Integrated miRNA-mRNA Atlas Reveals Temperature-Graded Brain Neuroendocrine Adaptation to Cold Stress in Silvery Pomfret (Pampus argenteus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design and Sample Collection
2.3. mRNA and miRNA Sequencing and Analysis
2.3.1. Library Construction and Sequencing
2.3.2. Differential Expression Analysis
2.3.3. Target Genes Identification and Functional Enrichment
2.3.4. Regulatory miRNA-mRNA Network Construction
2.4. Experimental Validation
3. Results
3.1. miRNA Sequencing and Identification
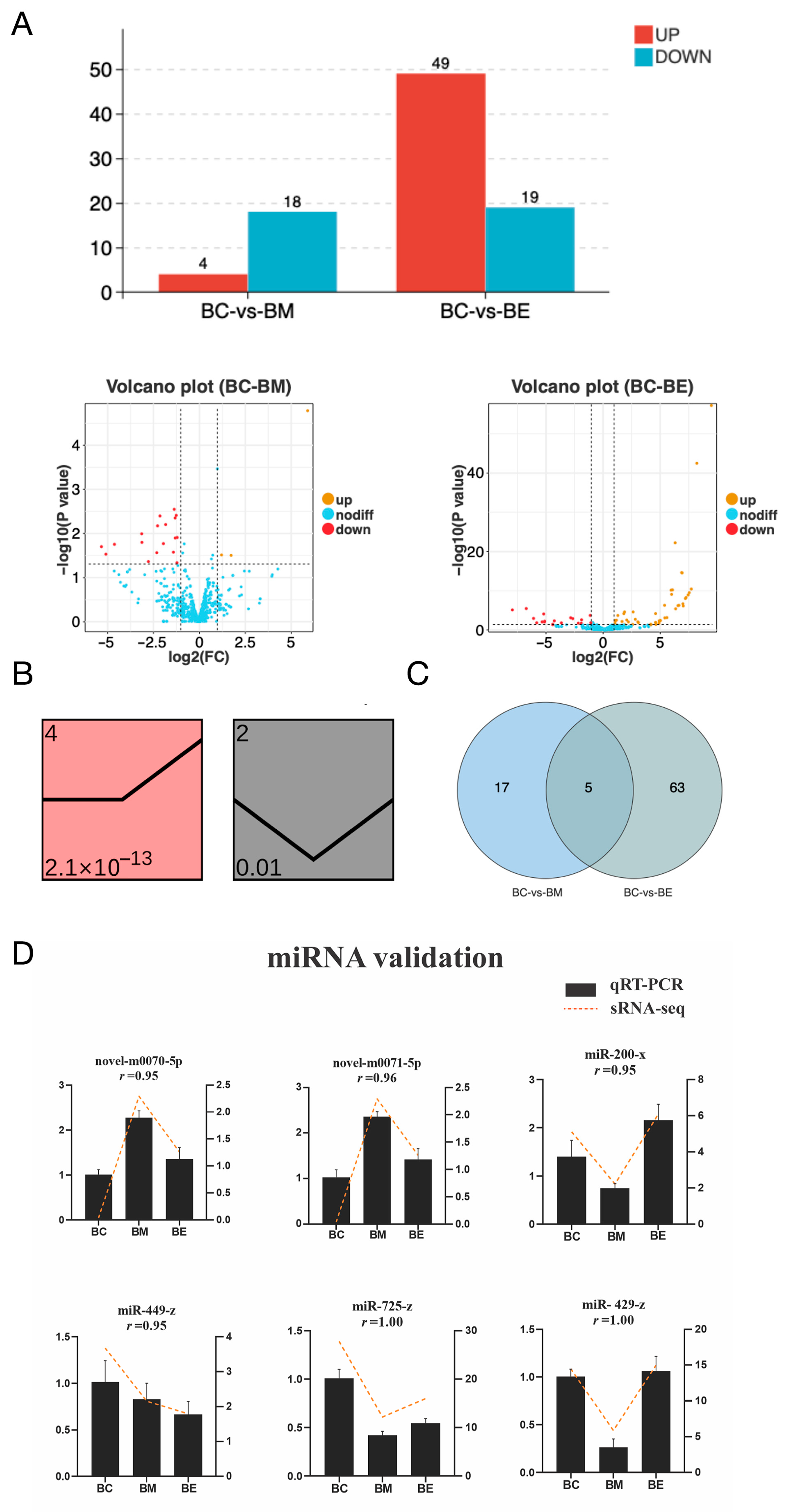
3.2. Cold Stress Induces Significant Brain miRNA Responses
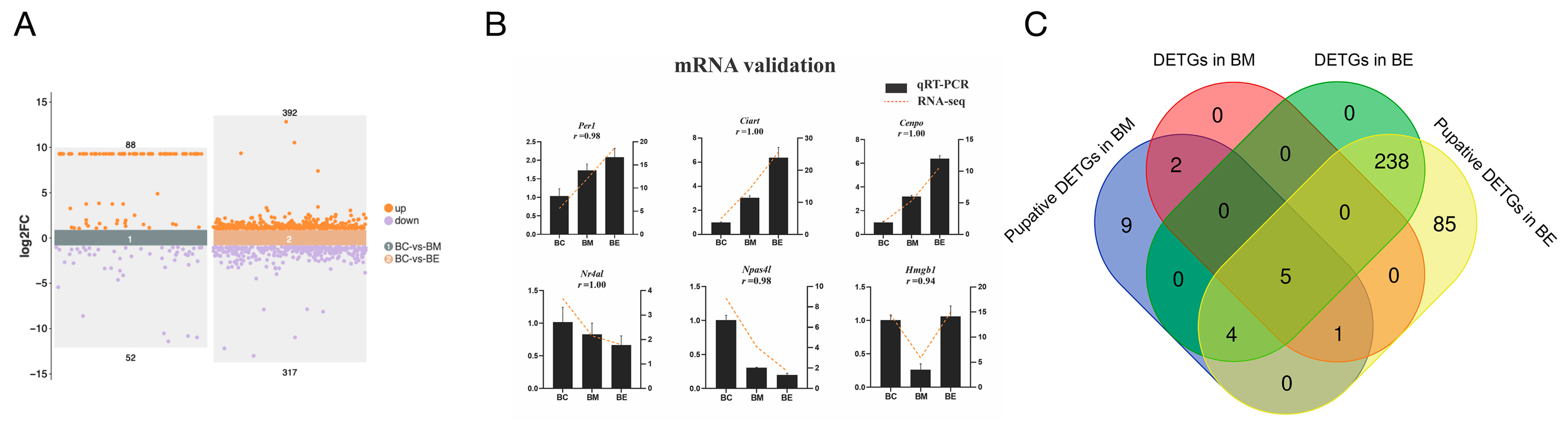
3.3. Integrated Analysis of miRNA-mRNA During Cold Stress
3.4. Functional Landscapes of DEmiR-Mediated Pathways
3.5. Cold-Responsive miRNA-mRNA Regulatory Networks
4. Discussion
4.1. Circadian Rhythm
4.2. Immune Response
4.3. Endocrine Adaptation Strategy
4.4. Potential Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Name | Primer (5′ to 3′) | Primer Tm | Product Length | Accession Number | Amplification Efficiency |
|---|---|---|---|---|---|
| novel-m0070-5p | CCCTGTGGTACCTGTACTACCTG | 56.7 | / | / | / |
| novel-m0071-5p | GCCTGTGGTACCTGTACTACCT | 56.7 | / | / | / |
| miR-429-y | GCCGTAATACTGTCTGGTAATGCCG | 51.4 | / | / | / |
| miR-725-z | CGCTTCAGTCATTGTTTCTGGTCGT | 56.6 | / | / | / |
| miR-200-x | GCATCTTACCTGACAGTGCTGGA | 60.6 | / | / | / |
| miR-449-z | ATTATTGGCAGTGTACTGCTGCTGG | 60.1 | / | / | / |
| Nr4a1 | CATCGGATGCACGATTAG | 50.4 | 201 | evm.TU.Chr04.473 | 90.01% |
| TGTGGGAGATTGGTCAGG | 54.2 | ||||
| Npas4 | TTCACCGCATGAACCTAG | 51.8 | 197 | evm.TU.Chr12.134 | 106.95% |
| TACCCAGAAGACGAGAAAGATA | 51.8 | ||||
| Hmgb1 | TGATTACCCTGGGCTTTC | 51.6 | 172 | evm.TU.Chr15.666 | 93.12% |
| TGATGCCGTATCCACTTTA | 50.1 | ||||
| Per1 | CGGAAGGTGGCGTTTATT | 52.7 | 155 | evm.TU.Chr01.395 | 105.80% |
| ACGGGCTGTACCAGGAGA | 58.7 | ||||
| Ciart | CTTTGCTTGGGCTTCTAC | 65.8 | 232 | evm.TU.Chr20.664 | 106.27% |
| GGAGTGATGGTCTGGGTT | 54.2 | ||||
| Cenpo | TGACCTGAAGCCAACTCT | 53.0 | 232 | evm.TU.Chr14.568 | 106.02% |
| CAGCACCAATACTGAACATA | 49.3 | ||||
| U6 | TGACCTGAAGCCAACTCT | 59.4 | / | 107.99% | |
| AACGCTTCACGAATTTGCGT | 55.6 |
References
- Beitinger, T.L.; Fitzpatrick, L.C. Physiological and ecological correlates of preferred temperature in Fish. Am. Zool. 2015, 19, 319–329. [Google Scholar] [CrossRef]
