Simple Summary
This study examines the diversity and distribution of shrimp and crabs in a river region where freshwater meets the sea, in southeastern Brazil. Monthly collections were carried out over one year in three different areas of the Ribeira de Iguape River, using hand nets and traps. These methods helped capture species living in different habitats along the river. The survey recorded more than 42,000 individuals, comprising twelve species. Most were freshwater shrimp, and one species, originally from another region, was identified for the first time in this area. This finding highlights the importance of continuous monitoring. Two shrimp species were especially abundant, each dominating different sections of the river. The area near the river mouth had the highest number of species, likely due to its transitional nature and the influence of both fresh- and saltwater. The total number of individuals varied by season, with greater numbers observed in winter and spring. Although environmental factors were related to species presence, other biological aspects not addressed in this study may also influence the distribution of these animals. These results significantly contribute to a better understanding of river ecosystems and provide valuable insights to support efforts in conserving biodiversity.
Abstract
Freshwater ecosystems are essential for biodiversity conservation but face increasing threats from human activities. This study assessed the species richness, diversity, and spatial and temporal distribution of decapod crustaceans (Caridea and Brachyura) in the lower Ribeira de Iguape River, southeastern Brazil, a globally recognized freshwater ecoregion. Monthly sampling took place over one year at three sites using both active and passive capture methods. Environmental parameters were measured in the field, and multivariate analyses (correspondence analysis, PERMANOVA, and multiple regression) were applied to explore species–environment relationships and community structure. A total of 42,897 individuals were recorded, including eight Caridea and four Brachyura species. Carideans accounted for over 97 percent of the total, with Potimirim spp. and Macrobrachium acanthurus as the most abundant. The site nearest the river mouth showed the highest species richness and diversity, likely due to its transitional characteristics and moderate salinity variation. Environmental variables such as dissolved oxygen and salinity influenced species occurrence, although biological interactions may also contribute. Seasonal changes in abundance appeared most evident during winter and spring, driven by shifts in dominant taxa. The presence of the non-native Macrobrachium rosenbergii suggests early establishment and reinforces the need for long-term ecological monitoring in transitional freshwater environments.
1. Introduction
Freshwater environments cover approximately 0.8% of the Earth’s surface but harbor at least 6% of all species described [1]. However, these ecosystems are among the most threatened, experiencing significant biodiversity loss due to several threats, including dam construction, dredging, pollution, the introduction of non-native invasive species, and intensive agricultural practices [2,3,4,5].
In the southeastern region of São Paulo State, the Ribeira de Iguape River basin plays a fundamental role in the socioeconomic dynamics of Vale do Ribeira communities, where artisanal fishing is particularly prominent. The Cananéia–Iguape lagoon system stands out as a central fishing hub, with activities concentrated in the Ribeira River and primarily targeting species such as manjuba (Engraulidae: anchovy), robalo (Centropomidae: snook), and the freshwater shrimps Macrobrachium acanthurus and Macrobrachium carcinus, which are harvested for use as live bait and for human consumption, respectively [6,7,8,9,10,11,12,13,14]. Human activities strongly impact this basin and have suffered environmental degradation in recent decades due to unregulated mining [15]. As a result, residues containing heavy metals remain accumulated in the river sediments throughout its course to the estuarine region [16,17]. Additionally, much of the riparian vegetation has been replaced by banana plantations and pastures [18], leading to the release of agrochemicals such as carbamates and glyphosate-based herbicides, among others, into aquatic environments [19,20]. However, there is a notable lack of ecotoxicological studies specifically addressing the impacts of these contaminants on key native species within the Ribeira de Iguape basin.
In addition to land-use impacts and water contamination, the introduction of non-native species represents another significant threat to this ecosystem. A notable example of freshwater crustacean dispersal due to human activities in Brazil is the presence of the giant freshwater prawn (Macrobrachium rosenbergii) in the eastern Amazon region and the state of Espírito Santo [21,22]. In São Paulo State, although there is no evidence of successful reproduction in natural environments to date, escapes of individuals from aquaculture farms have been reported [23].
The “Ribeira de Iguape region” is recognized by WWF as an ecoregion due to its ecological uniqueness and importance for aquatic biodiversity [24]. This area contains a critical freshwater reserve in São Paulo State and one of Brazil’s best-preserved genetic reservoirs [25]. The Vale do Ribeira includes 45 active conservation units across its territory [26]. It also retains more than 50 percent of the remaining native vegetation of the Atlantic Forest in Brazil [27], underscoring the essential role of these protected areas in conserving this biome. By the late 1990s, researchers had already stressed the need to intensify studies and collection efforts to better document species richness in São Paulo State, identifying the Vale do Ribeira as a priority region [28].
Although recognized as a conservation priority, the region still lacks comprehensive data on the biodiversity and distribution patterns of aquatic fauna, which limits the ability to predict ecological impacts and develop effective mitigation strategies. Notable studies on crustaceans include a compilation of freshwater decapod diversity in São Paulo State that listed 32 species from five families and eight genera [29]; an analysis of the high phylogenetic diversity among anomurans of the genus Aegla in the Ribeira do Iguape ecoregion, which highlighted the need for targeted conservation of these organisms and their habitats [30]; and a survey of decapod species richness and distribution across seven conservation areas in the Vale do Ribeira, which documented nine caridean shrimp species, five aeglid anomurans, and four brachyurans [31]. Additional studies have explored the biology and ecology of freshwater shrimp, including M. acanthurus, M. carcinus [32,33,34,35,36], and Macrobrachium olfersii [37,38].
Thus, despite having high species richness, significant endemism, and vulnerability, freshwater ecosystems still receive limited attention in large-scale conservation efforts [24]. This limited focus is partly due to the lack of comprehensive and organized data on the distribution of freshwater species [39]. Considering this, the present study assesses the species richness, diversity, and spatial and temporal distribution of the decapod crustacean community (Caridea and Brachyura) in the lower course of the Ribeira de Iguape River. The findings support management plans and conservation strategies for species and their habitats, enable the detection of non-native species, and help maintain the ecosystem’s integrity and stability.
2. Materials and Methods
2.1. Study Area
The Ribeira de Iguape River basin (24°42′29″ S; 47°33′19″ W) is one of the largest along the Brazilian coast, covering about 25,000 km2 of drainage area, of which 17,068 km2 lie within the state of São Paulo, covering 28 municipalities. Its headwaters are in the state of Paraná, and the river flows for about 470 km before reaching the Atlantic Ocean in the city of Iguape, on the southern coast of São Paulo State (SP) [40,41]. Near the coastal region, the river delivers a high freshwater load to the estuarine environment, forming the Cananéia–Iguape system, a network of lagoon channels situated within the United Nations Educational, Scientific and Cultural Organization (UNESCO) Biosphere Reserve [42].
The region is classified as having a humid subtropical climate according to the Köppen climate classification system [40]. Based on data from the automatic meteorological station (A712) of the National Institute of Meteorology (INMET, https://bdmep.inmet.gov.br/ accessed on 28 July 2025) in the city of Iguape, collected between April 2014 and January 2015, the average annual precipitation was 1267 mm, and the mean temperature was 21.4 ± 3.3 °C, ranging from 13.8 °C to 34.4 °C across seasons.
In the mid-18th century, a significant hydrological alteration occurred in the Ribeira de Iguape River, leading to the construction of the Valo Grande Channel near the urban center of Iguape [13]. This intervention diverted approximately 60% of the river’s flow into the lagoon system, causing a significant drop in salinity levels [13,40,42,43]. Consequently, the region is characterized by moderate tidal influence, with current velocities reaching 0.6 m/s during flood tides and 0.7 m/s during ebb tides [44]. This anthropogenic alteration also led to a decline in the diversity of fishery resources in the town of Iguape, compared to the neighboring city of Cananéia, where marine species still dominate local fisheries. In Iguape, by contrast, fishing has become increasingly based on freshwater species due to the reduced salinity levels [13].
2.2. Collection of Specimens and Environmental Parameters
From February 2014 to January 2015, monthly sampling was conducted during low tides in three areas along the lower course of the Ribeira de Iguape River, in Iguape, São Paulo State, Brazil. The first sampling area was located on the Valo Grande Channel, which is 250 m wide and 7 m deep. In contrast, the second and third areas were located along the river’s natural course, approximately 15 km and 8 km from the river mouth, respectively (Figure 1). Although the data were collected nearly a decade ago, there is no indication of anthropogenic changes or land-use alterations in the region during this period. Ongoing research efforts by the authors in the area have not revealed substantial shifts in environmental conditions nor the implementation of new conservation policies. Therefore, the dataset remains a valuable and representative baseline for characterizing this poorly studied estuarine system.
