Dietary Glycine and Methyl Donors Remodel Gut Microbiota to Enhance Collagen Synthesis in Sea Cucumber (Apostichopus japonicus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Sample Processing
2.3. Collagen Content Determination
2.4. Hydrolyzed Amino Acid Content Determination
2.5. High-Throughput Sequencing
2.6. Data Processing
2.7. Statistical Analysis
3. Results
3.1. Collagen Content in the Body Wall of Sea Cucumber
3.2. Hydrolyzed Amino Acid Content of Sea Cucumber Gut
3.3. Structural and Functional Characteristics of Gut Bacterial Community
3.3.1. Analysis of High-Throughput Sequencing Results
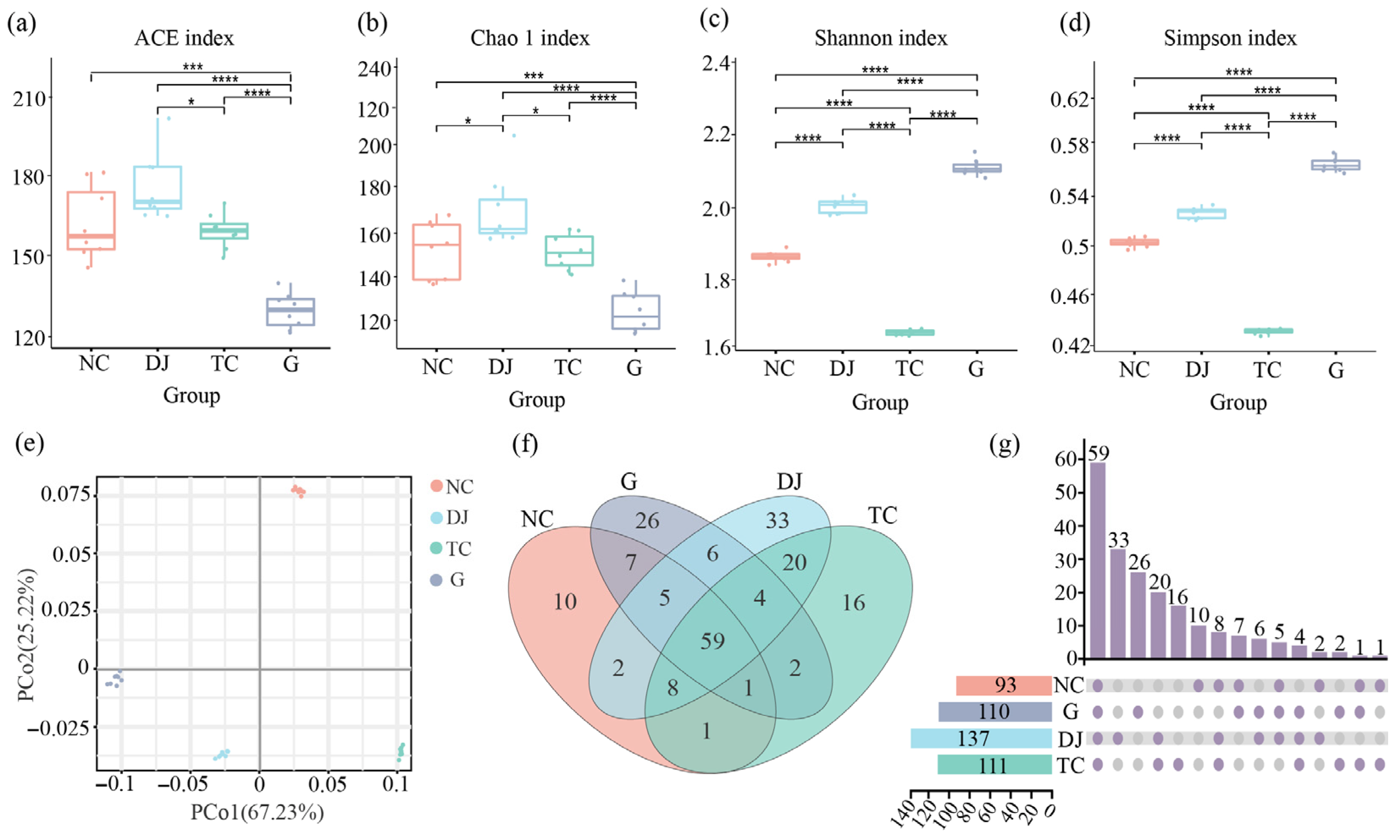
3.3.2. Diversity Analysis of Bacterial Community
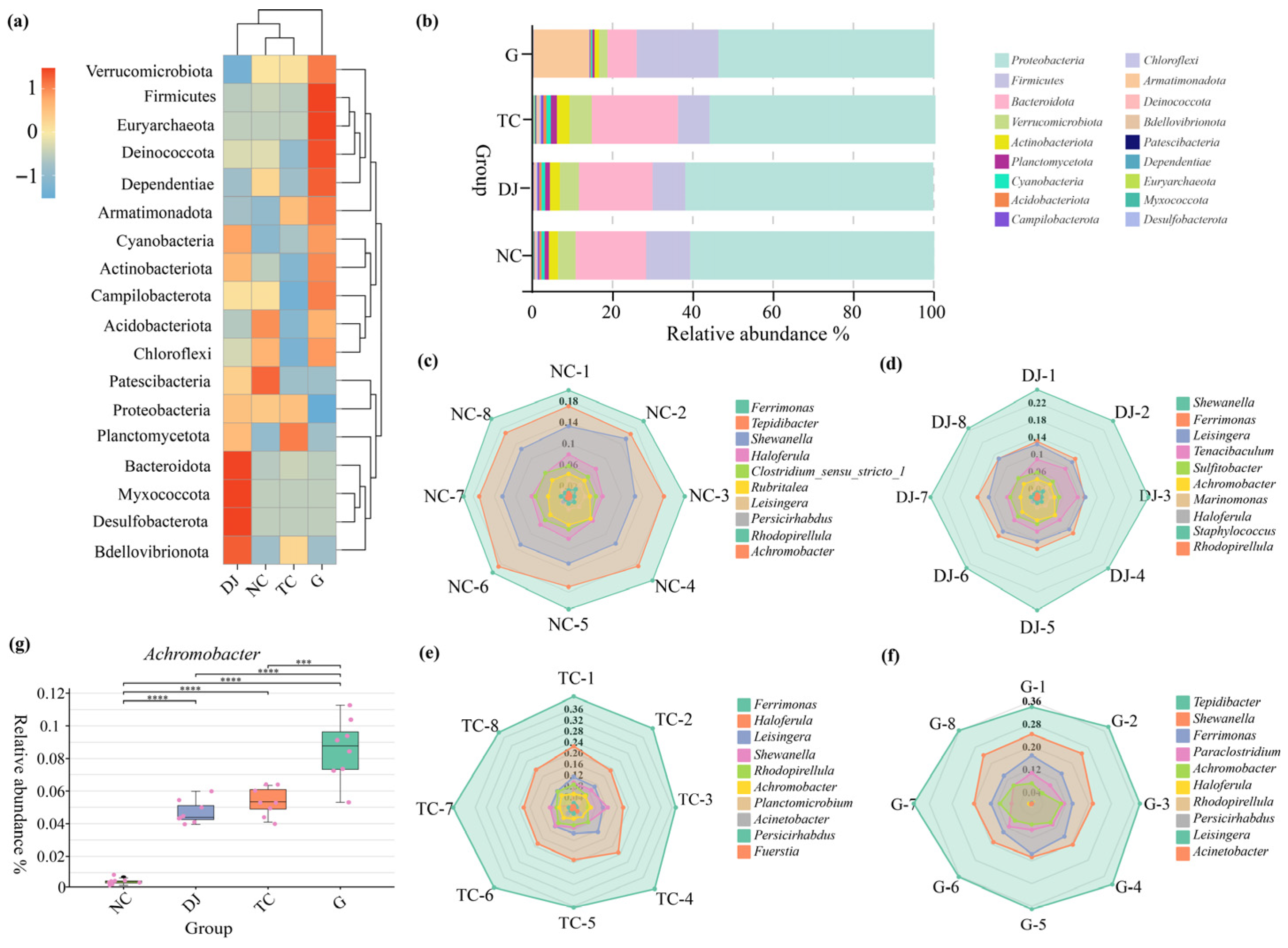
3.3.3. Structural Characteristics of Bacterial Community
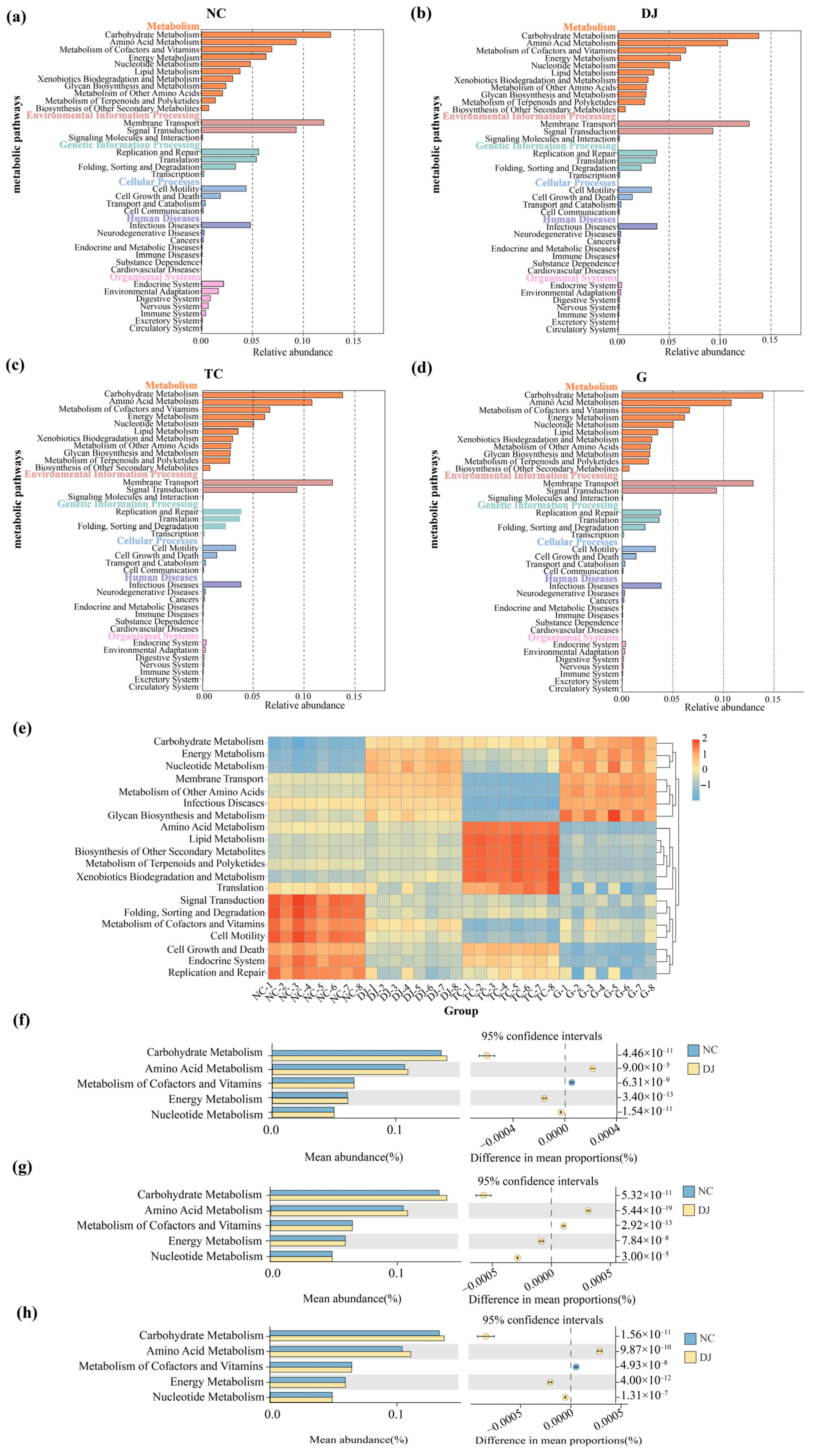
3.3.4. Functional Characteristics of Bacterial Community
3.4. Correlation Analysis Between Gut Bacterial Community and Body Wall Collagen in Sea Cucumber
4. Discussion
4.1. Dietary Supplementation Modulates the Gut Bacterial Community
4.2. Gut Microbiota Modulates Hydrolyzed Amino Acid Synthesis
4.3. Hydrolyzed Glycine Promotes Collagen Synthesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Li, Z.; Qi, Y.; Guo, Z.; Lin, Y.; Li, W.; Zhao, Q. Proximate composition and nutritional quality of deep sea growth sea cucumbers (Stichopus japonicus) from different origins. J. Sci. Food Agr. 2006, 96, 2378–2383. [Google Scholar] [CrossRef]
- Tian, M.; Xue, C.; Chang, Y.; Shen, J.; Zhang, Y.; Li, Z.; Wang, Y. Collagen fibrils of sea cucumber (Apostichopus japonicus) are heterotypic. Food Chem. 2020, 316, 126272. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Collier, F.; Mohebbi, M.; Pasco, J.A.; Shivappa, N.; Hébert, J.R.; Loughman, A. The associations of butyrate-producing bacteria of the gut microbiome with diet quality and muscle health. Gut Microbiome 2021, 2, e2. [Google Scholar] [CrossRef]
- Omar, A.A.; Marzouk, M.S.; Mahfouz, N.B.; Massoud, A.M.; Shukry, M.; Farrag, F.; Moustafa, E.M. Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performance. Open Vet. J. 2024, 14, 144. [Google Scholar] [CrossRef]
- Leeper, A.; Sauphar, C.; Berlizot, B.; Ladurée, G.; Koppe, W.; Knobloch, S.; Skírnisdóttir, S.; Björnsdóttir, R.; Øverland, M.; Benhaïm, D. Enhancement of soybean meal alters gut microbiome and influences behavior of farmed atlantic salmon (Salmo salar). Animals 2023, 13, 2591. [Google Scholar] [CrossRef]