- Crawshaw, L.I. Physiological and behavioral reactions of fishes to temperature change. J. De L’Office Des Rech. Sur Les Pêcheries Du Can. 1977, 34, 730–734. [Google Scholar] [CrossRef]
- Beitinger, T.L.; Bennett, W.A.; Mccauley, R.W. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fishes 2000, 58, 237–275. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, X.; Jiao, S.; You, F.; Zhang, P.J. Analyzing cold tolerance mechanism in transgenic zebrafish (Danio rerio). PLoS ONE 2014, 9, e102492. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, D.L.; Yu, D.H.; Lv, C.H.; Luo, H.Y.; Wang, Z.Y. Molecular cloning and expression analysis of scd1 gene from large yellow croaker Larimichthys crocea under cold stress. Gene 2015, 568, 100–108. [Google Scholar] [CrossRef]
- Reid, C.H.; Patrick, P.H.; Rytwinski, T.; Taylor, J.J.; Willmore, W.G.; Reesor, B.; Cooke, S.J. An updated review of cold shock and cold stress in fish. J. Fish Biol. 2022, 100, 1102–1137. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, R.; Wang, X.; Zhu, H.; Tian, Z. Transcriptome analysis reveals molecular mechanisms responsive to acute cold stress in the tropical stenothermal fish tiger barb (Puntius tetrazona). BMC Genom. 2020, 21, 737. [Google Scholar] [CrossRef]
- Cheng, A.M.; Byrom, M.W.; Shelton, J.; Ford, L.P. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005, 33, 1290–1297. [Google Scholar] [CrossRef]
- Pilotte, J.; Dupont-Versteegden, E.E.; Vanderklish, P.W. Widespread Regulation of miRNA Biogenesis at the Dicer Step by the Cold-Inducible RNA-Binding Protein, RBM3. PLoS ONE 2011, 6, e28446. [Google Scholar] [CrossRef]
- Seeliger, C.; Krauss, T.; Honecker, J.; Mengel, L.A.; Buekens, L.; Mesas-Fernández, A.; Skurk, T.; Claussnitzer, M.; Hauner, H. miR-375 is cold exposure sensitive and drives thermogenesis in visceral adipose tissue derived stem cells. Sci. Rep. 2022, 12, 9557. [Google Scholar] [CrossRef]
- Xu, J.; Cui, L.; Wang, J.; Zheng, S.; Zhang, H.; Ke, S.; Cao, X.; Shi, Y.; Li, J.; Zen, K. Cold-activated brown fat-derived extracellular vesicle-miR-378a-3p stimulates hepatic gluconeogenesis in male mice. Nat. Commun. 2023, 14, 5480. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gloria, J.L.; Carbó, R.; Buelna-Chontal, M.; Osorio-Alonso, H.; Henández-Díazcouder, A.; Fuente-León RLdl Sandoval, J.; Sánchez, F.; Rubio-Gayosso, I.; Sánchez-Muoz, F. Cold exposure aggravates pulmonary arterial hypertension through increased miR-146a-5p, miR-155-5p and cytokines TNF-α, IL-1β, and IL-6. Life Sci. 2021, 287, 120091. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.C.; Hsiao, Y.C.; Sun, H.S.; Chen, T.M.; Lee, S.J. MicroRNAs regulate gene plasticity during cold shock in zebrafish larvae. BMC Genom. 2016, 17, 922. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, S.; Liu, Y.; Chu, P.; Han, B.; Ning, X.; Wang, T.; Yin, S. Acute cold stress leads to zebrafish ovarian dysfunction by regulating miRNA and mRNA. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 48, 101139. [Google Scholar] [CrossRef]
- Gao, Q.; Gao, Q.; Min, M.; Zhang, C.; Peng, S.; Shi, Z. Ability of Lactobacillus plantarum lipoteichoic acid to inhibit Vibrio anguillarum -induced inflammation and apoptosis in silvery pomfret (Pampus argenteus) intestinal epithelial cells. Fish Shellfish Immunol. 2016, 54, 573–579. [Google Scholar] [CrossRef]
- Gao, Q.; Xiao, Y.; Zhang, C.; Min, M.; Peng, S.; Shi, Z. Molecular characterization and expression analysis of toll-like receptor 2 in response to bacteria in silvery pomfret intestinal epithelial cells. Fish Shellfish Immunol. 2016, 58, 1–9. [Google Scholar] [CrossRef]
- Gao, Q.; Yin, F.; Zhang, C.; Yue, Y.; Sun, P.; Min, M.; Peng, S.; Shi, Z.; Lv, J. Cloning, characterization, and function of MyD88 in silvery pomfret (Pampus argenteus) in response to bacterial challenge. Int. J. Biol. Macromol. Struct. Funct. Interact. 2017, 103, 327–337. [Google Scholar] [CrossRef]
- Gao, Q.; Yue, Y.; Min, M.; Peng, S.; Shi, Z.; Sheng, W.; Zhang, T. Characterization of TLR5 and TLR9 from silver pomfret (Pampus argenteus) and expression profiling in response to bacterial components. Fish. Shellfish. Immunol. 2018, 80, 241–249. [Google Scholar] [CrossRef]
- Divya, P.R.; Gopalakrishnan, A.; Basheer, V.S.; Swaminathan, R.; Mohitha, C.; Joy, L.; Kumar, R.; Manoj, P.; Jena, J.K. Mitochondrial ATPase 6/8 genes to infer the population genetic structure of silver pomfret fish Pampus argenteus along the Indian waters. Mitochondrial DNA 2015, 26, 189–194. [Google Scholar] [CrossRef]
- Almomin, S.; Kumar, V.; Al-Amad, S.; Al-Hussaini, M.; Akbar, A. Draft genome sequence of the silver pomfret fish, Pampus argenteus. Genome 2016, 59, 51–58. [Google Scholar] [CrossRef]
