin, H.; LeBlanc, P.; Gauthier, S. Local knowledge in ecological modeling. Ecol. Soc. 2018, 23, 11. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 2 September 2025).
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Harrison, R.D.; Sreekar, R.; Brodie, J.F.; Brook, S.; Luskin, M.; O’Kelly, H.; Velho, N. Impacts of hunting on tropical forests in Southeast Asia. Conserv. Biol. 2016, 30, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Rija, A.A.; Critchlow, R.; Thomas, C.D.; Beale, C.M. Global extent and drivers of mammal population declines in protected areas under illegal hunting pressure. PLoS ONE 2020, 15, e0227163. [Google Scholar] [CrossRef]
- Bhattarai, B.P.; Katuwal, H.B.; Regmi, S.; Nepali, A.; Suwal, R.N.; Acharya, R.; Sharma, H.P. Knowledge, attitudes, and conservation threats to globally vulnerable Sarus Cranes in Lumbini Province, Nepal. Discov. Conserv. 2025, 2, 15. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Adams, W.M.; Aronson, R.B.; Aveling, R.; Blackburn, T.M.; Broad, S.; Watkinson, A.R. One Hundred Questions of Importance to the Conservation of Global Biological Diversity. Conserv. Biol. 2009, 23, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Song, A.Y.; Yao, Y. To Ban or Not to Ban: China’s Trade in Endangered Species. J. Contemp. China 2022, 31, 153–167. [Google Scholar]
- Nicole, B.F. An Assessment of the Human-Wildlife Conflict Across Africa. In Wildlife Population Monitoring; IntechOpen: London, UK, 2019; pp. 1–10. [Google Scholar]
- Margaryan, L.; Prince, S.; Ioannides, D.; Röslmaier, M. Dancing with Cranes: A Humanist Perspective on Cultural Ecosystem Services of Wetlands. Tour. Geogr. 2022, 24, 501–522. [Google Scholar] [CrossRef]
- Brotherton, S.; Joyce, C.B.; Scharlemann, J.P. Global Offtake of Wild Animals from Wetlands: Critical Issues for Fish and Birds. Hydrobiologia 2020, 847, 1631–1649. [Google Scholar] [CrossRef]
- Benítez-López, A.; Alkemade, R.; Schipper, A.M.; Ingram, D.J.; Verweij, P.A.; Eikelboom, J.A.J.; Huijbregts, M.A.J. The Impact of Hunting on Tropical Mammal and Bird Populations. Science 2017, 356, 180–183. [Google Scholar] [CrossRef]
- Ahmad, S.; Bari, F.; Kabir, M.; Baig, M.A.; Khan, T.; Khattak, R.H.; Rehman, E.U. Population Status and Roost Site Selection of Endangered Egyptian Vultures (Neophronpercnopterus) in Poonch River Mahasheer National Park, Azad Jammu and Kashmir, Pakistan. J. Raptor Res. 2021, 55, 99–105. [Google Scholar] [CrossRef]
- Shah, J.; Ahmad, B.; Nawaz, H.; Khan, F.U.; Khan, J.; Ayaz, M.; Ali, Y. Hunting of Demoiselle Cranes in District Bannu, Khyber Pakhtunkhwa, Pakistan. J. Liaoning Tech. Univ. 2024, 18, 134–137. [Google Scholar]
- Veltheim, I.; Chavez-Ramirez, F.; Hill, R.; Cook, S. Assessing Capture and Tagging Methods for Brolgas, Antigone rubicunda (Gruidae). Wildl. Res. 2015, 42, 373–381. [Google Scholar] [CrossRef]