- Cardona, E.; Gueguen, Y.; Magré, K.; Lorgeoux, B.; Piquemal, D.; Pierrat, F.; Saulnier, D. Bacterial community characterization of water and gut of the shrimp Litopenaeus stylirostris in a biofloc system. BMC Microbio. 2016, 16, 157. [Google Scholar]
- Dai, Z.L.; Wu, G.; Zhu, W.Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef] [PubMed]
- Diwan, A.D.; Harke, S.N.; Panche, A.N. Host-microbiome interaction in fish and shellfish: An overview. Fish Shellfish Immune. Rep. 2023, 4, 100091. [Google Scholar] [CrossRef]
- Wang, L.; Pei, H.; Xing, T.; Chen, D.; Chen, Y.; Hao, Z.; Ding, J. Gut bacteria and host metabolism: The keys to sea cucumber (Apostichopus japonicus) quality traits. Food Chem. 2025, 482, 144178. [Google Scholar] [CrossRef]
- Lu, J.; Tao, X.; Luo, J.; Zhu, T.; Jiao, L.; Sun, P.; Zhou, Q.; Tocher, D.R.; Jin, M. Dietary choline activates the Ampk/Srebp signaling pathway and decreases lipid levels in Pacific white shrimp (Litopenaeus vannamei). Anim. Nutr. 2023, 15, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.F.; Feng, L.; Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Flesh shear force, cooking loss, muscle antioxidant status and relative expression of signaling molecules (Nrf2, Keap1, TOR, and CK2) and their target genes in Young Grass Carp (Ctenopharyngodon idella) muscle fed with graded levels of choline. PLoS ONE 2015, 10, e0142915. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Patra, A.; Mandal, A.; Mondal, N.S.; Dey, S.; Mirjan, S.K.; Ghosh, A.R. Alterations in biochemical parameters of fish species under choline administration directly into the pond water in a semi-intensive fish farming system: A comparative study. Int. J. Fish. Aquat. Stud. 2020, 8, 8–15. [Google Scholar] [CrossRef]
- Hansen, A.K.G.; Kortner, T.M.; Krasnov, A.; Björkhem, I.; Penn, M.; Krogdahl, Å. Choline supplementation prevents diet induced gut mucosa lipid accumulation in post-smolt Atlantic salmon (Salmo salar L.). BMC Vet Res. 2020, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shen, Y.; Pan, T.; Zhu, T.; Li, X.; Xu, F.; Betancor, M.B.; Jiao, L.; Tocher, D.R.; Zhou, Q. Dietary betaine mitigates hepatic steatosis and inflammation induced by a high-fat-fiet by modulating the Sirt1/Srebp-1/Pparα pathway in Juvenile Black Seabream (Acanthopagrus schlegelii). Front. Immunol. 