Overexpression of LIM Homeodomain Gene Arrowhead Induces Pleiotropic Developmental Alterations in the Silkworm, Bombyx mori
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Silkworm Rearing
2.2. Construction of PiggyBac Transgenic Vectors and Generation of Transgenic Silkworms
2.3. RNA Extraction and qRT-PCR
2.4. Protein Extraction and SDS-PAGE Analysis
2.5. Measurement of Juvenile Hormone (JH) Titer Level
2.6. Phylogenetic Analysis
2.7. Statistical Analysis
3. Results
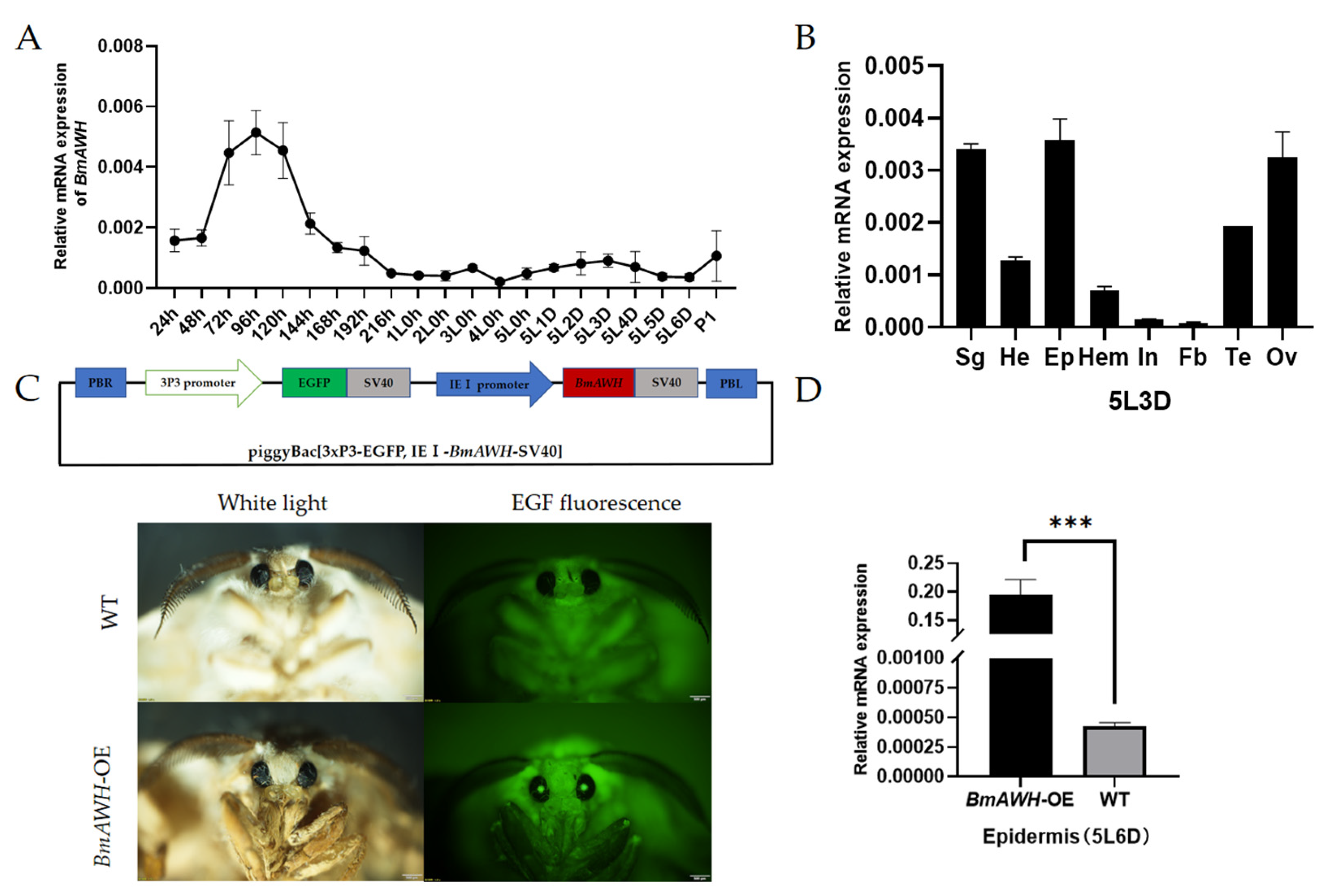
3.1. Gene Cloning, Molecular Characterization, and Expression Level of BmAWH
3.2. Construction of BmAWH Transgenic Vector and Transgenic Silkworms
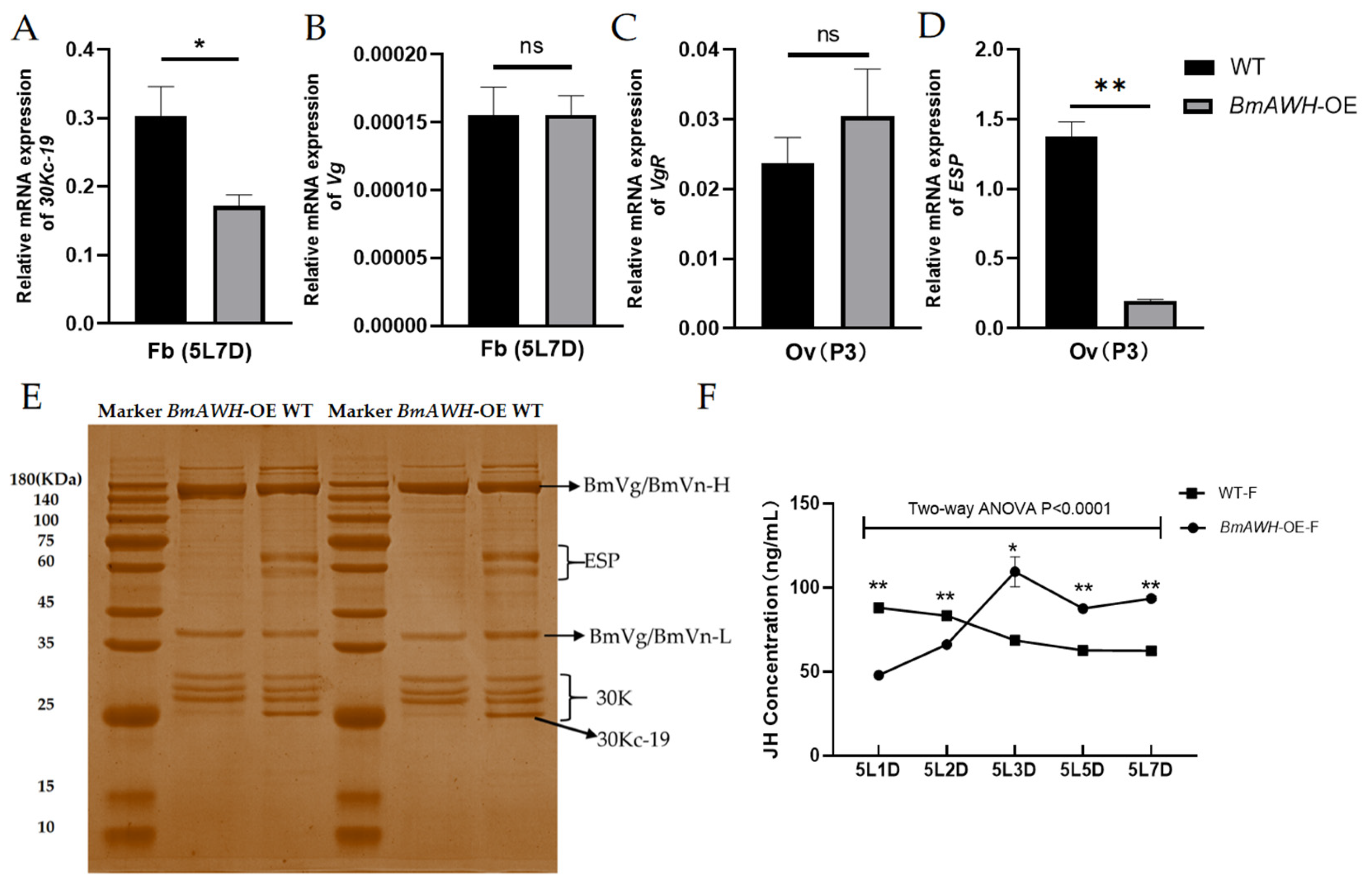
3.3. BmAWH Overexpression Reduced the Size of the Silk Gland and Reduced Cocoon Traits
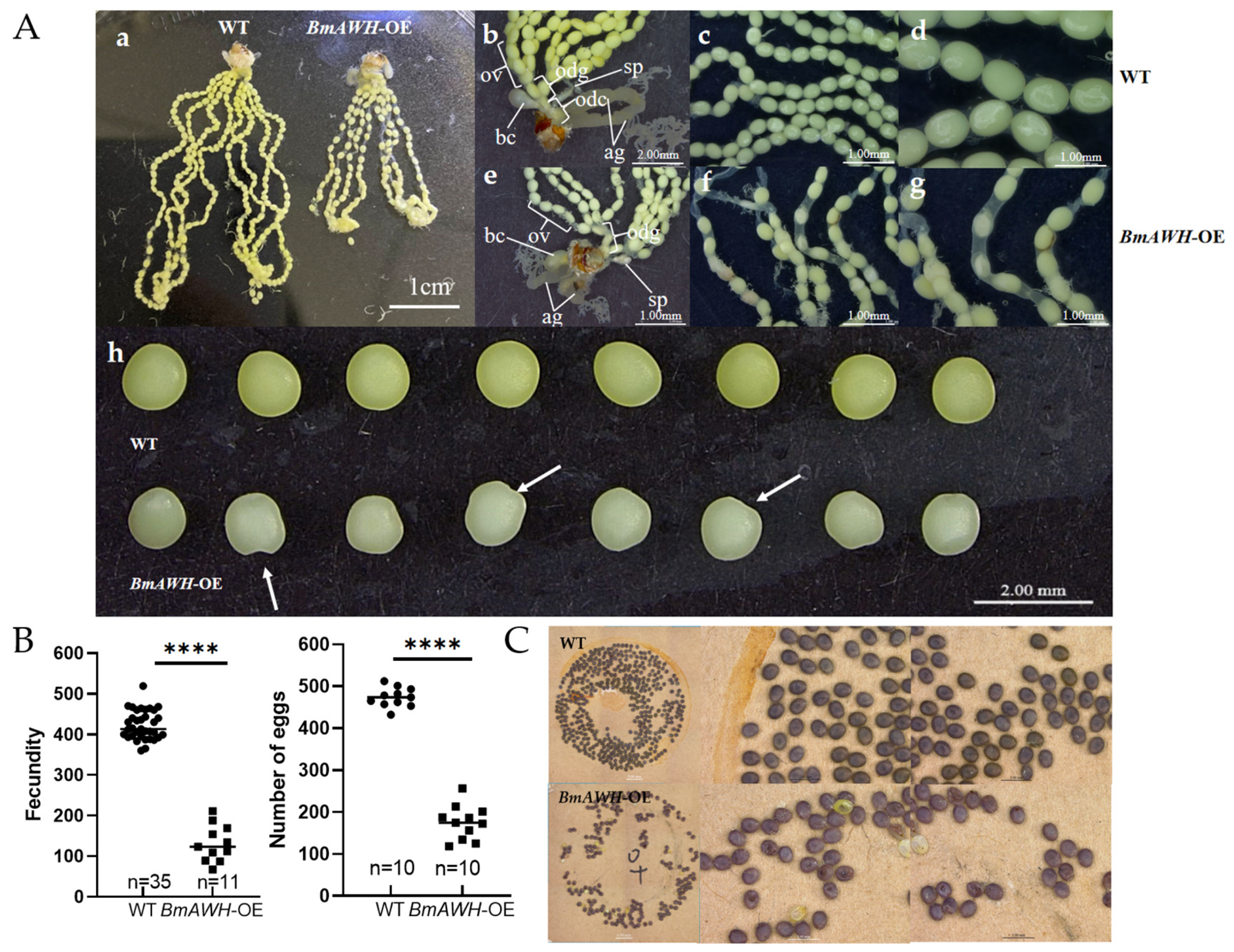
3.4. BmAWH Overexpression Leads to Female Reproductive Defects in Adult Moths
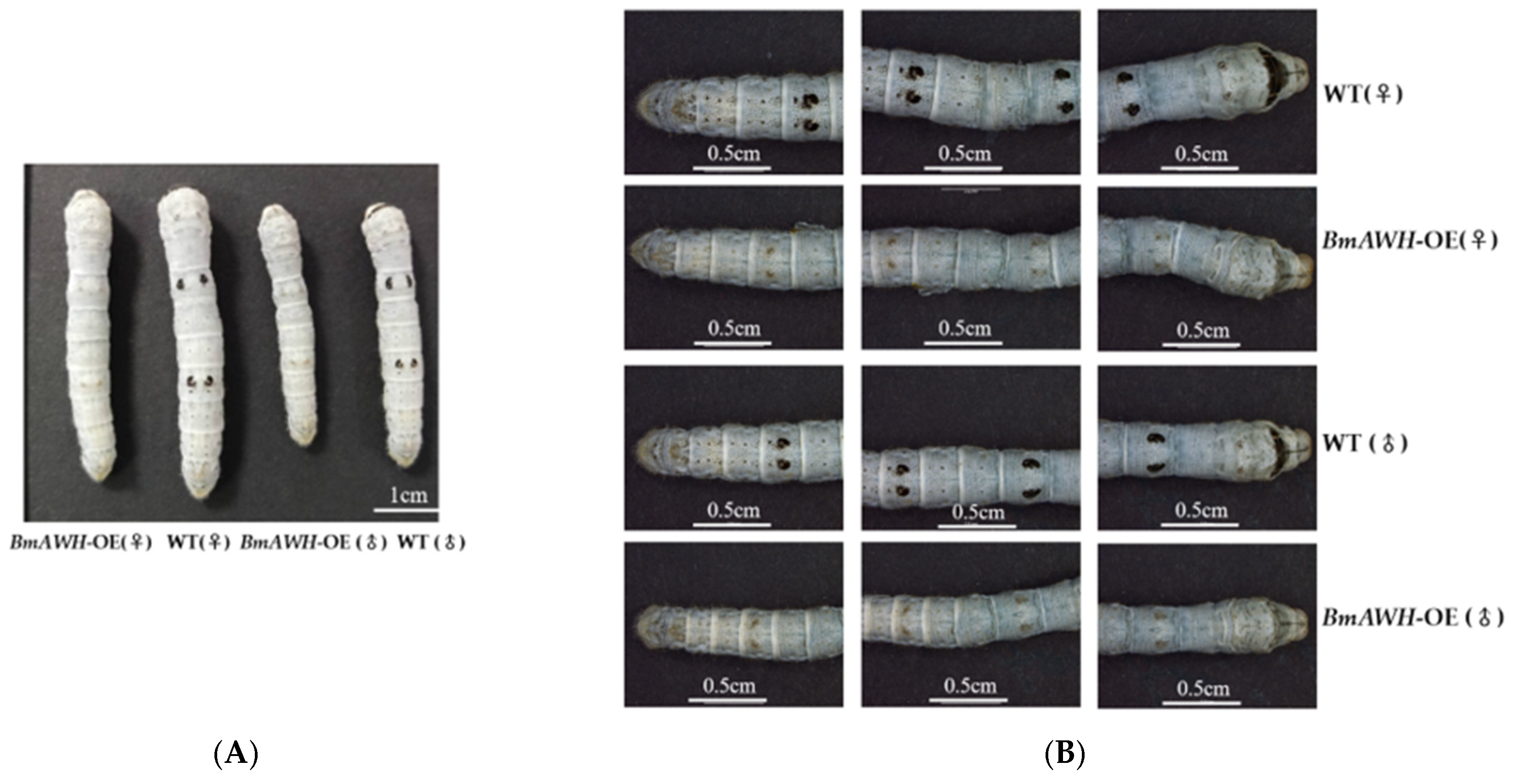
3.5. BmAWH Overexpression Resulted in Loss of Larval Melanin Pigmentation
3.6. Phylogenetic Analysis of BmAWH Across Different Insect Orders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gehring, W.J.; Hiromi, Y. Homeotic genes and the homeobox. Annu. Rev. Genet. 1986, 20, 147–173. [Google Scholar] [CrossRef]
- McGinnis, W.; Garber, R.L.; Wirz, J.; Kuroiwa, A.; Gehring, W.J. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell 1984, 37, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.P.; Weiner, A., J. Structural relationships among genes that control development: Sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proc. Natl. Acad. Sci. USA 1984, 81, 4115–4119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banerjee-Basu, S.; Baxevanis, A.D. Molecular evolution of the homeodomain family of transcription factors. Nucleic Acids Res. 2001, 29, 3258–3269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dawid, I.B.; Toyama, R.; Taira, M. LIM domain proteins. C. R. Acad. Sci. III 1995, 318, 295–306. [Google Scholar] [PubMed]
