Chromosomal Instability and Periodontal Disease in Idiopathic Infertility: Evidence of a Possible Association
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Participants and Eligibility Criteria
2.3. Cytogenetic and Molecular Assessment
2.4. Periodontal Examination
2.5. Statistical Analysis
3. Results
3.1. Demographic and Baseline Characteristics
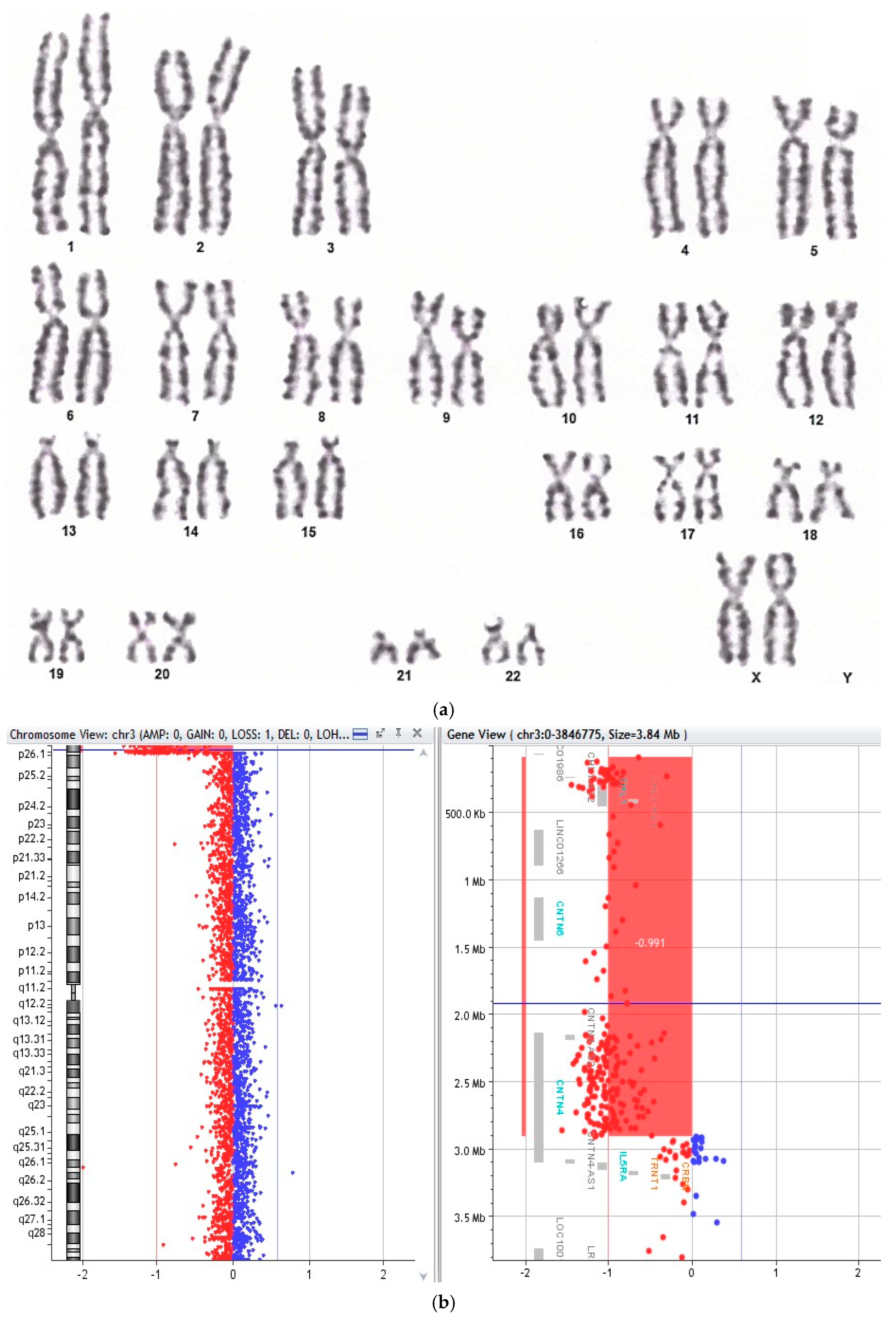
3.2. Cytogenetic Findings and Chromosomal Instability
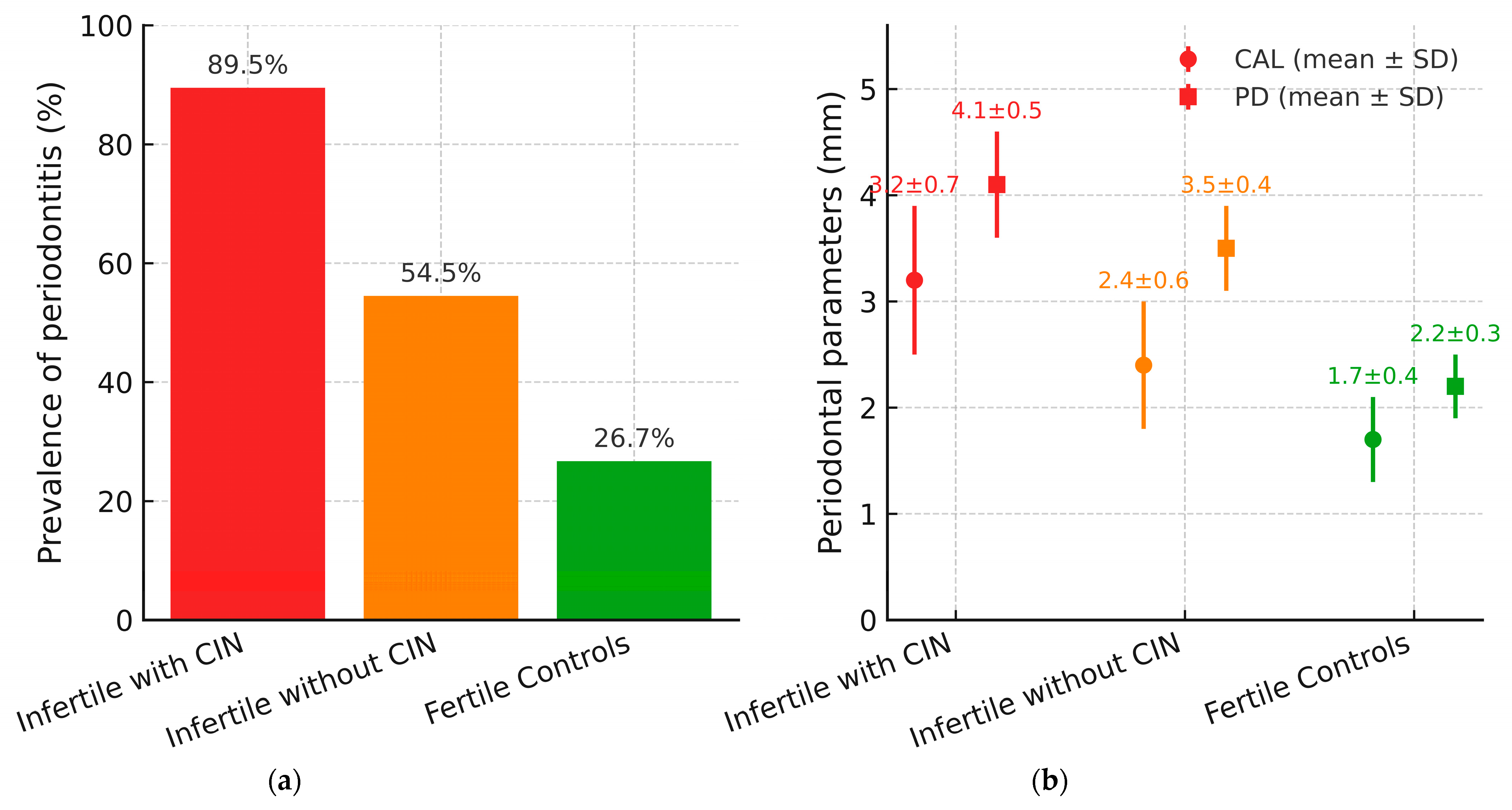
3.3. Periodontal Status and Disease Severity
3.4. Comparative Table of Clinical and Genetic Parameters
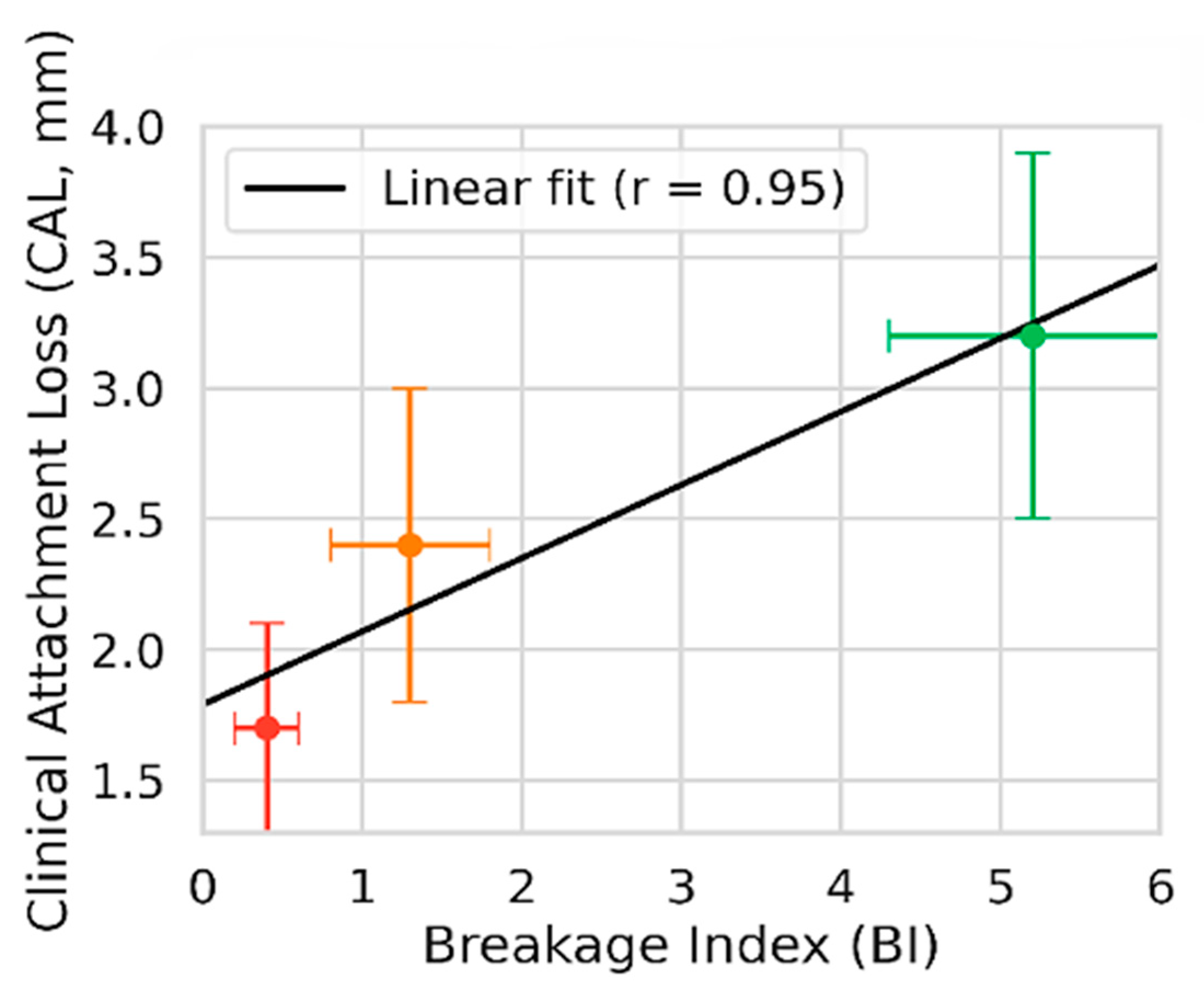
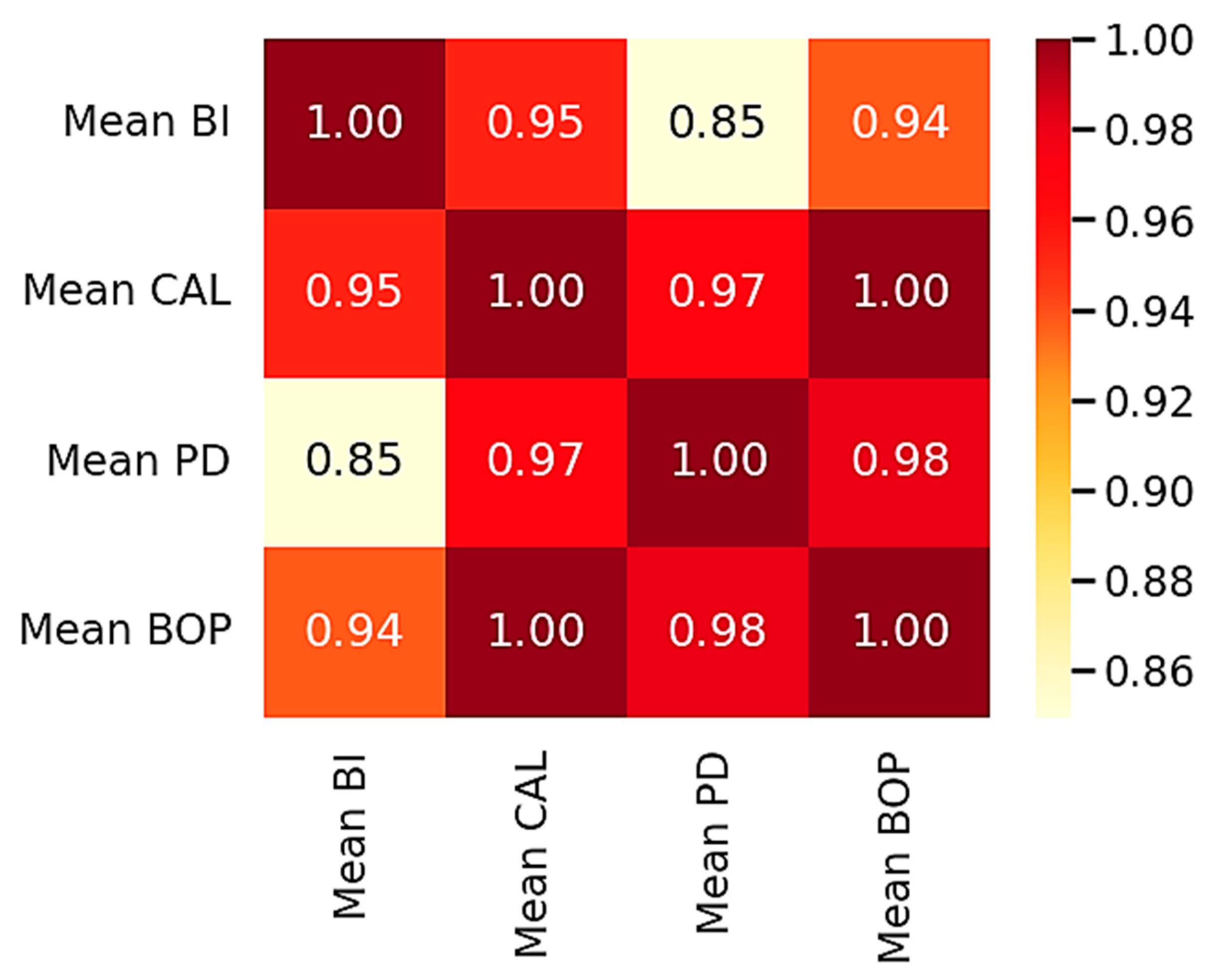
3.5. Graphical Representations