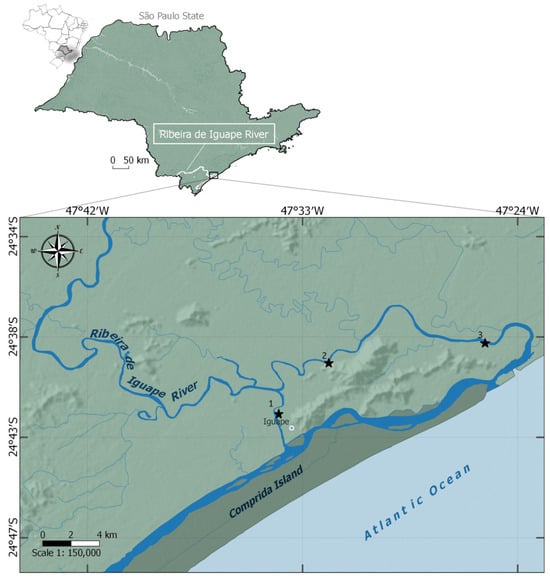
Figure 1.
Map of the Ribeira de Iguape River basin, São Paulo, Brazil, showing the decapod community sampling areas (1, 2, and 3) from 14 February to 15 January in the Iguape region.
No riparian forest is present along the riverbanks at any of the three sampled sites. In Area 1, the bank consists of a mixture of grasses (Brachiaria spp.) and aquatic plants, including floating water hyacinth (Eichhornia crassipes). In Area 2, aquatic plants are the most common, and filamentous algae appear at certain times of the year. In Area 3, grasses are the dominant vegetation.
The collections aimed to provide both qualitative and quantitative assessments of the decapod fauna through passive and active capture methods, applied monthly across three areas over a 12-month period, totaling 36 standardized events. This methodological approach follows the procedures established by [36,37], and was designed to minimize sampling bias by targeting different ecological niches and body size classes within the decapod community. As an active method, a sieve measuring 0.9 m in diameter with a 3 mm mesh size was used. Two people swept the sieve through partially submerged marginal vegetation for 20 min to capture individuals associated with this vegetation. Additionally, twelve traps were employed for passive sampling: six minnow traps and six box traps. The minnow traps (1.0 m long, 0.5 m in diameter, and 8 mm mesh size) were placed on the riverbed to capture bottom-dwelling individuals. The box traps (0.55 m long × 0.36 m wide × 0.25 m high), covered with 8 mm mesh netting and equipped with two lateral openings of 6 cm in diameter, were positioned and tied beneath the marginal vegetation. All traps were left in the water for approximately 24 h, baited with fish remains and cow bones.
At each collection site, data on water temperature (°C), salinity (ppt), pH, dissolved oxygen (mg L−1), and conductivity (µS cm−1) were obtained using a multiparameter probe (YSI-ProPlus, made in Yellow Springs, OH, USA) inserted into the water column approximately 30 cm below the surface. This depth is considered adequate for establishing relationships between environmental parameters and the decapod community, as previously applied in estuarine floodplain studies in the Brazilian Amazonia [45]. The transparency of the water was checked with a Secchi disk with a cable graduated in centimeters.
After capture, the animals were transported to the laboratory and frozen until further processing. Species identification followed established taxonomic references [46,47,48]. Shrimps of the genus Potimirim were abundant in the collections, making it impractical to identify and measure all individuals. Therefore, identification and morphometric analyses were performed during randomly selected months to determine species within this genus, while considering the total number of individuals in the subsequent analyses.
To evaluate shrimp size, subsampling was conducted on the total number of individuals collected for each species, by area and month. All individuals were examined in samples containing up to 80 specimens. If samples exceeded this number, a random subsample was taken, following an adaptation of established procedures [36,37], as follows: 80 individuals were selected from samples with 80–160 shrimps; 50% of individuals were used from samples with 160–320; 25% from samples with 320–500; and 10% from samples with more than 500 individuals. Carapace length (CL) was measured as the distance from the orbital angle to the posterior margin of the carapace. All Brachyura specimens were fully analyzed, with measurements taken at the carapace width (CW). A caliper with a precision of 0.01 mm was used for all measurements.
2.3. Data Analysis
2.3.1. Environmental Factors
A generalized linear model (GLM) was used to evaluate the effects of sampling area and season (summer: January to March; autumn: April to June; winter: July to September; spring: October to December) on environmental parameters. Model selection was based on the Akaike Information Criterion (AIC), choosing the model with the lowest AIC value [49]. For temperature, pH, and dissolved oxygen, a normal distribution with an identity link function was applied. For salinity, conductivity, and water transparency, a gamma distribution with a log link function was used. All analyses were performed using IBM SPSS Statistics® (trial version, Armonk, NY, USA).
2.3.2. Community Analysis
Diversity, Evenness, and Species Richness
Species richness was measured by counting the number of species in the study area and the total abundance of individuals recorded in the samples [50]. Species diversity (H′) was calculated using the Shannon–Wiener index [51], which considers both species richness and the relative abundance of each species [52]. Evenness (J′) was determined using a method that accounts for the level of uniformity in species distribution [53,54]. This index ranges from 0 to 1, with values closer to 1 indicating a more even distribution of individuals among species [52]. All analyses were performed using the free software PAST (version 3.25, Oslo, Norway).
Multivariate Analysis
Correspondence analysis (CA) was employed to link total species abundance to the sampling areas across the entire study period. In this analysis, absolute abundance values were used, considering each species as an independent data group, which minimized the impact of the sampling design [55]. This analysis was conducted using the freely available software PAST (version 3.25).
A two-way permutational multivariate analysis of variance (PERMANOVA) was applied to compare the total abundance of decapods and the most abundant Caridea and Brachyura species across areas and seasons. This analysis was performed using the PC-ORD software (version 6, Gleneden Beach, OR, USA) [56].
Multiple linear regression analyses were used to examine how environmental variables affected the relative abundance of the most common decapod species. The predictor variables included water temperature, dissolved oxygen, electrical conductivity, salinity, pH, and water transparency. The dependent variables represented the most prevalent decapod species. Predictor variables were selected based on entry probability ≤ 0.05 and removal probability ≥ 0.10. Model fit was assessed using the adjusted R2, the F-value for overall significance, and p-values < 0.05. Standardized coefficients (Beta) indicated each predictor’s relative contribution, and tolerance values were examined to verify multicollinearity, with no serious issues detected [57]. All analyses used IBM SPSS Statistics® (trial version).
3. Results
3.1. Environmental Factors
The seasonal and spatial variation in the mean values of environmental parameters is illustrated in Figure 2. The results from the generalized linear model (GLM) showed a significant interaction between sampling area and season only for salinity (Wal χ2 = 32.17; p < 0.05). This interaction was primarily driven by the higher mean salinity observed in Area 3 during spring (0.71), which was significantly different from all other area–season combinations. Additionally, significant differences were found between areas for dissolved oxygen (Wald χ2 = 10.748; p < 0.05) and conductivity (Wald χ2 = 8.401; p < 0.05). Concerning seasonal variation, temperature (Wald χ2 = 98.551; p < 0.05), dissolved oxygen (Wald χ2 = 111.520; p < 0.05), conductivity (Wald χ2 = 18.482; p < 0.05), pH (Wald χ2 = 3.477; p < 0.05), and water transparency (Wald χ2 = 33.565; p < 0.05) also exhibited significant differences (Table 1).
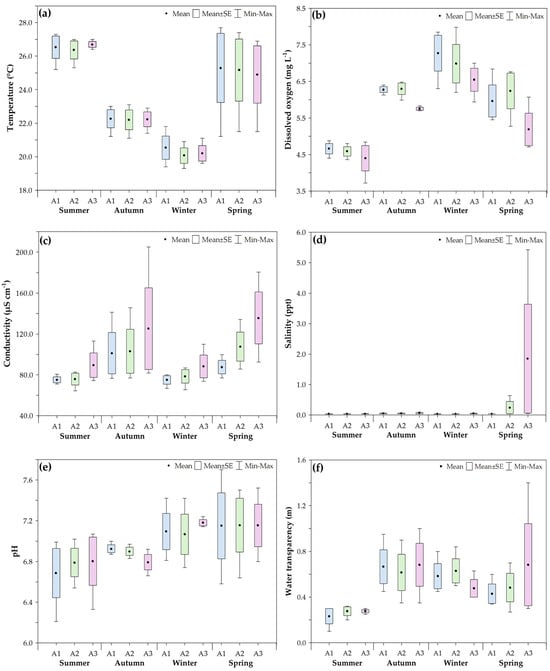
Figure 2.