2021, 12, 694720. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Zhao, M.; Du, X.; Fan, H.; Fu, Y.; Gong, Z.; Miao, S. Betaine alleviates high-fat diet induced excessive lipid deposition in Gibel Carp hepatopancreas and L8824 cells by enhancing VLDL secretion through HNF4α/MTTP pathway. Aquac. Nutr. 2024, 2024, 8886237. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Nassef, E.; Hegazi, E.; Bakr, A.N.; Moustafa, E.M.; Abdo, W.; Elbialy, Z.I. The modulatory effect of dietary betaine on intestinal absorptive capacity, lipogenesis and expression of lipid metabolism-and growth-related genes in Nile tilapia fed on soybean meal-based diet. Slov. Vet Res. 2019, 56, 22. [Google Scholar] [CrossRef]
- Suehs, B.A.; Gatlin, D.M., 3rd; Wu, G. Glycine nutrition and biochemistry from an aquaculture perspective. Anim. Front. 2024, 14, 17–23. [Google Scholar] [CrossRef]
- He, W.; Li, X.; Wu, G. Dietary glycine supplementation enhances syntheses of creatine and glutathione by tissues of hybrid striped bass (Morone saxatilis ♀ × Morone chrysops ♂) fed soybean meal-based diets. J. Anim. Sci. Biotechnol. 2024, 15, 67. [Google Scholar] [CrossRef]
- Yi, L.; Liu, J.; Yang, H.; Mo, A.; Zhai, Y.; Wang, S.; Yuan, Y. Effects of dietary glycinin on oxidative damage, apoptosis and tight junction in the gut of juvenile Hybrid Yellow Catfish, Pelteobagrus fulvidraco ♀ × Pelteobaggrus vachelli ♂. Int. J. Mol. Sci. 2022, 23, 11198. [Google Scholar] [CrossRef]
- Richard, L.; Vachot, C.; Surget, A.; Rigolet, V.; Kaushik, S.J.; Geurden, I. The effect of choline and cystine on the utilisation of methionine for protein accretion, remethylation and trans-sulfuration in juvenile shrimp Penaeus monodon. Br. J. Nutr. 2011, 106, 825–835. [Google Scholar] [CrossRef]
- Song, T.; Liang, X.; Wang, H.; Xue, M.; Wang, J. Gut mi-crobiota-bile acid crosstalk and metabolic fatty liver in spotted seabass (Lateolabrax maculatus): The role of a cholesterol, taurine and glycine supplement. Anim. Nutr. 2024, 17, 87–99. [Google Scholar] [CrossRef]
- Yamana, Y.; Hamano, T.; Yamamoto, K.I. Anesthetizer of the adult sea cucumber Apostichopus japonicus. Bull. Jpn. Soc. Sci. Fish. 2005, 71, 299–306. [Google Scholar] [CrossRef][Green Version]