- Sánchez-García, I.; Rabbitts, T.H. The LIM domain: A new structural motif found in zinc-finger-like proteins. Trends Genet. 1994, 10, 315–320. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, W.; Krumlauf, R. Homeobox genes and axial patterning. Cell 1992, 68, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, I.; Osada, H.; Forster, A.; Rabbitts, T.H. The cysteine-rich LIM domains inhibit DNA binding by the associated homeodomain in Isl-1. EMBO J. 1993, 12, 4243–4250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taira, M.; Hayes, W.P.; Otani, H.; Dawid, I.B. Expression of LIM class homeobox gene Xlim-3 in Xenopus development is limited to neural and neuroendocrine tissues. Dev. Biol. 1993, 159, 245–256. [Google Scholar] [CrossRef] [PubMed]
- German, M.S.; Wang, J.; Chadwick, R.B.; Rutter, W.J. Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: Building a functional insulin minienhancer complex. Genes Dev. 1992, 6, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Grigoriou, M.; Tucker, A.S.; Sharpe, P.T.; Pachnis, V. Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development 1998, 125, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lun, Y.; Ovchinnikov, D.; Kokubo, H.; Oberg, K.C.; Pepicelli, C.V.; Gan, L.; Lee, B.; Johnson, R.L. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat. Genet. 1998, 19, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Ensini, M.; Morton, S.B.; Baldassare, M.; Edlund, T.; Jessell, T.M.; Pfaff, S.L. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell 1994, 79, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Witte, D.P.; Branford, W.W.; Aronow, B.J.; Weinstein, M.; Kaur, S.; Wert, S.; Singh, G.; Schreiner, C.M.; Whitsett, J.A.; et al. Gsh-4 encodes a LIM-type homeodomain, is expressed in the developing central nervous system and is required for early postnatal survival. EMBO J. 1994, 13, 2876–2885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toyama, R.; Curtiss, P.E.; Otani, H.; Kimura, M.; Dawid, I.B.; Taira, M. The LIM class homeobox gene lim5: Implied role in CNS patterning in Xenopus and zebrafish. Dev. Biol. 1995, 170, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.D.; Abduljabbar, D.; Guixeras, A.; Fraguas, S.; Cebrià, F. LIM-HD transcription factors control axial patterning and specify distinct neuronal and intestinal cell identities in planarians. Open Biol. 2023, 13, 230327. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Modi, S.D.D. LIM Homeodomain (LIM-HD) genes and their co-regulators in developing reproductive system and disorders of sex development. Sex Dev. 2022, 16, 147–161. [Google Scholar] [CrossRef]
- Robert, O.; Westphal, H. Functions of LIM-homeobox genes. Trends Genet. 2000, 16, 75–83. [Google Scholar] [CrossRef]
- Kimoto, M.; Tsubota, T.; Uchino, K.; Sezutsu, H.; Takiya, S. LIM-homeodomain transcription factor Awh is a key component activating all three fibroin genes, fibH, fibL and fhx, in the silk gland of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2015, 56, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Takiya, S.; Tsubota, T.; Kimoto, M. Regulation of silk genes by hox and homeodomain proteins in the terminal differentiated silk gland of the Silkworm Bombyx mori. J. Dev. Biol. 