4. Discussion
5. Limitations
6. Suggestions for Future Directions
- Causality and trials: Prospective longitudinal cohorts and preregistered randomized trials testing whether standardized periodontal therapy lowers BI and improves reproductive outcomes (time-to-pregnancy, ART success, live birth).
- Bias control: Collection of validated SES and environmental exposure measures, coupled with causal-inference methods (propensity scores, DAGs, marginal structural models).
- Mechanisms and standardization: Evaluation of dose–response and mediation via inflammatory/oxidative biomarkers and telomere metrics, and multicenter studies with harmonized periodontal protocols, examiner calibration, centralized cytogenetics, and transparent plans (core outcomes, a priori sample size, data/code sharing).
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAP | American Academy of Periodontology |
| aCGH | Array-based Comparative Genomic Hybridization |
| BI | Breakage Index |
| BOP | Bleeding on Probing |
| BMI | Body Mass Index |
| CAL | Clinical Attachment Loss |
| CIN | Chromosomal Instability |
| CI | Confidence Interval |
| DNA | Deoxyribonucleic Acid |
| EFP | European Federation of Periodontology |
| ICC | Intraclass Correlation Coefficient |
| IL | Interleukin |
| IRB | Institutional Review Board |
| IQR | Interquartile Range |
| MMC | Mitomycin C |
| NSAID | Nonsteroidal Anti-Inflammatory Drug |
| OTC | Over-the-Counter |
| PCR | Polymerase Chain Reaction |
| PD | Probing Depth |
| PI | Plaque Index |
| RD | Risk Difference |
| ROS | Reactive Oxygen Species |
| SD | Standard Deviation |
| SES | Socioeconomic Status |
| SPSS | Statistical Package for the Social Sciences |
| TNF-α | Tumor Necrosis Factor-alpha |
| UNC-15 | University of North Carolina-15 periodontal probe |
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Grande, G. Human infertility and reproductive endocrinology. Life 2024, 14, 1550. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.H.; Tobler, K.J. Female Infertility; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556033/ (accessed on 16 July 2025).