- Nigro, L.M.; Elling, F.J.; Hinrichs, K.U.; Joye, S.B.; Teske, A. Microbial ecology and biogeochemistry of hypersaline sediments in Orca Basin. PLoS ONE 2020, 15, e0231676. [Google Scholar] [CrossRef]
- Xie, Z.; Du, J.; Gan, M.; Zhou, C.; Li, M.; Liu, C.; Shen, L. Short-term dietary choline supplementation alters the gut microbiota and liver metabolism of finishing pigs. Front. Microbiol. 2023, 14, 1266042. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Fang, S.; Yang, X.; Feng, J. Betaine improves intestinal functions by enhancing digestive enzymes, ameliorating intestinal morphology, and enriching intestinal microbiota in high-salt stressed rats. Nutrients 2018, 10, 907. [Google Scholar] [CrossRef]
- Al Wahid, Z.; Pradista, L.A.; Prastowo, S.; Ratriyanto, A. Betaine alter SCFA-producing bacteria population in laying pullet reared in tropical climate. IOP Conf. Ser. Earth Environ. Sci. 2024, 1341, 012060. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Wu, H.; Zhu, H.; Liu, C.; Hou, Y.; Dai, B.; Liu, X.; Liu, Y. Glycine relieves intestinal injury by maintaining mTOR signaling and suppressing AMPK, TLR4, and NOD signaling in weaned piglets after lipopolysaccharide challenge. Int. J. Mol. Sci. 2018, 19, 1980. [Google Scholar] [CrossRef] [PubMed]
- Marzec-Grządziel, A.; Gałązka, A. Sequencing of the whole genome of a bacterium of the genus Achromobacter reveals its potential for xenobiotics biodegradation. Agriculture 2023, 13, 1519. [Google Scholar] [CrossRef]
- Obeid, R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 2013, 5, 3481–3495. [Google Scholar] [CrossRef]
- Deng, C.; Zheng, J.; Zhou, H.; You, J.; Li, G. Dietary glycine supplementation prevents heat stress-induced impairment of antioxidant status and intestinal barrier function in broilers. Poult. Sci. 2023, 102, 102408. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, S.; Zhao, Y.; Wang, H.; Feng, J. Dietary betaine addition promotes hepatic cholesterol synthesis, bile acid conversion, and export in rats. Nutrients 2020, 12, 1399. [Google Scholar] [CrossRef]
- Jeukens, J.; Freschi, L.; Vincent, A.T.; Emond-Rheault, J.G.; Kukavica-Ibrulj, I.; Charette, S.J.; Levesque, R.C. A pan-genomic approach to understand the basis of host adaptation in Achromobacter. Genome Biol. Evol. 2017, 9, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Ketkar, O.A.; Irfan, S.; Rana, V.; Rahi, P.; Deshmukh, R.; Kaur, J.; Dhar, H. Genomic insights into omega-3 polyunsaturated fatty acid producing Shewanella sp. N2AIL from fish gut. Biology 2022, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.; Jung, J.; Kim, J.M.; Bayburt, H.; Han, D.M.; Jeon, C.O. Shewanella phaeophyticola sp. nov. and Vibrio algarum sp. nov., isolated from marine brown algae. Int. J. Syst. Evol. Microbiol. 