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curtiss, J.; Heilig, J.S. Arrowhead encodes a LIM homeodomain protein that distinguishes subsets of Drosophila imaginal cells. Dev. Biol. 1997, 190, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, J.; Heilig, J.S. Establishment of Drosophila imaginal precursor cells is controlled by the Arrowhead gene. Development 1995, 121, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Fu, G.; Jin, L.; Guo, Q.; Li, Z.; Niu, B.; Meng, Z.; Morrison, N.I.; Alphey, L.; Huang, Y. Transgene-based, female-specific lethality system for genetic sexing of the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 2013, 110, 6766–6770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamura, T.; Thibert, C.; Royer, C.; Kanda, T.; Eappen, A.; Kamba, M.; Kômoto, N.; Thomas, J.-L.; Mauchamp, B.; Chavancy, G.; et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat. Biotechnol. 2000, 18, 81–84. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Pan, Y.; Shen, J.; Yang, L.; Zhan, C.; Liang, S.; Tai, S.; Wan, L.; Li, T.; Cheng, T.; et al. SilkMeta: A comprehensive platform for sharing and exploiting pan-genomic and multi-omic silkworm data. Nucleic Acids Res. 2024, 52, D1024–D1032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Zhao, P.; Liu, H.; Dong, Z.; Yang, Q.; Wang, D.; Xia, Q. The synthesis, transportation and degradation of BmLP3 and BmLP7, two highly homologous Bombyx mori 30K proteins. Insect. Biochem. Mol. Biol. 2012, 42, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Futahashi, R.; Banno, Y.; Fujiwara, H. Caterpillar color patterns are determined by a two-phase melanin gene prepatterning process: New evidence from tan and laccase2. Evol. Dev. 2010, 12, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Bello, B.; Couble, P. Specific expression of a silk-encoding gene of Bombyx in the anterior salivary gland of Drosophila. Nature 1990, 346, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Takasu, Y.; Hata, T.; Uchino, K.; Zhang, Q. Identification of Ser2 proteins as major sericin components in the non-cocoon silk of Bombyx mori. Insect Biochem. Mol Biol. 2010, 40, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Novitski, E. The Genetics and Biology of Drosophila; Academic Press: London, UK; New York, NY, USA, 1976. [Google Scholar]