- Li, J.; Xue, L.Y.; Cao, M.Y.; Zhang, Y.; Wang, Y.J.; Xu, S.L.; Zheng, B.X.; Lou, Z.J. Gill transcriptomes reveal expression changes of genes related with immune and ion transport under salinity stress in silvery pomfret (Pampus argenteus). Fish Physiol. Biochem. 2020, 46, 1255–1277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zang, X.; Zhao, C.; Chu, P.; Chen, H.; Fu, D.; Wang, S.; Zhang, K.; Wang, T.; Yin, S. Cold stress changes the intestinal oxidative stress-mediated MAPK pathways and lipid metabolism in Takifugu fasciatus. Aquaculture 2025, 598, 742034. [Google Scholar] [CrossRef]
- Han, B.; Zhou, L.X.; Shi, Y.X.; Zhao, F.; Ji, J.; Zhang, K.; Yin, S.W.; Ning, X.H. LncRNA432-miR-21-y-DAPK2 ceRNA crosstalk regulates antibacterial response in hypoxia stress through mediating mitochondrial apoptosis in teleost fish. Int. J. Biol. Macromol. 2025, 295, 139694. [Google Scholar] [CrossRef]
- Ning, X.H.; Peng, Y.; Tang, P.; Zhang, Y.R.; Wang, L.L.; Zhang, W.W.; Zhang, K.; Ji, J.; Yin, S.W. Integrated analysis of transcriptome and metabolome reveals distinct responses of Pelteobagrus fulvidraco against Aeromonas veronii infection at invaded and recovering stage. Int. J. Mol. Sci. 2022, 23, 10121. [Google Scholar] [CrossRef]
- Peng, Y.; Han, B.; Zhang, K.; Tang, P.; Zhang, Y.R.; Ji, J.; Yin, S.W.; Ning, X.H. ceRNA network mediated by lncRNA-miRNA-mRNA of Pelteobagrus fulvidraco plays a dual function of immunity and lipid metabolism in response to Aeromonas veronii infection. Aquaculture 2023, 571, 739475. [Google Scholar] [CrossRef]
- Langmead, B. Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinform. 2010, 32, 11.1–11.17. [Google Scholar] [CrossRef]
- Ning, X.; Sun, L. Micro-transcriptome analysis reveals immune-related microRNA regulatory networks of Paralichthys olivaceus induced by Vibrio anguillarum infection. Int. J. Mol. Sci. 2020, 21, 4252. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Q.; Wang, J.; Guo, X.; Jiang, X.; Ren, Z.; Weng, C.; Sun, G.; Wang, X.; Liu, Y.; et al. Identification and characterization of novel amphioxus microRNAs by Solexa sequencing. Genome Biol. 2009, 10, R78. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods A Companion Methods Enzymol. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhen, L.; Guo, W.J.; Peng, M.L.; Liu, Y.Z.; Zang, S.C.; Ji, H.; Li, S.Z.; Yang, H.M. Identification of cold-responsive miRNAs in rats by deep sequencing. J. Therm. Biol. 2017, 66, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D.; Cheng, L.; Ma, J.X.; Wang, X.M.; Zhao, Y.Y. Chronic cold exposure leads to daytime preference in the circadian expression of hepatic metabolic genes. Front. Physiol. 2022, 13, 865627. [Google Scholar] [CrossRef] [PubMed]