2024, 74, 006378. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Alves, M.N.; Neto, S.E.; Alves, A.S.; Fonseca, B.M.; Carrêlo, A.; Pacheco, I.; Paquete, C.M.; Soares, C.M.; Louro, R.O. Characterization of the periplasmic redox network that sustains the versatile anaerobic metabolism of Shewanella oneidensis MR-1. Front. Microbiol. 2015, 6, 665. [Google Scholar] [CrossRef]
- Qasim, M.S.; Lampi, M.; Heinonen, M.K.; Garrido-Zabala, B.; Bamford, D.H.; Käkelä, R.; Roine, E.; Sarin, L.P. Cold-Active Shewanella glacialimarina TZS-4T nov. features a temperature-dependent fatty acid profile and putative sialic acid metabolism. Front. Microbiol. 2021, 12, 737641. [Google Scholar] [CrossRef]
- Nolan, M.; Sikorski, J.; Davenport, K.; Lucas, S.; Del Rio, T.G.; Tice, H.; Cheng, J.F.; Goodwin, L.; Pitluck, S.; Liolios, K.; et al. Complete genome sequence of Ferrimonas balearica type strain (PAT). Stand. Genom. Sci. 2010, 3, 174–182. [Google Scholar] [CrossRef]
- Ji, S.; Zhao, R.; Li, Z.; Li, B.; Shi, X.; Zhang, X.H. Ferrimonas sediminum sp. nov., isolated from coastal sediment of an amphioxus breeding zone. Int. J. Syst. Eevo. Microbiol. 2013, 63 Pt 3, 977–981. [Google Scholar] [CrossRef]
- Ratriyanto, A.; Mosenthin, R.; Bauer, E.; Eklund, M. Metabolic, osmoregulatory and nutritional functions of betaine in monogastric animals. Asian-Australas. J. Anim. Sci. 2009, 22, 1461–1476. [Google Scholar] [CrossRef]
- Abdelsattar, M.M.; Abd El-Ati, M.N.; Hussein, A.M.A.; Saleem, A.M. Impact of betaine as a feed additive on livestock performance, carcass characteristics and meat quality-a review. SVU-Internat. J. Agricult. Sci. 2019, 1, 33–42. [Google Scholar]
- Urios, L.; Cueff, V.; Pignet, P.; Barbier, G. Tepidibacter formicigenes sp. nov., a novel spore-forming bacterium isolated from a Mid-Atlantic Ridge hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2004, 54, 439–443. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.; Yi, S.; Kim, W.H.; Kim, Y.; Namgoong, B.; Choe, A.; Cho, G.; Shin, J.; Park, Y.; et al. Red ginseng dietary fiber shows prebiotic potential by modulating gut microbiota in dogs. Microbiol. Spectr. 2023, 11, e0094923. [Google Scholar] [CrossRef]