- Andrew, D.J.; Henderson, K.D.; Seshaiah, P. Salivary gland development in Drosophila melanogaster. Mech. Dev. 2000, 92, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Henderson, K.D.; Andrew, D.J. Regulation and function of Scr, exd, and hth in the Drosophila salivary gland. Dev. Biol. 2000, 217, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Sun, X.; Lu, R.; Qu, Z.; Ding, X.; Dai, T.; Qiu, J.; Tan, Y.; Zhu, R.; Pan, Z.; et al. The LIM domain protein BmFHL2 inhibits egg production in female silkworm, Bombyx mori. Cells 2023, 12, 452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, R.; Li, S.; Liu, J.; Wu, H.; Yao, G.; Sun, Y.; Chen, Z.J.; Li, W.; Du, Y. Up-regulated FHL2 inhibits ovulation through interacting with androgen receptor and ERK1/2 in polycystic ovary syndrome. EBioMedicine 2020, 52, 102635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Li, Z.; Zhong, Z.; Li, J.; Chen, Q.; Gao, R.; Zhou, X.; Zhang, Y.; Yang, C.; Pan, H. Silencing of LIM homeodomain transcription factor 1 alpha (Lmx1a) gene caused larval molting failure and adult reproductive deficiency in Henosepilachna vigintioctopunctata. Insect Sci. 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Futahashi, R.; Okamoto, S.; Kawasaki, H.; Zhong, Y.S.; Iwanaga, M.; Mita, K.; Fujiwara, H. Genome-wide identification of cuticular protein genes in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2008, 38, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Qiao, L.; Luo, J.; Ding, X.; Wu, S. The evolution and genetics of lepidopteran egg and caterpillar coloration. Curr. Opin. Genet. Dev. 2021, 69, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tong, X.; Peng, C.; Luo, J.; Zhang, C.; Lu, K.; Li, C.; Ding, X.; Duan, X.; Lu, Y.; et al. The BTB-ZF gene Bm-mamo regulates pigmentation in silkworm caterpillars. eLife 2024, 8, RP90795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stearns, F.W. One hundred years of pleiotropy: A retrospective. Genetics 2011, 187, 355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Q.; Zhao, Y. The diverse biofunctions of LIM domain proteins: Determined by subcellular localization and protein-protein interaction. Biol. Cell 2007, 99, 489–502. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idris, N.F.B.; Hou, C.; Liu, Z.; Liu, L.; Yang, C.; Yang, Z.; Hu, H.; Dai, F.; Tong, X. Overexpression of LIM Homeodomain Gene Arrowhead Induces Pleiotropic Developmental Alterations in the Silkworm, Bombyx mori. Biology 2025, 14, 1248. https://doi.org/10.3390/biology14091248
Idris NFB, Hou C, Liu Z, Liu L, Yang C, Yang Z, Hu H, Dai F, Tong X. Overexpression of LIM Homeodomain Gene Arrowhead Induces Pleiotropic Developmental Alterations in the Silkworm, Bombyx mori. Biology. 2025; 14(9):1248. https://doi.org/10.3390/biology14091248
Chicago/Turabian StyleIdris, Nur Fazleen Binti, Chunping Hou, Zhongyi Liu, Lulu Liu, Chunyan Yang, Zongmeng Yang, Hai Hu, Fangyin Dai, and Xiaoling Tong. 2025. "Overexpression of LIM Homeodomain Gene Arrowhead Induces Pleiotropic Developmental Alterations in the Silkworm, Bombyx mori" Biology 14, no. 9: 1248. https://doi.org/10.3390/biology14091248
APA StyleIdris, N. F. B., Hou, C., Liu, Z., Liu, L., Yang, C., Yang, Z., Hu, H., Dai, F., & Tong, X. (2025). Overexpression of LIM Homeodomain Gene Arrowhead Induces Pleiotropic Developmental Alterations in the Silkworm, Bombyx mori. Biology, 14(9), 1248. https://doi.org/10.3390/biology14091248