- Bari, F.; Rehman, E.U.; Kabir, M.; Ahmad, S. An Extension to the Known Wintering Range of the Steppe Eagle (Aquila nipalensis) in the Poonch and Jhelum Valleys, Azad Jammu and Kashmir, Pakistan. Ardeola 2020, 67, 415–422. [Google Scholar] [CrossRef]
- Morar, F.; Peterlicean, A. The Role and Importance of Educating Youth Regarding Biodiversity Conservation in Protected Natural Areas. Procedia Econ. Financ. 2012, 3, 1117–1121. [Google Scholar] [CrossRef]
| Threat Type | Mean Frequency | Mean Severity |
|---|---|---|
| Poaching (Illegal Killing) | 4.9 | 4.8 |
| Illegal Wildlife Trading | 4.7 | 4.5 |
| Taming | 4.6 | 4.3 |
| Collision with Power Lines | 4.4 | 4.0 |
| Human-subsidized Predation | 2.3 | 2.2 |
| Threat Comparison | Mean Difference | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|
| Poaching (illegal killing)—Human-subsidized predation | 2.6200 | 2.0860 | 3.1541 | <0.001 |
| Poaching (illegal killing)—Collision with power lines | 1.8021 | 1.3509 | 2.2533 | <0.001 |
| Illegal trade—Human-subsidized predation | 1.4754 | 0.8444 | 2.1064 | <0.001 |
| Taming—Human-subsidized predation | 1.4375 | 0.8678 | 2.0072 | <0.001 |
| Poaching (illegal killing)—Illegal wildlife trade | 1.1447 | 0.7612 | 1.5281 | <0.001 |
| Illegal trade—Collision with power lines | 0.6574 | 0.0948 | 1.2201 | 0.013 |
| Taming—Collision with power lines | 0.6196 | 0.1266 | 1.1125 | 0.006 |
| Taming—Illegal wildlife trade | −0.0379 | −0.4697 | 0.3939 | 0.999 |
| Human-subsidized predation—Collision with power lines | −0.8179 | −1.4922 | −0.1436 | 0.009 |
| Taming—Poaching (illegal killing) | −1.1825 | −1.4536 | −0.9115 | <0.001 |
| Threat Comparison | Mean Difference (Diff) | Lower CI (Lwr) | Upper CI (Upr) | Adjusted p-Value (p Adj) |
|---|---|---|---|---|
| Poaching (illegal killing)—Human-subsidized predation | 2.9256 | 2.4327 | 3.4185 | <0.001 |
| Poaching (illegal killing)—Collision with power lines | 1.9446 | 1.5282 | 2.3610 | <0.001 |
| Taming—Human-subsidized predation | 1.7138 | 1.1880 | 2.2397 | <0.001 |
| Illegal trade—Human-subsidized predation | 1.4830 | 0.9006 | 2.0654 | <0.001 |
| Poaching (illegal killing)—Illegal wildlife trade | 1.4426 | 1.0887 | 1.7966 | <0.001 |
| Taming—Collision with power lines | 0.7328 | 0.2779 | 1.1878 | <0.001 |
| Illegal trade—Collision with power lines | 0.5020 | −0.0173 | 1.0213 | 0.064 |
| Taming—Illegal wildlife trade | 0.2309 | −0.1677 | 0.6294 | 0.506 |
| Human-subsidized predation—Collision with power lines | −0.9810 | −1.6033 | −0.3586 | <0.001 |
| Taming—Poaching (illegal killing) | −1.2118 | −1.4620 | −0.9616 | <0.001 |
| Threat Type | Correlation (r) | p-Value | 95% CI (Low) | 95% CI (High) | Method |
|---|---|---|---|---|---|
| Collision with power lines | 0.930 | <0.001 | 0.8395 | 0.9702 | Spearman |
| Illegal wildlife trading | 0.915 | <0.001 | 0.8333 | 0.9575 | Spearman |
| Poaching (Illegal Killing) | 0.557 | <0.001 | 0.4652 | 0.6365 | Spearman |
| Taming | 0.868 | <0.001 | 0.7991 | 0.9146 | Spearman |