- Tan, H.Q.; Wu, X.Y.; Zhang, X.Q.; Wu, M.; Zhu, X.F. Tepidibacter mesophilus sp. nov., a mesophilic fermentative anaerobe isolated from soil polluted by crude oil, and emended description of the genus Tepidibacter. Int. J. Syst. Evol. Microbiol. 2012, 62 Pt 1, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, R.T.; Drake, H.L. Glycine synthase of the purinolytic bacterium, Clostridium acidiurici. Purification of the glycine-CO2 exchange system. J. Biol. Chem. 1984, 259, 6085–6089. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Quan, G.; Jiang, X.; Yang, Y.; Ding, X.; Zhang, D.; Wang, X.; Hardwidge, P.R.; Ren, W.; Zhu, G. Effects of metabolites derived from gut microbiota and hosts on pathogens. Frontiers 2018, 8, 314. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Blachier, F. Amino acid-derived bacterial metabolites in the colorectal luminal fluid: Effects on microbial communication, metabolism, physiology, and growth. Microorganisms 2023, 11, 1317. [Google Scholar] [CrossRef] [PubMed]
- Gojda, J.; Cahova, M. Gut microbiota as the link between elevated BCAA serum levels and insulin resistance. Biomolecules 2021, 11, 1414. [Google Scholar] [CrossRef]
- Lowe, C.D.; Mello, L.V.; Samatar, N.; Martin, L.E.; Montagnes, D.J.; Watts, P.C. The transcriptome of the novel dinoflagellate Oxyrrhis marina (Alveolata: Dinophyceae): Response to salinity examined by 454 sequencing. BMC. Genom. 2011, 12, 519. [Google Scholar] [CrossRef]
- Bae, S.H.; Han, H.W.; Moon, J. Functional analysis of the molecular interactions of TATA box-containing genes and essential genes. PLoS ONE 2015, 10, e0120848. [Google Scholar] [CrossRef]
- Woźniak, M.; Tyśkiewicz, R.; Siebielec, S.; Gałązka, A.; Jaroszuk-Ściseł, J. Metabolic profiling of endophytic bacteria in relation to their potential application as components of multi-task biopreparations. Microb. Ecol. 2023, 86, 2527–2540. [Google Scholar] [CrossRef]
- Jakovljević, V.D.; Radojević, I.D.; Grujić, S.M.; Ostojić, A.M. Response of selected microbial strains and their consortia to the presence of automobile paints: Biofilm growth, matrix protein content and hydrolytic enzyme activity. Saudi J. Biol. Sci. 2022, 29, 103347. [Google Scholar] [CrossRef] [PubMed]