Boxplots showing the seasonal variation in environmental parameters ((a). water temperature; (b). dissolved oxygen; (c). electrical conductivity; (d). salinity; (e). pH; (f). water transparency) recorded in the three sampling areas from February 2014 to January 2015 in the Ribeira de Iguape River, Iguape (SP). A1 = Area 1, A2 = Area 2, A3 = Area 3.

Table 1.
Overall annual averages and standard deviations of environmental variables by area and season, recorded from February 2014 to January 2015 in the Ribeira de Iguape River, Iguape (SP). (D.O. = dissolved oxygen; Transp. = transparency). Differences were assessed using a generalized linear model (GLM). For variables without a significant area–season interaction, identical lowercase letters in the same column indicate no significant differences. * For salinity, a significant interaction was detected, with Area 3 in spring differing from all other combinations.
3.2. Community Structure
A total of 42,897 individuals from the infraorders Caridea and Brachyura were collected, representing 12 species. Caridea was the most common group, making up 97.4% of all decapods (41,764 individuals), including members of the families Atyidae and Palaemonidae. Brachyura consisted of 1133 individuals (2.6% of the total), spread across four families: Sesarmidae, Portunidae, Panopeidae, and Ocypodidae. The complete species list, along with the total number of individuals and their minimum, maximum, and average sizes (mm), is shown in Table 2.

Table 2.
Species composition, total abundance, size range (minimum and maximum), and total number of individuals by capture method for decapod species (Caridea and Brachyura) recorded in the Ribeira de Iguape River, Iguape (SP), from February 2014 to January 2015. N = total number of individuals. IUCN status: LC = Least Concern; DD = Data Deficient according to Livro Vermelho dos Crustáceos do Brasil [Red List of Brazilian Crustaceans] [58].
The overall diversity index for the region was 1.12 bits per individual, with an evenness value of 0.47. Among the sampled areas, Area 3 had the highest species richness (11 species), diversity index (H′ = 1.22), and evenness (J′ = 0.51) (Figure 3a). Seasonally, summer and autumn showed the highest diversity and evenness values compared to other seasons (Figure 3b).
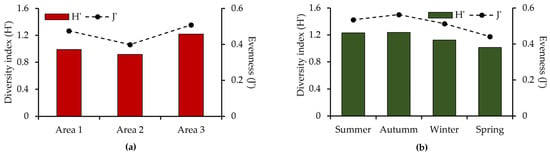
Figure 3.
Spatial and temporal variation in the diversity index (H′) and evenness (J′) from February 2014 to January 2015 in the Ribeira de Iguape River, Iguape (SP). (a) Variation by area; (b) variation by season.
Among the carideans, the family Atyidae accounted for 48.5% of the total shrimp collected, including Potimirim potimirim and Potimirim brasiliana, collectively called Potimirim spp. The family Palaemonidae was represented by five species: four from the genus Macrobrachium and one from the genus Palaemon. Macrobrachium acanthurus had the second highest abundance among the carideans (41.4%), followed by Palaemon pandaliformis (6.6%), Macrobrachium olfersii (3.4%), and Macrobrachium carcinus (0.1%). The non-native species, Macrobrachium rosenbergii, was represented by two individuals (0.005%) (Table 3).

Table 3.
Composition and abundance of decapod species by sampling area and season from February 2014 to January 2015 in the Ribeira de Iguape River, Iguape (SP).
Five species of brachyurans were observed, belonging to the genera Armases, Callinectes, Minuca, and Panopeus. The most common species was Armases rubripes (87.2%), followed by Callinectes sapidus (7.6%), Callinectes bocourti (3.8%), Panopeus rugosus (1.3%), and Minuca mordax, which was represented by a single individual (0.1%) (Table 3).
Among the sampling methods used, active capture with a sieve was the only effective way to sample Potimirim spp. This method was also the most efficient for catching the shrimps P. pandaliformis, M. acanthurus, and M. olfersii, as well as the crab A. rubripes. In contrast, passive methods (box and minnow traps) were effective in capturing larger-bodied shrimps and brachyuran crabs (Table 3).
The composition and total abundance of decapod species by area and season are shown in Table 3. Overall decapod crustacean abundance varied only across seasons (PERMANOVA; Pseudo F = 6.93, p < 0.05) (Figure 4a). Among the carideans, the abundance of Potimirim spp. showed significant differences between areas (Pseudo F = 7.63, p < 0.05) and seasons (Pseudo F = 2.44, p < 0.05) (Figure 4b). A similar pattern was observed for M. acanthurus (areas: Pseudo F = 3.73, p < 0.05; seasons: Pseudo F = 3.27, p < 0.05) (Figure 4c). For M. olfersii, no significant differences were found for either factor (p > 0.05) (Figure 4d), whereas P. pandaliformis exhibited an interaction between area and season (Pseudo F = 1.76, p < 0.05), with a higher number of individuals recorded in Area 3 during winter and spring (Figure 4e). For the most abundant brachyuran, A. rubripes, the distribution differed only among areas (Pseudo F = 2.70, p < 0.05) (Figure 4f).
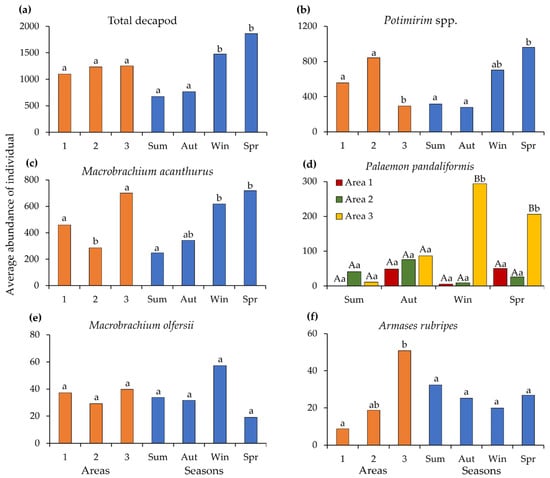
Figure 4.
The results of the permutational multivariate analysis of variance (PERMANOVA) for the total number of decapods and the most abundant species, examining the effects of sampling area and season and their interaction: (a) total decapod; (b) Potimirim spp.; (c) Macrobrachium acanthurus; (d) Palaemon pandaliformis; (e) Macrobrachium olfersii; (f) Armases rubripes. (Sum—summer, Aut—autumn, Win—Winter, Spr—spring). Since no significant interaction between area and season was found, identical lowercase letters between columns of the same color (area or season) indicate no statistically significant differences (p > 0.05). For Palaemon pandaliformis, where a significant interaction between area and season was observed, capital letters indicate significant differences among areas within each season, whereas lowercase letters indicate significant differences among seasons within each area (p < 0.05).
Correspondence analysis by area showed that axis 1 explained 53.6% of the total data variation, and axis 2 explained 26.9% (χ2 = 21.014, p < 0.05). The species P. pandiformis, M. olfersii, and all brachyurans showed a stronger association with Area 3, while M. acanthurus was more closely linked to Areas 1 and 3 (Figure 5).
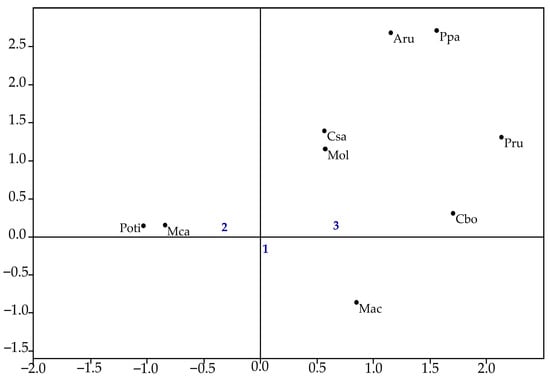
Figure 5.
Correspondence analysis of decapod species abundance across sampling areas. (Mol—Macrobrachium olfersii, Aru—Armases rubripes, Ppa—Palaemon pandaliformis, Mac—Macrobrachium acanthurus, Poti—Potimirim spp., Mca—Macrobrachium carcinus, Cbo—Callinectes bocourti, Csa—Callinectes sapidus, Pru—Panopeus rugosus).