- Gery, S.; Koeffler, H.P. The role of circadian regulation in cancer. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 459–464. [Google Scholar] [CrossRef]
- Chappuis, S.; Ripperger, J.A.; Schnell, A.; Rando, G.; Jud, C.; Wahli, W.; Albrecht, U. Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol. Metab. 2013, 2, 184–193. [Google Scholar] [CrossRef]
- Anafi, R.C.; Lee, Y.; Sato, T.K.; Venkataraman, A.; Ramanathan, C.; Kavakli, I.H.; Hughes, M.E.; Baggs, J.E.; Growe, J.; Liu, A.C. Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol. 2014, 12, e1001840. [Google Scholar] [CrossRef]
- Chau, P.K.; Ryan, E.; Dalen, K.T.; Haugen, F. Timing of acute cold exposure determines UCP1 and FGF21 expression—Possible interactions between the thermal environment, thermoregulatory responses, and peripheral clocks. J. Therm. Biol. 2024, 124, 14. [Google Scholar] [CrossRef]
- Messmer, M.N.; Kokolus, K.M.; Eng, J.W.; Abrams, S.I.; Repasky, E.A. Mild cold-stress depresses immune responses: Implications for cancer models involving laboratory mice. BioEssays 2015, 36, 884–891. [Google Scholar] [CrossRef]
- Ulasov, I.; Yi, R.; Guo, D.; Sarvaiya, P.; Cobbs, C. The emerging role of MMP14 in brain tumorigenesis and future therapeutics. Biochim. Biophys. Acta 2014, 1846, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Zhang, S.M.; Ruan, Z.H.; Liu, X.L.; Yang, G.; Jia, Y.L.; Li, Y.K.; Pan, P.; Wang, W.B.; Li, G.; et al. AP-1 signaling pathway promotes pro-IL-1β transcription to facilitate NLRP3 inflammasome activation upon influenza A virus infection. Virulence 2022, 13, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.H.; He, Y. The NLRP3 Inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Ward, M.F.; Sama, A.E.; Wang, H.C. Extracellular HMGB1 as a proinflammatory cytokine. J. Interf. Cytokine Res. 2004, 24, 329–333. [Google Scholar] [CrossRef]
- Knigge, T.; Leblanc, G.A.; Ford, A.T. A Crab Is Not a Fish: Unique aspects of the crustacean endocrine system and considerations for endocrine toxicology. Front. Endocrinol. 2021, 12, 587608. [Google Scholar] [CrossRef]
- Blanco, A.M. Hypothalamic- and pituitary-derived growth and reproductive hormones and the control of energy balance in fish. Gen. Comp. Endocr. 2020, 287, 113322. [Google Scholar] [CrossRef]