| Human-subsidized predation | 0.189 | 0.482 | −0.3380 | 0.6263 | Spearman |
| Region | Mean Frequency | SD Frequency | Mean Severity | SD Severity | Count |
|---|---|---|---|---|---|
| Attock | 4.62 | 1.01 | 4.62 | 1.01 | 50 |
| Bhakkar | 4.1 | 1.25 | 4.1 | 1.25 | 50 |
| DG Khan | 4.54 | 0.862 | 4.5 | 0.974 | 50 |
| Khushab | 3.76 | 1.15 | 4.02 | 1.22 | 50 |
| Layyah | 4.22 | 1.27 | 4.26 | 1.27 | 50 |
| Mianwali | 4.68 | 0.768 | 4.48 | 0.863 | 50 |
| Muzaffargarh | 4.44 | 1.05 | 4.48 | 1.01 | 50 |
| Rajanpur | 4.88 | 0.521 | 4.82 | 0.72 | 50 |
| Location of Threat | Threat Type | Count |
|---|---|---|
| River Bank | Taming | 3 |
| River Bank | Poaching (illegal killing) | 42 |
| River Bank | Human-subsidized predation | 1 |
| River Bank | Illegal wildlife trading | 3 |
| River Bank | Collision with power lines | 1 |
| Poaching Camps | Average Poaching Parties | Count |
|---|---|---|
| Camp 2 | 3.8 | 5 |
| Camp 3 | 6 | 2 |
| Camp 4 | 6.66 | 15 |
| Camp 5 | 8 | 14 |
| Camp 6 | 8.4 | 10 |
| Camp 7 | 9 | 1 |
| None | 2 | 3 |
| Source of Variation | df | Sum Sq | Mean Sq | F-Value | p-Value |
|---|---|---|---|---|---|
| Threat Type | 4 | 222.73 | 55.68 | 104.92 *** | <0.001 |
| Occupation | 9 | 7.82 | 0.87 | 1.64 | 0.103 |
| Education Level | 4 | 1.21 | 0.30 | 0.57 | 0.685 |
| Preferred Crane Species | 2 | 0.40 | 0.20 | 0.38 | 0.687 |
| Poaching Method | 3 | 16.15 | 5.38 | 10.14 *** | <0.001 |
| Residuals | 377 | 200.08 | 0.53 |
| Source of Variation | df | Sum Sq | Mean Sq | F-Value | p-Value |
|---|---|---|---|---|---|
| Threat Type | 4 | 270.52 | 67.63 | 153.64 *** | <0.001 |
| Occupation | 9 | 4.12 | 0.46 | 1.04 | 0.407 |
| Education Level | 4 | 0.75 | 0.19 | 0.43 | 0.789 |
| Preferred Crane Species | 2 | 1.03 | 0.52 | 1.17 | 0.310 |
| Poaching Method | 3 | 20.38 | 6.79 | 15.43 *** | <0.001 |
| Residuals | 377 | 165.95 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulfiqar, A.; Sun, X.; Wu, Q.; Rehman, A.; Khan, N.; Khan, M.N. Comparative Analysis of Perceived Threat Threshold from Various Drivers to Cranes Along Indus Flyway, Punjab, Pakistan. Biology 2025, 14, 1275. https://doi.org/10.3390/biology14091275
Zulfiqar A, Sun X, Wu Q, Rehman A, Khan N, Khan MN. Comparative Analysis of Perceived Threat Threshold from Various Drivers to Cranes Along Indus Flyway, Punjab, Pakistan. Biology. 2025; 14(9):1275. https://doi.org/10.3390/biology14091275
Chicago/Turabian StyleZulfiqar, Ayesha, Xueying Sun, Qingming Wu, Abdul Rehman, Nasrullah Khan, and Mah Noor Khan. 2025. "Comparative Analysis of Perceived Threat Threshold from Various Drivers to Cranes Along Indus Flyway, Punjab, Pakistan" Biology 14, no. 9: 1275. https://doi.org/10.3390/biology14091275
APA StyleZulfiqar, A., Sun, X., Wu, Q., Rehman, A., Khan, N., & Khan, M. N. (2025). Comparative Analysis of Perceived Threat Threshold from Various Drivers to Cranes Along Indus Flyway, Punjab, Pakistan. Biology, 14(9), 1275. https://doi.org/10.3390/biology14091275