The results of the multiple linear regression analyses showed that different environmental variables were significantly and positively linked to species abundance (Table 4). Dissolved oxygen levels were significantly associated with the abundance of Potimirim spp. (p = 0.013), while pH was the predictor for M. acanthurus (p = 0.004). Water salinity was identified as an explanatory variable for M. olfersii, P. pandaliformis, and A. rubripes. Although the adjusted coefficients of determination (adjusted r2) ranged from 13.7% to 21.5%, the models revealed trends in the relationship between water physicochemical conditions and species distribution. For M. carcinus, C. bocourti, C. sapidus, and P. rugosus, the stepwise method did not select any environmental variables for the models, indicating no statistically significant associations with the tested variables.

Table 4.
Results of multiple linear regressions between environmental variables and abundance for each decapod species. All significant associations observed were positive. (D.O.—dissolved oxygen.).
4. Discussion
This study advances environmental monitoring and biodiversity protection in the Vale do Ribeira region. Regional faunal surveys are crucial for understanding ecosystem structure and function, especially as pressures from human activities and climate change increase. Such studies are vital for developing environmental monitoring programs and provide a basis for effective biodiversity conservation efforts [59].
The results are consistent with previous studies that identified the Ribeira de Iguape ecoregion as a conservation priority due to its high concentration of genetic diversity within a single watercourse, the Ribeira de Iguape River [30]. This classification followed a conservation assessment framework that considered species richness, endemism, threat status, and both genetic and phylogenetic diversity across South American freshwater ecoregions [30]. The observed patterns in decapod crustacean diversity and distribution reinforce the region’s ecological importance and emphasize the need for sustained protection and responsible management.
Comparing species richness across different regions is complex, as species counts vary depending on factors such as sampling effort, area size, climate, and substrate types [60]. In freshwater habitats, the complexity increases due to the high internal diversity of these systems, which often requires multiple sampling methods, including active techniques (e.g., sieving and manual searching) and passive ones (e.g., traps), both of which directly influence the number of species recorded [61,62,63].
The combination of sieving and traps used in this study, designed to target the specific niches occupied by different species, allowed for a broader understanding of decapod composition in the region, as each method was essential for capturing different species. In a study on the diversity and biology of swimming crabs (Portunidae) in the Estuarine–Lagoon Complex of Iguape, Ilha Comprida, and Cananéia, ten species were documented, including Callinectes sapidus and Callinectes bocourti, which were also found in the present study, reinforcing the estuarine influence on the lower course of the Ribeira de Iguape River [64]. However, surveys specifically focused on brachyuran composition in the region, especially in the Ribeira de Iguape River, remain limited. A previous study conducted in the Ribeira de Iguape basin and neighboring coastal areas did not include sampling within the river itself [31], which may explain the lack of records for species such as Armases rubripes, Minuca mordax, and Panopeus rugosus. These species are reported here for the first time in this area. Although primarily marine, these crabs are commonly found in brackish environments like lagoons and estuaries, and they may also be present in freshwater systems, including river deltas, during certain life stages. This distribution pattern reflects their ability to tolerate variations in salinity and temperature [65,66].
Regarding the carideans, all Atyidae and Palaemonidae species previously recorded in headwater and mid-course areas of the Ribeira de Iguape River basin [31], including Potimirim potimirim, Potimirim brasiliana (=Potimirim glabra), Macrobrachium acanthurus, Macrobrachium olfersii, and Macrobrachium carcinus, that were also found in the present study, except Macrobrachium heterochirus and Macrobrachium potiuna, which were absent because they are associated with headwater habitats characterized by rocky substrates and better-preserved riparian vegetation.
Among the species recorded in this study, M. carcinus, M. acanthurus, and C. sapidus warrant special attention for conservation, as reflected in their listing in the Livro Vermelho dos Crustáceos do Brasil [Red List of Brazilian Crustaceans] [58]. These species are categorized as Data Deficient (DD), indicating a lack of reliable data on their population status and calling for detailed assessments of their population trends, particularly given their commercial value. National action plans aimed at protecting aquatic and mangrove-associated fauna also identify these species as conservation priorities. The other species recorded in this study are classified as Least Concern (LC), suggesting that their populations are currently stable. Nevertheless, their inclusion on the Red List reinforces the need for continuous monitoring, especially in environments subject to gradual changes that may compromise long-term species persistence.
The detection of Macrobrachium rosenbergii, a non-native species introduced to Brazil in the 1970s for aquaculture, remains significant due to its ongoing spread [23,67]. Its presence has been recorded in 43 municipalities across eight Brazilian states, with the most occurrences reported in natural environments in Pará [68,69,70,71]. In São Paulo, only one previous record exists in natural waters, documenting a single specimen in the Tietê River basin. In the Vale do Ribeira region, the cultivation of this species was authorized in 1993 in the Etá River (Eldorado, SP), a tributary of the Ribeira de Iguape River [72], which may have been the initial introduction point along São Paulo’s southern coast. Recent sampling efforts in the Iguape region (February 2025) recorded small individuals (12.4 and 24.5 mm CL; Bertini, pers. comm.), and local fishers frequently report capturing the species, including ovigerous females, indicating that its presence may be more widespread than previously documented. Despite the low abundance observed, this population may be in the early stages of establishment, possibly limited by competition with native species such as M. acanthurus and M. carcinus, which exhibit highly territorial behavior. In northern Brazil, reproductive populations are already established, with records of ovigerous females in estuarine habitats, the identification of juveniles through molecular analyses, and the occurrence of all male morphotypes, indicating successful reproduction and recruitment in the wild [73]. The species also shows higher fecundity than native prawns, territorial behavior, and omnivorous feeding habits, which may contribute to competition for space and food resources [73]. The occurrence of M. rosenbergii in transitional zones such as the lower Ribeira de Iguape River reinforces the need for continuous monitoring of non-native species and their potential consequences for native communities.
The environmental variations observed in the lower course of the Ribeira de Iguape River result from the combined influence of saltwater intrusion and the region’s rainfall regime. Although saline water reaches this part of the river, the high freshwater flow limits the progression of the salt wedge, especially during the rainy season. Salinity ranged from nearly zero to five, with the highest levels recorded in Areas 2 and 3 during spring, the season with the least recorded rainfall (331.8 mm, INMET). A similar trend was observed in electrical conductivity, which rose due to the higher concentration of dissolved ions associated with marine influence during this period. Despite the narrow salinity range, these values are consistent with the behavior of subtropical estuaries under strong freshwater influence, such as the Ribeira de Iguape River. The observed increases, especially in Area 3 during the drier months, indicate a localized but significant marine influence, enough to define this section as a transitional zone between freshwater and estuarine environments. The year-round presence of marine species, such as swimming crabs (Brachyura), in this area further supports this classification. This hydrological pattern resembles that observed in Guajará Bay, which, because of its high freshwater input from the Amazon River, acts like a river for most of the year, effectively preventing the salt wedge from advancing during the rainy season [45]. Similarly, the other physicochemical parameters recorded in the Ribeira de Iguape River, including temperature, pH, water transparency, and dissolved oxygen, follow patterns commonly reported for estuarine systems, where seasonal variation and proximity to the coast jointly influence environmental dynamics [45,74,75,76].
The seasonality observed in the total decapod community, with higher abundances during winter and spring, mainly reflects the population dynamics of the dominant species, especially the caridean shrimps. These patterns also influenced diversity indices, with the highest diversity and evenness observed in autumn and the lowest in winter and spring. These periods coincided with higher abundances of M. acanthurus and Potimirim spp., indicating that seasonal decreases in diversity are due to the numerical dominance of these taxa. Such patterns are consistent with the amphidromous behavior of M. acanthurus, which migrates upstream as individuals grow, as documented along more than 80 km of the Ribeira de Iguape River [36]. In this system, juveniles typically concentrate near the river mouth and gradually migrate upstream as they grow. This ontogenetic migration shapes the spatial distribution and size structure of the population. A similar longitudinal migration can be inferred for Potimirim spp., supported by studies in other river systems where individuals at different life stages are found along various parts of the river, consistent with an amphidromous life cycle [77].
Studies conducted in other tropical and temperate estuarine systems support the influence of seasonal fluctuations on decapod communities. In the Mundaú/Manguaba lagoon complex (Alagoas, Brazil), higher abundances of M. acanthurus and Palaemon pandaliformis were recorded during the rainy season, in association with environmental factors [74]. Similarly, long-term studies in the temperate Guadalquivir River estuary (Spain) showed significant seasonal and yearly changes in community composition, with peaks in abundance, diversity, and biomass during spring and autumn, mainly driven by fluctuations in temperature and salinity [76,78]. These findings indicate that seasonal environmental changes modulate the composition, dominance, and distribution of decapod species. Among the caridean shrimps with migratory behavior observed in the present study, such variations may directly influence recruitment, growth, and spatial distribution processes, resulting in seasonal fluctuations in community structure.