- Ge, G.D.; Long, Y.; Song, G.L.; Li, Q.; Cui, Z.B.; Yan, H.W. Transcriptomic profiling revealed signaling pathways associated with the spawning of female zebrafish under cold stress. Int. J. Mol. Sci. 2022, 23, 7494. [Google Scholar] [CrossRef]
- Eshkevari, L.; Permaul, E.; Mulroney, S.E. Acupuncture blocks cold stress-induced increases in the hypothalamus-pituitary-adrenal axis in the rat. J. Endocrinol. 2013, 217, 95–104. [Google Scholar] [CrossRef]
- Karn, T.; Ruckhäberle, E.; Hanker, L.; Müller, V.; Schmidt, M.; Solbach, C.; Gätje, R.; Gehrmann, M.; Holtrich, U.; Kaufmann, M.; et al. Gene expression profiling of luminal B breast cancers reveals NHERF1 as a new marker of endocrine resistance. Breast Cancer Res. Treat. 2011, 130, 409–420. [Google Scholar] [CrossRef]
- Gee, J.M.W.; Willsher, P.C.; Kenny, F.S.; Robertson, J.F.R.; Pinder, S.E.; Ellis, I.O.; Nicholson, R.I. Endocrine response and resistance in breast cancer: A role for the transcription factor Fos. Int. J. Cancer 1999, 84, 54–61. [Google Scholar] [CrossRef]
- Dash, S.N.; Hakonen, E.; Ustinov, J.; Otonkoski, T.; Andersson, O.; Lehtonen, S. sept7b is required for the differentiation of pancreatic endocrine progenitors. Sci. Rep. 2016, 6, 24992. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, J.Q.; Wu, S.J.; Li, Y.J.; Pan, Y.C. Integrative analysis of miRNA and mRNA expression associated with the immune response in the intestine of rainbow trout infected with infectious hematopoietic necrosis virus. Fish Shellfish Immunol. 2022, 131, 54–66. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, L.; Wang, X.; Jin, H. RNA-based therapeutics: An overview and prospectus. Cell Death Dis. 2022, 13, 644. [Google Scholar] [CrossRef]
- Avendaño-Portugal, C.; Montaño-Samaniego, M.; Guttman-Bazbaz, R.; Bravo-Estupiñan, D.M.; Ibáñez-Hernández, M. Therapeutic applications of poly-miRNAs and miRNA sponges. Int. J. Mol. Sci. 2025, 26, 4535. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, X.; Zhu, T.; Luo, Q.; Zhao, Y.; Xu, T. A novel protein NLRP12-119aa that prevents rhabdovirus replication by disrupting the RNP complex formation. Adv. Sci. 2025, 12, e2409953. [Google Scholar] [CrossRef]