- Nadă, E.S.; Albu, D.F.; Pătraşcu, A.; Albu, Ş.D.; Gogănău, A.M.; Albu, C.C. Current opportunities and new horizons into the genetic study of infertility. Rom. J. Morphol. Embryol. 2021, 62, 191–200. [Google Scholar] [CrossRef]
- Carson, S.A.; Kallen, A.N. Diagnosis and management of infertility: A review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef]
- Popescu, C.D.; Hamoud, B.H.; Sima, R.M.; Bobirca, A.; Balalau, O.D.; Amza, M.; Micu, R.; Gorecki, G.P.; Ples, L. Infertility as a possible multifactorial condition; The experience of a single center. J. Mind Med. Sci. 2024, 11, 466–474. [Google Scholar] [CrossRef]
- Comaills, V.; Castellano-Pozo, M. Chromosomal instability in genome evolution: From cancer to macroevolution. Biology 2023, 12, 671. [Google Scholar] [CrossRef]
- Shipley, J.; Rodeck, C.H.; Garrett, C.; Galbraith, J.; Giannelli, F. Mitomycin C-induced chromosome damage in fetal blood cultures and prenatal diagnosis of Fanconi‘s anaemia. Prenat. Diagn. 1984, 4, 217–221. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A multifaceted disease of tooth-supporting tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Albu, Ş.-D.; Suciu, I.; Albu, C.-C.; Dragomirescu, A.-O.; Ionescu, E. Impact of malocclusions on periodontopathogenic bacterial load and progression of periodontal disease: A quantitative analysis. Microorganisms 2024, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Albu, Ş.-D.; Dragomirescu, A.-O.; Albu, C.-C.; Suciu, I.; Ionescu, E. Genetic polymorphisms of interleukins IL-1A, IL-1B, and IL-1RN in patients with periodontal disease and dentomaxillary anomalies. Rom. J. Oral Rehabil. 2024, 16, 253–266. [Google Scholar] [CrossRef]
- Nwhator, S.; Opeodu, O.; Ayanbadejo, P.; Umeizudike, K.; Olamijulo, J.; Alade, G.; Agbelusi, G.; Arowojolu, M.; Sorsa, T. Could periodontitis affect time to conception? Ann. Med. Health Sci. Res. 2014, 4, 817–822. [Google Scholar] [CrossRef]
- Hart, R.; Doherty, D.A.; Pennell, C.E.; Newnham, I.A.; Newnham, J.P. Periodontal disease: A potential modifiable risk factor limiting conception. Hum. Reprod. 2012, 27, 1332–1342. [Google Scholar] [CrossRef]
- Nwhator, S.O.; Umeizudike, K.A.; Samuel, T.A.; Soroye, M.O.; Umeizudike, T.I. Periodontitis and subfertility: Opinions and practices of Nigerian specialists. West Afr. J. Med. 2013, 32, 267–271. [Google Scholar]
- Arce, R.M.; Barros, S.P.; Wacker, B.; Peters, B.; Moss, K.; Offenbacher, S. Increased TLR4 expression in murine placentas after oral infection with periodontal pathogens. Placenta 2009, 30, 156–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fogacci, M.F.; Barbirato, D.S.; Amaral, C.S.; da Silva, P.G.; Coelho, M.O.; Bertozi, G.; de Carvalho, D.P.; Leão, A.T. No association between periodontitis, preterm birth, or intrauterine growth restriction: Experimental study in Wistar rats. Am. J. Obstet. Gynecol. 2016, 214, 749.e1–749.e11. [Google Scholar] [CrossRef]
- Padilla-Cáceres, T.; Arbildo-Vega, H.I.; Caballero-Apaza, L.; Cruzado-Oliva, F.; Mamani-Cori, V.; Cervantes-Alagón, S.; Munayco-Pantoja, E.; Panda, S.; Vásquez-Rodrigo, H.; Castro-Mejía, P.; et al. Association between the risk of preterm birth and low birth weight with periodontal disease in pregnant women: An umbrella review. Dent. J. 2023, 11, 74. [Google Scholar] [CrossRef]
- Temur, I.; Ozcan Bulut, S.; Dönertas, S.N.; Dal Dönertas, A.; Temur, K.T.; Magat, G. Are inflammatory markers and periodontitis effective in predicting miscarriage? Healthcare 2025, 13, 1565. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Arrico, C.F.; Silvestre, F.J.; Fernández-Reyes, M.; Silvestre-Rangil, J.; Rocha, M. Is there an association between periodontal disease and infertility? A systematic review. Med. Oral Patol. Oral Cir. Bucal 2024, 29, e866–e875. [Google Scholar] [CrossRef] [PubMed]
- Benedek, C.; Kerekes-Máthé, B.; Bardocz-Veres, Z.; Szabó, B.; Iacob, A.; Stoica, A.; Dako, T.; Kovács, M.; Dénes, L.; Bereșescu, L. Association between genotoxic effects of ageing dental restorations and micronuclei in oral mucosal cells. Medicina 2025, 61, 1363. [Google Scholar] [CrossRef] [PubMed]
- Tadin, A.; Gavić, L.; Roguljić, M.; Babić, M.; Galić, I.; Želježić, D. Assessment of cytogenetic damage to exfoliated gingival cells in patients with chronic generalized periodontitis. Acta Clin. Croat. 2021, 60, 209–215. [Google Scholar] [CrossRef]