The spatial distribution of the community did not reveal significant differences in overall abundance across areas, but dominant species showed distinct patterns of occupancy. Area 3, situated near the river mouth and influenced by marine conditions, provided favorable environments for both marine and freshwater species to coexist. This area had the highest values of species richness, diversity, and evenness, with all recorded taxa present. In contrast, Area 2 exhibited lower diversity due to the numerical dominance of Potimirim spp. These patterns emphasize the ecological importance of Area 3 and suggest that spatial variation in community structure was closely related to species evenness.
The distribution of the dominant species, in turn, was associated with variations in environmental factors, particularly salinity, dissolved oxygen, and pH. Patterns of association between these species, environmental gradients, and sampling areas emerged from both the multiple linear regression models and the correspondence analysis. However, the regression models showed low coefficients of determination, indicating that the selected variables accounted for only part of the observed variation. A comparable pattern occurred in the Guajará River estuary (Amazon region, Pará, Brazil), where abiotic factors had only weak correlations with species distribution [45]. These findings indicate that community structure results from a dynamic interaction of abiotic, biotic, and habitat features, including vegetation cover and the availability of organic debris, which influence both local diversity and species abundance.
Among the species contributing most to the spatial patterns observed, A. rubripes, P. pandaliformis, and M. olfersii stood out for their strong association with Area 3 and their positive relationship with salinity. Similar patterns have been documented in other regions of Brazil. In the case of A. rubripes, the association with salinity was evident in the salt marshes of the Patos Lagoon in southern Brazil, where ovigerous females occurred in greater numbers during periods of elevated salinity [79]. In addition, environmental features such as the presence of grasses in the marginal vegetation appear to favor the occurrence of this species in estuaries [80], a pattern also observed in the present study, as Area 3 had a high abundance of Brachiaria spp. For P. pandaliformis, its salinity-influenced distribution aligns with studies indicating a preference for estuarine environments [81,82]. However, no significant correlation was reported between this species and salinity in areas ranging from 2 to 9 ppt [83]. This divergence may reflect the high salinity tolerance of its larvae, which can withstand ranges from 0 to 35 ppt [84]. Salinities around 28 ppt are considered ideal for larval development, although lower values are necessary to trigger the process [84], which is compatible with the conditions observed in the sampled area. For M. olfersii, although adults are predominantly found in freshwater habitats, reproduction depends on brackish environments, as shown in previous studies [85,86]. Thus, salinity acts as a key factor for the presence of this species in transitional zones such as the one identified in the present study.
The distribution of Potimirim spp. showed a positive correlation with dissolved oxygen levels and was more abundant in Area 2, where greater coverage of filamentous algae and aquatic plants occurred. Such environments offer favorable conditions by providing shelter and food. According to [87], shrimps belonging to the family Atyidae use substrates covered with algae and decaying leaves as shelter. These surfaces host microbial biofilms, which the shrimps feed on by scraping or filtering particles, linking habitat selection directly to their feeding strategy. Higher algal biomass may also contribute to increased dissolved oxygen concentrations in the water, benefiting species that rely on well-oxygenated environments. In addition, the lower abundance of Macrobrachium species (such as M. acanthurus and M. olfersii) in Area 2 may reflect an avoidance mechanism by atyids, which may avoid areas with a higher risk of predation. This migration behavior in response to predator presence was demonstrated experimentally, with Atya lanipes responding to chemical and tactile cues from M. carcinus by altering its movement patterns and microhabitat use [88]. Similar results occurred in neotropical streams, where the presence of predatory fish inhibited the foraging activity of P. glabra, leading to the accumulation of periphyton [89]. These findings support the idea that non-consumptive, behaviorally mediated trophic interactions, such as predator avoidance, can influence the spatial distribution of atyid shrimps. This mechanism likely contributes to the higher abundance of Potimirim spp. observed in Area 2.
In the case of M. acanthurus, although a significant association with pH was identified, this parameter remained nearly neutral across all study areas, consistently within the optimal range of 6.5 to 8.5 for most Macrobrachium species [90]. Most individuals were collected using sieves in marginal vegetation, especially in Areas 2 and 3. This pattern suggests that the species’ distribution is better explained by the high abundance of riparian grasses, such as Brachiaria spp., rather than by pH itself, indicating a preference for this type of vegetative cover. These habitats provide protection from predators [64,91] and offer favorable conditions for feeding and reproduction [92]. The structure and composition of aquatic vegetation have been shown to influence decapod abundance and community composition in river systems of the Paraná floodplain, reinforcing the functional role of macrophytes in habitat selection [93]. Consequently, beyond abiotic conditions, the distribution of freshwater decapods also depends on biotic factors, including ecological interactions, microhabitat diversity [93,94,95,96], predation [88,89], and food availability [87,97]. Therefore, the physiographic features of each area play a central role in shaping the spatial organization of decapod communities in the Ribeira de Iguape River. Species-specific associations with distinct vegetative microhabitats, such as riparian grasses and submerged macrophytes, directly influence community structure. These findings emphasize the ecological importance of habitat complexity in determining species distributions in transitional freshwater environments.
5. Conclusions
This study revealed that, despite historical and ongoing human pressures on the Ribeira de Iguape River [13,40,42,43], the region still supports a diverse decapod community composed of freshwater, estuarine, and marine species. The area closest to the river mouth showed the highest species richness and diversity, likely due to its transitional environment and moderate salinity fluctuations. Spatial and seasonal patterns indicated that community structure results from the interaction of abiotic conditions and habitat-related factors. The presence of the non-native Macrobrachium rosenbergii suggests an early stage of establishment and reinforces the importance of continued monitoring to assess potential ecological impacts.
Overall, these results reaffirm the ecological importance of freshwater–estuarine transitions and provide a foundation for conservation planning and biodiversity management in the region. The presence of native decapods listed in the Livro Vermelho dos Crustáceos do Brasil further increases the conservation value of this ecosystem and supports the need for long-term monitoring in transitional aquatic environments.
Author Contributions
Conceptualization, G.B., E.K.S.M. and M.R.R.S.G.; methodology, G.B., E.K.S.M. and L.C.F.d.A.; validation, G.B., E.K.S.M., M.R.R.S.G. and L.C.F.d.A.; formal analysis, G.B., E.K.S.M. and L.C.F.d.A.; research, G.B., E.K.S.M., M.R.R.S.G. and L.C.F.d.A.; resources, G.B.; data curation, G.B.; writing—original draft preparation, G.B., E.K.S.M. and M.R.R.S.G.; writing—review and editing, G.B., E.K.S.M., M.R.R.S.G. and L.C.F.d.A.; visualization, G.B., E.K.S.M., M.R.R.S.G. and L.C.F.d.A.; supervision, G.B.; project administration, G.B.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the São Paulo Research Foundation (FAPESP), Brazil, grant number 2013/06457-2.