- Shieh, H.S. Aerobic degradation of choline. i. fermentation of choline by a marine bacterium Achromobacter cholinophagum n. sp. Can. J. Microbiol. 1964, 10, 837–842. [Google Scholar] [CrossRef]
- Shieh, H.S. Further studies on the oxidation of betaine by a marine bacterium, Achromobacter cholinophagum. Can. J. Microbiol. 1966, 2, 299–302. [Google Scholar] [CrossRef]
- Magnusson, M.; Wang, T.J.; Clish, C.; Engström, G.; Nilsson, P.; Gerszten, R.E.; Melander, O. Dimethylglycine deficiency and the development of diabetes. Diabetes 2015, 64, 3010–3016. [Google Scholar] [CrossRef]
- Lecroisey, A.; Keil, B. Differences in the degradation of native collagen by two microbial collagenases. Biochem. J. 1979, 179, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Cronlund, A.L.; Woychik, J.H. Solubilization of collagen in restructured beef with collagenases and α-Amylase. J. Food Sci. 1987, 52, 857–860. [Google Scholar] [CrossRef]
- de Paz-Lugo, P.; Lupiáñez, J.A.; Meléndez-Hevia, E. High glycine concentration increases collagen synthesis by articular chondrocytes in vitro: Acute glycine deficiency could be an important cause of osteoarthritis. Amino Acids 2018, 50, 1357–1365. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Spencer, T.E. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious beneficial effect of nonessential amino acid, glycine: A review. Oxid. Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Posey, E.A.; Steele, C.C.; Savell, J.W.; Bazer, F.W.; Wu, G. Dietary glycine supplementation enhances postweaning growth and meat quality of pigs with intrauterine growth restriction. J. Anim. Sci. 2023, 101, skad354. [Google Scholar] [CrossRef] [PubMed]
- Corzo, A.; Fritts, C.A.; Kidd, M.T.; Kerr, B.J. Response of broiler chicks to essential and non-essential amino acid supplementation of low crude protein diets. Anim. Feed Sci. Technol. 2005, 118, 319–327. [Google Scholar] [CrossRef]
- Ospina-Rojas, I.C.; Murakami, A.E.; Oliveira, C.A.L.; Guerra, A.F.Q.G. Supplemental glycine and threonine effects on performance, intestinal mucosa development, and nutrient utilization of growing broiler chickens. Poult. Sci. 2013, 92, 2724–2731. [Google Scholar] [CrossRef]