- Alkan, B.; Koroglu-Aydin, P. The effects of smoking on genotoxic and histopathologic damage in exfoliated oral epithelial cells and the periodontium: A cross-sectional study. Medicine 2023, 102, e33140. [Google Scholar] [CrossRef]
- Muradyan, R.E.; Parsadanyan, G.; Nersesyan, A. Re: Evaluation of micronuclei and cytomorphometric changes in patients with different tobacco related habits using exfoliated buccal cells. Asian Pac. J. Cancer Prev. 2022, 23, 755–757. [Google Scholar] [CrossRef]
- Fenech, M.; Holland, N.; Zeiger, E.; Chang, P.W.; Kirsch-Volders, M.; Bolognesi, C.; Stopper, H.; Knudsen, L.E.; Knasmueller, S.; Nersesyan, A.; et al. Objectives and achievements of the HUMN project on its 26th anniversary. Mutat. Res. Rev. Mutat. Res. 2024, 794, 108511. [Google Scholar] [CrossRef]
- Borba, T.T.; Molz, P.; Schlickmann, D.S.; Santos, C.; Oliveira, C.F.; Prá, D.; Neto, L.K.; Franke, S.I.R. Periodontitis: Genomic instability implications and associated risk factors. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 840, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Van Dyke, T.E. The role of inflammation and genetics in periodontal disease. Periodontology 2000 2020, 83, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Swarnalakshmi, R.; Ramya, R.; Sabitha, B.; Bharanidharan; Nagarathinam, A.E.; Kumar, K.R. Analysis of DNA instability in patients with periodontitis. SRM J. Res. Dent. Sci. 2017, 8, 25–29. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. The role of genetics and oxidative stress in the etiology of male infertility—A unifying hypothesis? Front. Endocrinol. 2020, 11, 581838. [Google Scholar] [CrossRef]
- Lefort, C.T.; Kim, M. Human T lymphocyte isolation, culture and analysis of migration In Vitro. J. Vis. Exp. 2010, 40, 2017. [Google Scholar] [CrossRef]
- Lynch, A.R.; Bradford, S.; Zhou, A.S.; Oxendine, K.; Henderson, L.; Horner, V.L.; Weaver, B.A.; Burkard, M.E. A Survey of Chromosomal Instability Measures across Mechanistic Models. Proc. Natl. Acad. Sci. USA 2024, 121, e2309621121. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Hoefsloot, L.; Simoni, M.; Tüttelmann, F. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions: 2020 update. Andrology 2020, 8, 5–19. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Marusawa, H.; Endo, Y.; Chiba, T. Inflammation-mediated genomic instability: Roles of activation-induced cytidine deaminase in carcinogenesis. Cancer Sci. 2012, 103, 1201–1206. [Google Scholar] [CrossRef]
- Tijhuis, A.E.; Johnson, S.C.; McClelland, S.E. The emerging links between chromosomal instability (CIN), metastasis, inflammation and tumour immunity. Mol. Cytogenet. 2019, 12, 17. [Google Scholar] [CrossRef]
- Enășescu, D.A.; Moisescu, M.G.; Imre, M.; Greabu, M.; Ripszky Totan, A.; Stanescu-Spinu, I.; Burcea, M.; Albu, C.; Miricescu, D. Lutein treatment effects on the redox status and metalloproteinase-9 (MMP-9) in oral cancer squamous cells—Are there therapeutical hopes? Materials 2021, 14, 2968. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Allemang, A.; Audebert, M.; Chauhan, V.; Dertinger, S.; Hendriks, G.; Luijten, M.; Marchetti, F.; Minocherhomji, S.; Pfuhler, S.; et al. Oxidative DNA damage leading to chromosomal aberrations and mutations. OECD Ser. Advers. Outcome Pathw. 2023, 29, 154. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Lastra, J.M.P.d.l.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemical insights into oxidative and nitrative modifications of DNA. Int. J. Mol. Sci. 2023, 24, 15240. [Google Scholar] [CrossRef]
- Ripszky Totan, A.; Greabu, M.; Stanescu-Spinu, I.I.; Imre, M.; Spinu, T.C.; Miricescu, D.; Ilinca, R.; Coculescu, E.C.; Badoiu, S.C.; Coculescu, B.I.; et al. The Yin and Yang dualistic features of autophagy in thermal burn wound healing. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221125090. [Google Scholar] [CrossRef]
- Cannan, W.J.; Pederson, D.S. Mechanisms and consequences of double-strand DNA break formation in chromatin. J. Cell. Physiol. 2016, 231, 3–14. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Tu, Y.; Long, Z.; Liu, J.; Kong, D.; Peng, J.; Wu, H.; Zheng, G.; Zhao, J.; Chen, Y.; et al. Reactive oxygen species bridge the gap between chronic inflammation and tumor development. Oxid. Med. Cell. Longev. 2022, 2022, 2606928. [Google Scholar] [CrossRef]