Institutional Review Board Statement
The animal study protocol was conducted in accordance with Brazilian regulations and authorized by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), permit number 42713-1.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the many colleagues and students who helped with sampling and laboratory analysis, and the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for permission to conduct sampling (number 42713-1).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Revenga, C.; Campbell, I.; Abell, R.; de Villiers, P.; Bryer, M. Prospects for monitoring freshwater ecosystems towards the 2010 targets. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Davies, P.M. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 2021, 50, 85–94. [Google Scholar] [CrossRef]
- Mendonça, J.T.; Katsuragawa, M. Caracterização da pesca artesanal no complexo estuarino-lagunar de Cananéia-Iguape, Estado de São Paulo, Brasil (1995–1996). Acta Sci. Biol. Sci. 2001, 23, 535–547. [Google Scholar]
- Bertini, G.; Valenti, W.C. Importância econômica dos camarões-de-água-doce. In Relatos de Pesquisas e Outras Experiências Vividas no Vale do Ribeira; Silva, R.B., Ming, L.C., Eds.; Fundação de Estudos e Pesquisas em Agronomia, Medicina Veterinária e Zootecnia: Jaboticabal, Brazil, 2010; pp. 155–170. [Google Scholar]
- Mendonça, J.T.; Verani, J.R.; Nordi, N. Evaluation and management of blue crab Callinectes sapidus (Rathbun, 1896) (Decapoda: Portunidae) fishery in the Estuary of Cananéia, Iguape and Ilha Comprida, São Paulo. Braz. J. Biol. 2010, 70, 37–45. [Google Scholar] [CrossRef]
- Mendonça, J.T.; Bonfante, T.M. Assessment and management of white mullet Mugil curema (Valencienne, 1836) (Mugilidae) fisheries of the south coast of São Paulo state, Brazil. Braz. J. Biol. 2011, 71, 663–672. [Google Scholar] [CrossRef]
- Mendonça, J.T.; Machado, I.C.; Jensen, L.V.; Campolim, M.B.; Lucena, A.; Cardoso, T.A. Ordenamento da pesca com cercos fixos no estuário de Cananéia-Iguape-Ilha Comprida. Arq. Ciênc. Mar 2011, 44, 36–51. [Google Scholar]
- Ramires, M.; Barrella, W.; Esteves, A.M. Caracterização da pesca artesanal e o conhecimento pesqueiro local no Vale do Ribeira e Litoral Sul de São Paulo. Rev. Cecil. 2012, 4, 37–43. [Google Scholar]
- Mendonça, J.T. Caracterização da pesca artesanal no litoral sul de São Paulo–Brasil. Bol. Inst. Pesca 2015, 41, 479–492. [Google Scholar]
- Prado, H.M.; Schlindwein, M.N.; Murrieta, R.S.S.; Nascimento-Júnior, D.R.D.; Souza, E.P.D.; Cunha-Lignon, M.; Contente, R.F. O Canal do Valo Grande no Complexo Estuarino Cananéia-Iguape (SP, Brasil): História ambiental, ecologia e perspectivas futuras. Ambiente Soc. 2019, 22, e01822. [Google Scholar] [CrossRef]
- Cunha-Lignon, M.; Mendonça, J.T. Ecossistema manguezal: Seus recursos naturais e pesca. In Manguezais, Camarões-de-Água-Doce e Manjuba-de-Iguape: Patrimônios Natural e Cultural do Vale do Ribeira e Litoral Sul do Estado de São Paulo; Cunha-Lignon, M., Bertini, G., Montealegre-Quijano, S., Eds.; Unesp: Registro, Brazil, 2021; pp. 23–65. [Google Scholar]
- Rodrigues, V.G.S.; Fujikawa, A.; Abessa, D.M.D.S.; Hortellani, M.A.; Sarkis, J.E.S.; Sígolo, J.B. Uso do bivalve límnico Anodontites tenebricosus (LEA, 1834) no biomonitoramento de metais do Rio Ribeira de Iguape. Química Nova 2012, 35, 454–459. [Google Scholar] [CrossRef]
- Abessa, D.M.; Morais, L.G.; Perina, F.C.; Davanso, M.B.; Rodrigues, V.G.S.; Martins, L.M.; Sígolo, J.B. Sediment geochemistry and climatic influences in a river influenced by former mining activities: The case of Ribeira de Iguape River, SP-PR, Brazil. Open J. Water Pollut. Treat. 2014, 1, 43–53. [Google Scholar] [CrossRef]
- Bonnail, E.; Pérez-López, R.; Sarmiento, A.M.; Nieto, J.M.; Del-Valls, T.Á. A novel approach for acid mine drainage pollution biomonitoring using rare earth elements bioaccumulated in the freshwater clam Corbicula fluminea. J. Hazard. Mater. 2017, 338, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, A.F.G.; Tachibana, L.; Corrêa, C.F.; Koki, M.R.; Neto, A.L.; Baccarin, A.E. Qualidade da água do Rio Ribeira de Iguape da área de extração de areia no município de Registro, SP. Rev. Acad. Ciênc. Anim. 2008, 6, 483–492. [Google Scholar] [CrossRef][Green Version]
- Marques, M.N.; Badiru, A.I.; Beltrame, O.; Pires, M.A.F. Pesticide leaching and run-off hazard in the Ribeira de Iguape River Basin in São Paulo State, Brazil. J. Braz. Soc. Ecotoxicol. 2007, 2, 179–185. [Google Scholar] [CrossRef]
- Marques, M.N.; Cotrim, M.B.; Pires, M.A.F.; Beltrame-Filho, O. Avaliação do impacto da agricultura em áreas de proteção ambiental, pertencentes à bacia hidrográfica do rio Ribeira de Iguape, São Paulo. Química Nova 2007, 30, 1171–1178. [Google Scholar] [CrossRef]
- Barros-Silva, P. Distribuição de camarões exóticos no Brasil. Bol. Inst. Pesca 1997, 24, 33–45. [Google Scholar]
- Valenti, W.C.; New, M.B. Freshwater prawn culture: The Brazilian experience. World Aquacult. 2000, 31, 28–30. [Google Scholar]
- Magalhães, C.; Bueno, S.L.S.; Bond-Buckup, G.; Valenti, W.C.; Silva, H.L.M.D.; Kiyohara, F.; Ferreira, E.C.; Rocha, S.S. Exotic species of freshwater decapod crustaceans in the state of São Paulo, Brazil: Records and possible causes of their introduction. Biodivers. Conserv. 2005, 14, 1929–1945. [Google Scholar] [CrossRef]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Silva-Mattos, D.M.; Bovi, M.L. Understanding the threats to biological diversity in southeastern Brazil. Biodivers. Conserv. 2002, 11, 1747–1758. [Google Scholar] [CrossRef]
- SIGRH-Sistema Integrado de Gerenciamento de Recursos Hídricos do Estado de São Paulo. Relatório de Situação dos Recursos Hídricos da UGRHI 11–Comitê da Bacia Hidrográfica do Ribeira de Iguape e Litoral Sul 2020. Available online: https://sigrh.sp.gov.br/public/uploads/documents/CBH-RB/19455/relatorio-de-situacao-2020.pdf (accessed on 15 July 2025).
- Instituto Florestal. Inventário Florestal do Estado de São Paulo: Mapeamento da Cobertura Vegetal Nativa; Governo do Estado de São Paulo, Secretaria de Infraestrutura e Meio Ambiente do Estado de São Paulo: São Paulo, Brazil, 2020. Available online: https://smastr16.blob.core.windows.net/home/2020/07/inventarioflorestal2020.pdf (accessed on 15 March 2023).
- Magalhães, C. Fauna de Camarões do Estado de São Paulo: Revisão e Prioridades de Pesquisa; Unpublished Report; Museu de Zoologia da USP: São Paulo, Brazil, 1999. [Google Scholar]
- Magalhães, C. Diversity of freshwater decapods in São Paulo. Rev. Bras. Zool. 1999, 16, 181–193. [Google Scholar]
- Pérez-Losada, M.; Bond-Buckup, G.; Jara, C.G.; Crandall, K.A. Molecular systematics and biogeography of the freshwater crab genus Aegla. Cladistics 2009, 25, 386–405. [Google Scholar]
- Rocha, S.S.D.; Bueno, S.L.D.S. Crustáceos decápodes de água doce com ocorrência no Vale do Ribeira de Iguape e rios costeiros adjacentes, São Paulo, Brasil. Rev. Bras. Zool. 2004, 21, 1001–1010. [Google Scholar] [CrossRef]
- Valenti, W.C.; Mello, J.T.C.; Lobão, V.L. Dinâmica da reprodução de Macrobrachium acanthurus (Wiegmann, 1836) e Macrobrachium carcinus (Linnaeus, 1758) do Rio Ribeira de Iguape (Crustacea, Decapoda, Palaemonidae). Ciênc. Cult. 1986, 38, 349–355. [Google Scholar]
- Valenti, W.C.; Lobão, V.L.; Mello, J.T.C. Crescimento relativo de Macrobrachium acanthurus (Wiegmann, 1836) (Crustacea, Decapoda, Palaemonidae). Rev. Bras. Zool. 1989, 6, 1–8. [Google Scholar] [CrossRef]
- Valenti, W.C.; Mello, J.T.C.; Lobão, V.L. Maturation and growth curves of Macrobrachium carcinus (Linnaeus) (Crustacea, Decapoda, Palaemonidae) from Ribeira de Iguape River, southern Brazil. Rev. Bras. Zool. 1994, 11, 649–658. [Google Scholar] [CrossRef]
- Bertini, G.; Baeza, J.A. Fecundity and fertility in a freshwater population of the neotropical amphidromous shrimp Macrobrachium acanthurus from the southeastern Atlantic. Invert. Reprod. Dev. 2014, 58, 207–217. [Google Scholar] [CrossRef]
- Bertini, G.; Baeza, J.A.; Perez, E. A test of large-scale reproductive migration in females of the amphidromous shrimp Macrobrachium acanthurus (Caridea: Palaemonidae) from southeastern Brazil. Mar. Freshw. Res. 2014, 65, 81–93. [Google Scholar] [CrossRef]
- Ribeiro, C.C.; Lopes, V.H.P.; Bertini, G. Abundance and spatio-temporal distribution of the amphidromous shrimp Macrobrachium olfersii (Caridea: Palaemonidae) along the Ribeira de Iguape River (São Paulo, Brazil). Nauplius 2020, 28, e2020017. [Google Scholar] [CrossRef]
- Ribeiro, C.C.; Braga, A.A.; Miranda, E.K.S.; Bertini, G. Evaluation of population dynamics of Macrobrachium olfersii (Palaemonidae) along ~150 km extension of the Lower Ribeira de Iguape River, São Paulo, Brazil. Pan-Am. J. Aquat. Sci. 2023, 18, 8–34. [Google Scholar]
- Revenga, C.; Kura, Y. Status and Trends of Biodiversity of Inland Water Ecosystems; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2003; Technical Series No. 11. Available online: https://www.cbd.int/doc/publications/cbd-ts-11.pdf (accessed on 17 April 2025).