- Xie, S.W.; Tian, L.X.; Jin, Y.; Yang, H.J.; Liang, G.Y.; Liu, Y.J. Effect of glycine supplementation on growth performance, body composition and salinity stress of juvenile Pacific white shrimp, Litopenaeus vannamei fed low fishmeal diet. Aquaculture 2014, 418, 159–164. [Google Scholar] [CrossRef]
- Aksnes, A.; Mundheim, H.; Toppe, J.; Albrektsen, S. The effect of dietary hydroxyproline supplementation on salmon (Salmo salar L.) fed high plant protein diets. Aquaculture 2018, 275, 242–249. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Wang, Q.; Mai, K.; Xu, W.; Zhou, H. Hydroxyproline supplementation on the performances of high plant protein source based diets in turbot (Scophthalmus maximus L.). Aquaculture 2014, 433, 476–480. [Google Scholar] [CrossRef]

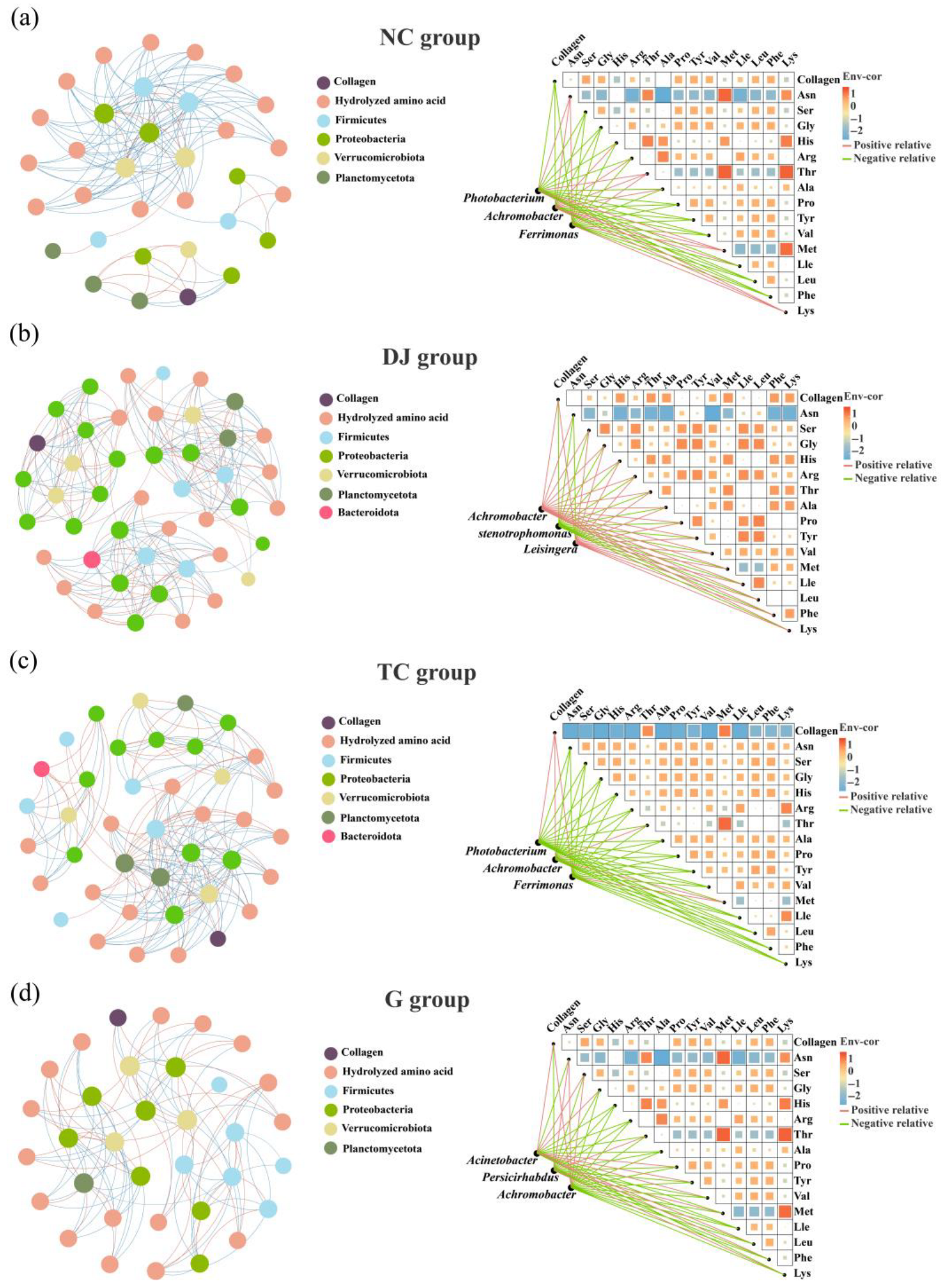
| Network Metrics/Group | DJ | G | NC | TC |
|---|---|---|---|---|
| Number of nodes | 45 | 34 | 33 | 40 |
| Number of edges | 239 | 113 | 129 | 190 |
| Number of positive | 104 | 48 | 29 | 75 |
| Number of negative | 134 | 65 | 100 | 115 |
| Graph Density | 0.24 | 0.201 | 0.244 | 0.244 |
| Network diameter | 4 | 4 | 5 | 5 |
| Average clustering coefficient | 0.844 | 0.844 | 0.847 | 0.871 |
| Average degree | 10.578 | 6.647 | 7.818 | 9.5 |
| Modularity | 0.642 | 0.652 | 0.293 | 0.483 |
| Number of nodes | 45 | 34 | 33 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Pei, H.; Chen, Y.; Liu, A.; Xing, T.; Zhang, H.; Wang, L. Dietary Glycine and Methyl Donors Remodel Gut Microbiota to Enhance Collagen Synthesis in Sea Cucumber (Apostichopus japonicus). Biology 2025, 14, 1246. https://doi.org/10.3390/biology14091246
Chen D, Pei H, Chen Y, Liu A, Xing T, Zhang H, Wang L. Dietary Glycine and Methyl Donors Remodel Gut Microbiota to Enhance Collagen Synthesis in Sea Cucumber (Apostichopus japonicus). Biology. 2025; 14(9):1246. https://doi.org/10.3390/biology14091246
Chicago/Turabian StyleChen, Dongsheng, Honglin Pei, Yuchen Chen, Anzheng Liu, Tengyu Xing, Hai Zhang, and Luo Wang. 2025. "Dietary Glycine and Methyl Donors Remodel Gut Microbiota to Enhance Collagen Synthesis in Sea Cucumber (Apostichopus japonicus)" Biology 14, no. 9: 1246. https://doi.org/10.3390/biology14091246
APA StyleChen, D., Pei, H., Chen, Y., Liu, A., Xing, T., Zhang, H., & Wang, L. (2025). Dietary Glycine and Methyl Donors Remodel Gut Microbiota to Enhance Collagen Synthesis in Sea Cucumber (Apostichopus japonicus). Biology, 14(9), 1246. https://doi.org/10.3390/biology14091246