- Kidane, D.; Chae, W.J.; Czochor, J.; Eckert, K.A.; Glazer, P.M.; Bothwell, A.L.; Sweasy, J.B. Interplay between DNA repair and inflammation, and the link to cancer. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 116–139. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-induced tumorigenesis and metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef]
- Petrescu, I.; Condrea, C.; Alexandru, A.; Dumitrescu, D.; Simion, G.; Severin, E.; Albu, C.; Albu, D. Diagnosis and treatment protocols of cutaneous melanoma: Latest approach 2010. Chirurgia 2010, 105, 637–643. [Google Scholar] [PubMed]
- Kozak, M.; Dabrowska-Zamojcin, E.; Mazurek-Mochol, M.; Pawlik, A. Cytokines and their genetic polymorphisms related to periodontal disease. J. Clin. Med. 2020, 9, 4045. [Google Scholar] [CrossRef] [PubMed]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Teles, R.P.; Likhari, V.; Socransky, S.S.; Haffajee, A.D. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: A cross-sectional study. J. Periodontal Res. 2009, 44, 411–417. [Google Scholar] [CrossRef]
- Sanders, A.E.; Divaris, K.; Naorungroj, S.; Heiss, G.; Risques, R.A. Telomere length attrition and chronic periodontitis: An ARIC Study nested case-control study. J. Clin. Periodontol. 2015, 42, 12–20. [Google Scholar] [CrossRef]
- Aquino-Martinez, R.; Khosla, S.; Farr, J.N.; Monroe, D.G. Periodontal disease and senescent cells: New players for an old oral health problem? Int. J. Mol. Sci. 2020, 21, 7441. [Google Scholar] [CrossRef]
- Masi, S.; Salpea, K.D.; Li, K.; Parkar, M.; Nibali, L.; Donos, N.; Patel, K.; Taddei, S.; Deanfield, J.E.; D’Aiuto, F.; et al. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic. Biol. Med. 2011, 50, 730–735. [Google Scholar] [CrossRef]
- Chakrabarti, S.K.; Chattopadhyay, D. The hidden drivers of aging: Microbial influence on genomic stability and telomere dynamics. Explor. Res. Hypothesis Med. 2025, 10, 122–134. [Google Scholar] [CrossRef]
- Isola, G.; Santonocito, S.; Lupi, S.M.; Polizzi, A.; Sclafani, R.; Patini, R.; Marchetti, E. Periodontal health and disease in the context of systemic diseases. Mediators Inflamm. 2023, 2023, 9720947. [Google Scholar] [CrossRef]
- Potiris, A.; Moustakli, E.; Trismpioti, E.; Drakaki, E.; Mavrogianni, D.; Matsas, A.; Zikopoulos, A.; Sfakianakis, A.; Tsakiridis, I.; Dagklis, T.; et al. From inflammation to infertility: How oxidative stress and infections disrupt male reproductive health. Metabolites 2025, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Kis-György, R.; Körtési, T.; Anicka, A.; Nagy-Grócz, G. The connection between the oral microbiota and the kynurenine pathway: Insights into oral and certain systemic disorders. Curr. Issues Mol. Biol. 2024, 46, 12641–12657. [Google Scholar] [CrossRef] [PubMed]
- Brăila, A.D.; Poalelungi, C.V.; Albu, C.C.; Damian, C.M.; Dȋră, L.M.; Bănățeanu, A.M.; Bogdan-Andreescu, C.F. The relationship between cervicovaginal infection, human papillomavirus infection and cervical intraepithelial neoplasia in romanian women. Diseases 2025, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, V.; Roozbeh, N.; Banaei, M.; Kutenaei, M.A. Exploring the link between periodontal disease and sperm quality: A comprehensive systematic review study. BMC Oral Health 2025, 25, 742. [Google Scholar] [CrossRef]
- Li, F.; Duan, X.; Li, M.; Ma, X. Sperm DNA fragmentation index affect pregnancy outcomes and offspring safety in assisted reproductive technology. Sci. Rep. 2024, 14, 356. [Google Scholar] [CrossRef]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef]
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Age | 20–40 years | <20 or >40 years |
| Cases (Group A) | Idiopathic infertility: ≥12 months of regular unprotected intercourse with no identifiable anatomical, hormonal, infectious, or endocrinological cause | Identified cause of infertility or duration <12 months |
| Controls (Group B) | Fertile: natural conception within the last 3 years; no history of infertility | History of infertility/subfertility or conception via assisted reproduction among controls |
| Smoking | Non-smoker or light smoker (<10 cigarettes/day) | Heavy smoking (≥10 cigarettes/day) |
| Systemic status | No chronic systemic inflammatory disease (e.g., diabetes mellitus, autoimmune disease, inflammatory bowel disease) | Presence of a chronic systemic inflammatory disease |