- Mahiques, M.M.D.; Figueira, R.C.; Alves, D.P.; Italiani, D.M.; Martins, C.C.; Dias, J. Coastline changes and sedimentation related with the opening of an artificial channel: The Valo Grande Delta, SE Brazil. An. Acad. Bras. Cienc. 2014, 86, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Panapaná. Elaboração do Plano da Bacia Hidrográfica do Ribeira de Iguape e Litoral Sul–UGRHI 11; Panapaná Informação e Educação: São Paulo, Brazil, 2016; p. 12. [Google Scholar]
- Mahiques, M.M.D.; Burone, L.; Figueira, R.C.L.; Lavenére-Wanderley, A.A.D.O.; Capellari, B.; Rogacheski, C.E.; Cussioli, M.C. Anthropogenic influences in a lagoonal environment: A multiproxy approach at the Valo Grande mouth, Cananéia-Iguape system (SE Brazil). Braz. J. Oceanogr. 2009, 57, 325–337. [Google Scholar] [CrossRef]
- Cunha-Lignon, M.; Kampel, M.; Menghini, R.P.; Schaeffer-Novelli, Y.; Cintrón, G.; Dahdouh-Guebas, F. Mangrove forests submitted to depositional processes and salinity variation investigated using satellite images and vegetation structure surveys. J. Coast. Res. 2011, 64, 344–348. [Google Scholar]
- Tessler, M.G.; Souza, R.B. Características hidrodinâmicas do estuário de Cananéia. Bol. Inst. Oceanogr. 1998, 46, 25–34. [Google Scholar]
- Cavalcante, D.V.; da Silva, B.B.; Martinelli-Lemos, J.M. Biodiversity of decapod crustaceans in the estuarine floodplain around the city of Belém (Pará) in Brazilian Amazonia. Zoologia 2012, 29, 203–209. [Google Scholar] [CrossRef]
- Holthuis, L.B. Marine Lobsters of the World: An Annotated and Illustrated Catalogue of Species of Interest to Fisheries Known to Date; FAO Fisheries Synopsis No. 125; FAO: Rome, Italy, 1993; pp. 1–292. [Google Scholar]
- Melo, G.A.S. Manual de Identificação dos Brachyura (Caranguejos e Siris) do Litoral Brasileiro; Plêiade: São Paulo, Brazil, 2003. [Google Scholar]
- Torati, L.S.; Mantelatto, F.L. Identification key for the species of the infraorder Anomura (Crustacea: Decapoda) from the coast of São Paulo State, Brazil. Biota Neotrop. 2012, 12, 147–158. [Google Scholar]
- Akaike, H. A new look at statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Harper & Row: New York, NY, USA, 1989; pp. 120–150. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Begon, M.; Townsend, C.R.; Harper, J.L. Ecology: From Individuals to Ecosystems, 4th ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 115–145. [Google Scholar]
- Pielou, E.C. Ecological Diversity, 1st ed.; Wiley and Sons: New York, NY, USA, 1975. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Science: Oxford, UK, 2011. [Google Scholar]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data; Version 6.255 Beta; MjM Software Design: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Pinheiro, M.; Boos, H. Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010–2014; Sociedade Brasileira de Carcinologia—SBC: Porto Alegre, Brazil, 2016. [Google Scholar]
- Braga, A.A.; Fransozo, A.; Bertini, G.; Fumis, P.B. Composition and abundance of the crabs (Decapoda Brachyura) off Ubatuba and Caraguatatuba, Northern Coast of São Paulo, Brasil. Biota Neotrop. 2005, 5, 45–78. [Google Scholar] [CrossRef]
- Almeida, A.O.; Costa-Souza, A.C.; Cunha, A.M.; Souza-Santos, P.; Oliveira, M.V.; Oliveira-Soledade, G. Estuarine caridean shrimps (Crustacea: Decapoda) from Ilhéus, Bahia, Brazil: Updated checklist and a key for their identification. Check List 2013, 9, 1396–1405. [Google Scholar] [CrossRef][Green Version]
- Silva, L.C.F.; Vieira, L.C.G.; Costa, D.A.; Lima Filho, G.F.D.; Vital, M.V.C.; de Carvalho, R.A.; Oliveira, L.C. Qualitative and quantitative benthic macroinvertebrate samplers in Cerrado streams: A comparative approach. Acta Limnol. Bras. 2005, 17, 123–128. [Google Scholar][Green Version]
- Rocha, S.S.D. Diferença entre dois métodos de coleta utilizados na captura de crustáceos decápodes em um rio da Estação Ecológica Juréia-Itatins, São Paulo. Iheringia Sér. Zool. 2010, 100, 116–122. [Google Scholar] [CrossRef][Green Version]
- Bertini, G.; Rodrigues, M.M.; Izumi, K.K.; Izumi, K.S. Camarões-de-água-doce do Vale do Ribeira: Riqueza, importância ecológica e economia. In Manguezais, Camarões-de-Água-Doce e Manjuba-de-Iguape: Patrimônios Natural e Cultural do Vale do Ribeira e Litoral Sul do Estado de São Paulo; Cunha-Lignon, M., Bertini, G., Montealegre-Quijano, S., Eds.; Unesp: Registro, Brazil, 2021; pp. 70–111. [Google Scholar]
- Severino-Rodrigues, E.; Soares, F.C.; Graça-Lopes, R.; Souza, K.H.; Canéo, V.O.C. Diversidade e biologia de espécies de Portunidae (Decapoda, Brachyura) no estuário de Iguape, Ilha Comprida e Cananéia, São Paulo, Brasil. Bol. Inst. Pesca 2009, 35, 47–60. [Google Scholar][Green Version]
- Almeida, A.O.; Coelho, P.A.; Luz, J.R.; Santos, J.T.A.; Ferraz, N.R. Decapod crustaceans in fresh waters of southeastern Bahia, Brazil. Rev. Biol. Trop. 2008, 56, 1225–1254. [Google Scholar][Green Version]
- Scalco, A.C.; Ramos, C.A.; Boos, H. Estrutura populacional de Uca (Minuca) mordax (Smith, 1870) (Decapoda: Ocypodidae) em um sistema estuarino impactado no Sul do Brasil. Rev. CEPSUL Biodivers. Conserv. Mar. 2016, 5, 17–28. [Google Scholar] [CrossRef]
- Valenti, W.C.; Barros, H.P.; Moraes-Valenti, P.; Bueno, G.W.; Cavalli, R.O. Aquaculture in Brazil: Past, present and future. Aquac. Rep. 2021, 19, 100611. [Google Scholar] [CrossRef]
- Iketani, G.; Aviz, M.A.B.; Maciel, C.; Valenti, W.C.; Schneider, H.; Sampaio, I. Successful invasion of the Amazon Coast by the giant river prawn, Macrobrachium rosenbergii: Evidence of a reproductively viable population. Aquat. Invasions 2016, 11, 277–286. [Google Scholar] [CrossRef]
- Silva, T.A.D.; Ballester, E.L.C.; Cunico, A.M. First records of non-native species Malaysian prawn Macrobrachium rosenbergii (Decapoda: Palaemonidae) in an important South American hydrographic system. Acta Limnol. Bras. 2020, 32, e25. [Google Scholar] [CrossRef]
- Oliveira, C.D.L.; Santos, L.V.R. Distribution of the giant river prawn Macrobrachium rosenbergii (De Man, 1879) in Brazil: 43 years after its introduction. Nauplius 2021, 29, e2021007. [Google Scholar] [CrossRef]
- Rodrigues, J.B.S.; Menezes, P.A.; Oliveira, A.L.; Lira, A.S.; Azevedo, J.W.J.; Lopes, D.F.C. Dinâmica populacional do Macrobrachium rosenbergii (de Man, 1879), na bacia do Rio Pericumã, Pinheiro, Maranhão. Rev. Bras. Eng. Pesca 2023, 14, 54–64. [Google Scholar]
- Panorama da Aquicultura. Inaugurado em São Paulo projeto da APT para cultivo de Macrobrachium e Tilápia Vermelha. Panor. da Aquicultura. 1993. Available online: https://panoramadaaquicultura.com.br/inaugurado-em-sao-paulo-projeto-da-apt-para-cultivo-de-macrobrachium-e-tilapia-vermelha/ (accessed on 22 May 2025).