- Saad, H.M.; Atef, E.; Elsayed, A.E. New Insights on the potential role of pyroptosis in Parkinson’s neuropathology and therapeutic targeting of NLRP3 inflammasome with recent advances in nanoparticle-based miRNA therapeutics. Mol. Neurobiol. 2025, 62, 9365–9384. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Cao, Y.; Hou, L.; Luo, T.; Li, M.; Gao, S.; Wang, L.; Sheng, K.; Zheng, L. Ginger exosome-like nanoparticle-derived miRNA therapeutics: A strategic inhibitor of intestinal inflammation. J. Adv. Res. 2025, 69, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Volpe, M.C.; Agostinis, C.; Vodret, S.; Ring, N.A.R.; Colliva, A.; Vuerich, R.; Braga, L.; Cook-Calvete, A.; Romano, F.; et al. Anti-miRNA therapeutics for uterine fibroids. Biomed. Pharmacother. 2025, 185, 117946. [Google Scholar] [CrossRef] [PubMed]
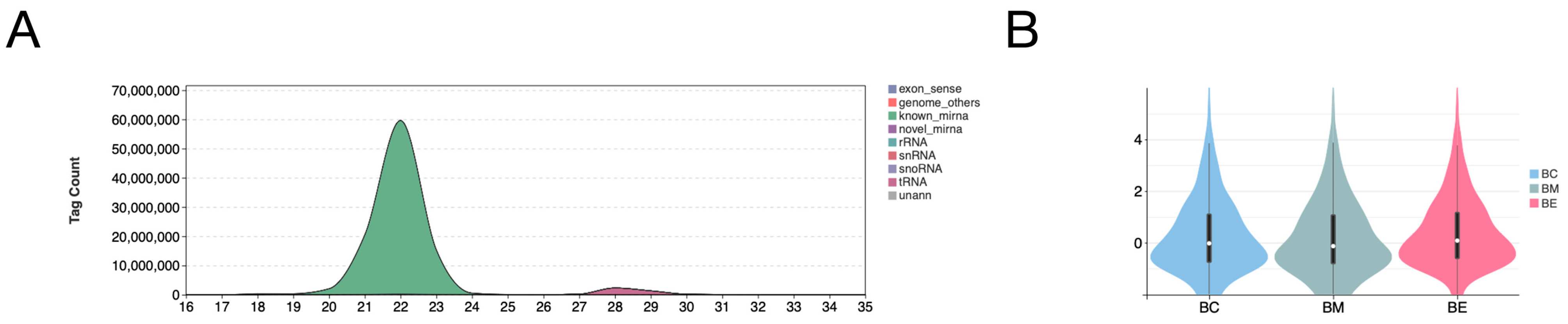



| Sample | Clean Reads | HQ Reads | HQ Ratio (%) | Clean Tags | Known miRNA Abundance | Novel miRNA Abundance |
|---|---|---|---|---|---|---|
| BC-1 | 11577604 | 11412232 | 98.57 | 11047812 | 10455376 | 7072 |
| BC-2 | 12040977 | 11974548 | 99.45 | 11437905 | 10604543 | 8307 |
| BC-3 | 12925738 | 12863379 | 99.52 | 12556139 | 12228779 | 9526 |
| BM-1 | 10779702 | 10731401 | 99.55 | 10414358 | 9969297 | 7680 |
| BM-2 | 12155435 | 12100330 | 99.55 | 11594623 | 10857439 | 8139 |
| BM-3 | 11946167 | 11872926 | 99.39 | 11498661 | 10947635 | 10184 |
| BE-1 | 12498393 | 12429937 | 99.45 | 11984423 | 10763958 | 10113 |
| BE-2 | 12408964 | 12340518 | 99.45 | 11693420 | 10590040 | 9840 |
| BE-3 | 11896489 | 11831975 | 99.46 | 11495540 | 11078132 | 10806 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, D.; Ning, X. Integrated miRNA-mRNA Atlas Reveals Temperature-Graded Brain Neuroendocrine Adaptation to Cold Stress in Silvery Pomfret (Pampus argenteus). Biology 2025, 14, 1265. https://doi.org/10.3390/biology14091265
Yin D, Ning X. Integrated miRNA-mRNA Atlas Reveals Temperature-Graded Brain Neuroendocrine Adaptation to Cold Stress in Silvery Pomfret (Pampus argenteus). Biology. 2025; 14(9):1265. https://doi.org/10.3390/biology14091265
Chicago/Turabian StyleYin, Danqing, and Xianhui Ning. 2025. "Integrated miRNA-mRNA Atlas Reveals Temperature-Graded Brain Neuroendocrine Adaptation to Cold Stress in Silvery Pomfret (Pampus argenteus)" Biology 14, no. 9: 1265. https://doi.org/10.3390/biology14091265
APA StyleYin, D., & Ning, X. (2025). Integrated miRNA-mRNA Atlas Reveals Temperature-Graded Brain Neuroendocrine Adaptation to Cold Stress in Silvery Pomfret (Pampus argenteus). Biology, 14(9), 1265. https://doi.org/10.3390/biology14091265