| Pregnancy/ Lactation | Not pregnant and not lactating | Pregnancy or lactation |
| Chromosomal disorders | No known chromosomal disorder | Known chromosomal disorder |
| Immunosuppressive therapy | No immunosuppressive therapy currently or within the past 3 months | Immunosuppressive therapy currently or within the past 3 months |
| Malignancy | No active malignancy/no ongoing oncologic treatment (remote history in complete remission allowed) | Active malignancy |
| Medications affecting periodontal tissues, bone, or immunity (≤3 months) | None of the following in the past 3 months | Any of the following in the past 3 months: systemic corticosteroids; calcineurin inhibitors (cyclosporine, tacrolimus); antineoplastic agents; antiresorptives (bisphosphonates, denosumab); calcium-channel blockers associated with gingival overgrowth (e.g., nifedipine); phenytoin; oral retinoids |
| Systemic antibiotics/NSAIDs (≤30 days) | No systemic antibiotics or NSAIDs within the previous 30 days | Systemic antibiotics or NSAIDs within the previous 30 days (temporary exclusion; rescreen after a 30-day washout) |
| Periodontal therapy (≤6 months) | No periodontal therapy within the previous 6 months | Periodontal therapy within the previous 6 months |
| (A) Group Summaries (n, Mean ± SD) | ||||
|---|---|---|---|---|
| Parameter | Infertile with CIN (n = 19) | Infertile Without CIN (n = 11) | Fertile Controls (n = 30) | |
| Mean Breakage Index (BI) | 5.2 ± 0.9 | 1.3 ± 0.5 | 0.4 ± 0.2 | |
| Periodontitis Prevalence | 89.5% (17/19) | 54.5% (6/11) | 26.7% (8/30) | |
| Mean CAL (mm) | 3.2 ± 0.7 | 2.4 ± 0.6 | 1.7 ± 0.4 | |
| Mean Probing Depth (mm) | 4.1 ± 0.5 | 3.5 ± 0.4 | 2.2 ± 0.3 | |
| Mean Plaque Index | 2.3 ± 0.4 | 1.9 ± 0.5 | 1.4 ± 0.3 | |
| Mean BOP (% sites) | 35.2 ± 8.6 | 26.4 ± 7.1 | 17.3 ± 6.8 | |
| (B) Between-group contrasts (mean difference with 95% CI; Hedges g) | ||||
| Metric | Comparison | Mean Difference | 95% CI | Hedges g |
| CAL (mm) | CIN vs. NoCIN | 0.80 | 0.30 to 1.30 | 1.17 |
| CIN vs. Controls | 1.50 | 1.14 to 1.86 | 2.76 | |
| NoCIN vs. Controls | 0.70 | 0.27 to 1.13 | 1.49 | |
| PD (mm) | CIN vs. NoCIN | 0.60 | 0.30 to 0.90 | 1.35 |
| CIN vs. Controls | 1.90 | 1.64 to 2.16 | 4.94 | |
| NoCIN vs. Controls | 1.30 | 1.07 to 1.53 | 3.38 | |
| Plaque Index | CIN vs. NoCIN | 0.40 | 0.03 to 0.77 | — |
| CIN vs. Controls | 0.90 | 0.68 to 1.12 | — | |
| NoCIN vs. Controls | 0.50 | 0.15 to 0.85 | — | |
| BOP (% sites) | CIN vs. NoCIN | +8.8 pp | +2.73 to +14.87 | — |
| CIN vs. Controls | +17.9 pp | +13.14 to +22.66 | — | |
| NoCIN vs. Controls | +9.1 pp | +3.83 to +14.37 | — | |
| Periodontitis prevalence (RD) | CIN vs. NoCIN | +0.35 | −0.10 to +0.69 | — |
| CIN vs. Controls | +0.63 | +0.24 to +0.83 | — | |
| NoCIN vs. Controls | +0.28 | −0.16 to +0.65 | — | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albu, C.-C.; Albu, Ş.-D.; Bogdan-Andreescu, C.F.; Poalelungi, C.-V.; Damian, C.M.; Burcea, A.; Bănățeanu, A.-M.; Cadar, E.; Slăvescu, D.A.; Brăila, A.D. Chromosomal Instability and Periodontal Disease in Idiopathic Infertility: Evidence of a Possible Association. Biology 2025, 14, 1256. https://doi.org/10.3390/biology14091256
Albu C-C, Albu Ş-D, Bogdan-Andreescu CF, Poalelungi C-V, Damian CM, Burcea A, Bănățeanu A-M, Cadar E, Slăvescu DA, Brăila AD. Chromosomal Instability and Periodontal Disease in Idiopathic Infertility: Evidence of a Possible Association. Biology. 2025; 14(9):1256. https://doi.org/10.3390/biology14091256
Chicago/Turabian StyleAlbu, Cristina-Crenguţa, Ştefan-Dimitrie Albu, Claudia Florina Bogdan-Andreescu, Cristian-Viorel Poalelungi, Constantin Marian Damian, Alexandru Burcea, Andreea-Mariana Bănățeanu, Emin Cadar, Dan Alexandru Slăvescu, and Anca Daniela Brăila. 2025. "Chromosomal Instability and Periodontal Disease in Idiopathic Infertility: Evidence of a Possible Association" Biology 14, no. 9: 1256. https://doi.org/10.3390/biology14091256
APA StyleAlbu, C.-C., Albu, Ş.-D., Bogdan-Andreescu, C. F., Poalelungi, C.-V., Damian, C. M., Burcea, A., Bănățeanu, A.-M., Cadar, E., Slăvescu, D. A., & Brăila, A. D. (2025). Chromosomal Instability and Periodontal Disease in Idiopathic Infertility: Evidence of a Possible Association. Biology, 14(9), 1256. https://doi.org/10.3390/biology14091256