- Silva-Oliveira, G.C.; Ready, J.S.; Iketani, G.; Bastos, S.; Gomes, G.; Sampaio, I.; Maciel, C. The invasive status of Macrobrachium rosenbergii (De Man, 1879) in Northern Brazil, with an estimation of areas at risk globally. Aquat. Invasions 2011, 6, 319–328. [Google Scholar] [CrossRef]
- Teixeira, R.L.; Sá, H.S. Abundância de macrocrustáceos decápodos nas áreas rasas do complexo lagunar Mundaú/Manguaba, AL. Rev. Bras. Biol. 1998, 58, 393–404. [Google Scholar] [CrossRef]
- Lutz, Í.; Nascimento, M.; Beasley, C.R.; Cardoso, C.; Isaac, V.; Bentes, B. Spatio-temporal variation in the density and diversity of decapods captured with artisanal traps in an Amazon estuary. Acta Limnol. Bras. 2022, 34, e9. [Google Scholar] [CrossRef]
- González-Ortegón, E.; de Carvalho-Souza, G.F.; Vilas, C.; Baldó, F.; Cuesta, J.A. Trends in the decapod crustacean community at the southernmost estuary of the Atlantic coast of Europe. Sci. Rep. 2023, 13, 22857. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.C.; Pantaleão, J.A.F.; Teles, J.N.; França, N.F.C.; da Costa, J.R.P.; Cabral, H.; Mantelatto, F.L. The influence of natural barriers on the amphidromous shrimp Potimirim brasiliana (Caridea, Atyidae) from two rivers in Southeastern Brazil. Biol. Bull. 2022, 242, 27–39. [Google Scholar] [CrossRef]
- Cuesta, J.A.; Fernández-González, V.; Drake, P. Decapod crustacean assemblages on subtidal soft bottoms in the Alboran Sea (western Mediterranean): Spatial and temporal variability. Sci. Mar. 2006, 70, 69–80. [Google Scholar]
- Rodrigues, L.S.; Pimenta, A.M.; Barutot, R.A. Population dynamics of Armases rubripes in southwestern Atlantic salt marshes: Seasonality, abiotic influence and reproductive biology. Reg. Stud. Mar. Sci. 2021, 41, 101567. [Google Scholar] [CrossRef]
- Lima, G.V.; Silveira, C.M.; Oshiro, L.M.Y. Estrutura populacional dos camarões simpátricos Potimirim glabra e Potimirim potimirim (Crustacea, Decapoda, Atyidae) no rio Sahy, Rio de Janeiro, Brasil. Iheringia Sér. Zool. 2006, 96, 81–87. [Google Scholar] [CrossRef]
- Mortari, R.C.; Nunes Pralon, B.G.; Negreiros-Fransozo, M.L. Reproductive biology of Palaemon pandaliformis (Stimpson, 1871) (Crustacea, Decapoda, Caridea) from two estuaries in southeastern Brazil. Invertebr. Reprod. Dev. 2009, 53, 223–232. [Google Scholar] [CrossRef]
- Rosa, L.C.D.; Passos, A.C.; Corrêa, M.F.M. Aspectos populacionais e reprodutivos de Palaemon pandaliformis (Crustacea: Palaemonidae) em uma marisma subtropical no sul do Brasil. Bol. Inst. Pesca 2015, 41, 849–857. [Google Scholar]
- Laino, P.D.S.; Musiello-Fernandes, J.; Braga, A.A. Population structure of the shrimp Palaemon pandaliformis (Stimpson, 1871) (Caridea: Palaemonidae) in the river Rio Itapemirim, in Southeastern Brazil. Biota Neotrop. 2020, 20, e20201020. [Google Scholar] [CrossRef]
- Moreira, G.S.; McNamara, J.C. Physiological responses of the early zoeal stages of Palaemon pandaliformis (Stimpson) and Palaemon northropi (Rankin) to salinity variation. Trop. Zooplankton 1984, 113, 165–169. [Google Scholar]
- Mossolin, E.C.; Bueno, S.L.S. Reproductive strategy of Macrobrachium olfersii. Nauplius 2002, 10, 65–73. [Google Scholar]
- Ferreira, R.S.; Vieira, R.R.R.; D’Incao, F. The marine and estuarine shrimps of the Palaemoninae (Crustacea: Decapoda: Caridea) from Brazil. Zootaxa 2010, 2606, 1–24. [Google Scholar] [CrossRef]
- Covich, A.P.; Crowl, T.A.; Johnson, S.L.; Varza, D.; Certain, D.L. Post-Hurricane Hugo increases in atyid shrimp abundances in a Puerto Rican montane stream. Biotropica 1991, 23, 448–454. [Google Scholar] [CrossRef]
- Crowl, T.A.; Covich, A.P. Responses of a freshwater shrimp to chemical and tactile stimuli from a large decapod predator. J. N. Am. Benthol. Soc. 1994, 13, 291–298. [Google Scholar] [CrossRef]
- Moulton, T.P.; Souza, M.L.; Silveira, R.M.L.; Krsulović, F.A.M.; Silveira, M.P.; Assis, J.C.F.; Francischetti, C.N. Patterns of periphyton are determined by cascading trophic relationships in two neotropical streams. Mar. Freshw. Res. 2010, 61, 57–64. [Google Scholar] [CrossRef]
- Jayachandran, K.V. Palaemonid Prawns: Biodiversity, Taxonomy, Biology and Management; Science Publishers Inc.: Enfield, NH, USA, 2001; pp. 1–624. [Google Scholar]
- Soares, M.R.; Oshiro, L.M.Y.; Toledo, J.C. Biologia reprodutiva de Macrobrachium jelskii (Crustacea, Decapoda, Palaemonidae) no rio São Francisco, Minas Gerais, Brasil. Iheringia Sér. Zool. 2015, 105, 307–315. [Google Scholar] [CrossRef]
- Montoya, J.V. Freshwater shrimps of the genus Macrobrachium associated with roots of Eichhornia crassipes (Water Hyacinth) in the Orinoco Delta (Venezuela). Caribb. J. Sci. 2003, 39, 155–159. [Google Scholar]
- Torres, M.V.; Giri, F.; Collins, P.A. Temporal and spatial patterns of freshwater decapods associated with aquatic vegetation from floodplain rivers. Hydrobiologia 2018, 823, 169–189. [Google Scholar] [CrossRef]
- Iwata, T.; Inoue, M.; Nakano, S.; Miyasaka, H.; Doi, A.; Covich, A.P. Shrimp abundance and habitat relationships in tropical rain-forest streams, Sarawak, Borneo. J. Trop. Ecol. 2003, 19, 387–395. [Google Scholar] [CrossRef]
- Kemenes, A.; Forsberg, B.R.; Magalhães, C.; dos Anjos, H. Environmental factors influencing the community structure of shrimps and crabs (Crustacea: Decapoda) in headwater streams of the Rio Jaú, Central Amazon, Brazil. Pan-Am. J. Aquat. Sci. 2010, 5, 36–46. [Google Scholar]
- Mejía-Ortiz, L.M.; Alvarez, F. Seasonal patterns in the distribution of three species of freshwater shrimp, Macrobrachium spp., along an altitudinal river gradient. Crustaceana 2010, 83, 385–397. [Google Scholar] [CrossRef]
- Silva-Junior, E.F.; Silva-Araújo, M.; Moulton, T.P. Distribution and abundance of freshwater decapods in an Atlantic rainforest catchment with a dammed future. Braz. J. Biol. 2017, 77, 820–829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).