Crosstalk Between Intestinal Microbiota and Host Defense Peptides in Fish
Simple Summary
Abstract
1. Introduction
2. Overview of HDPs in Fish
2.1. Definition and Classification
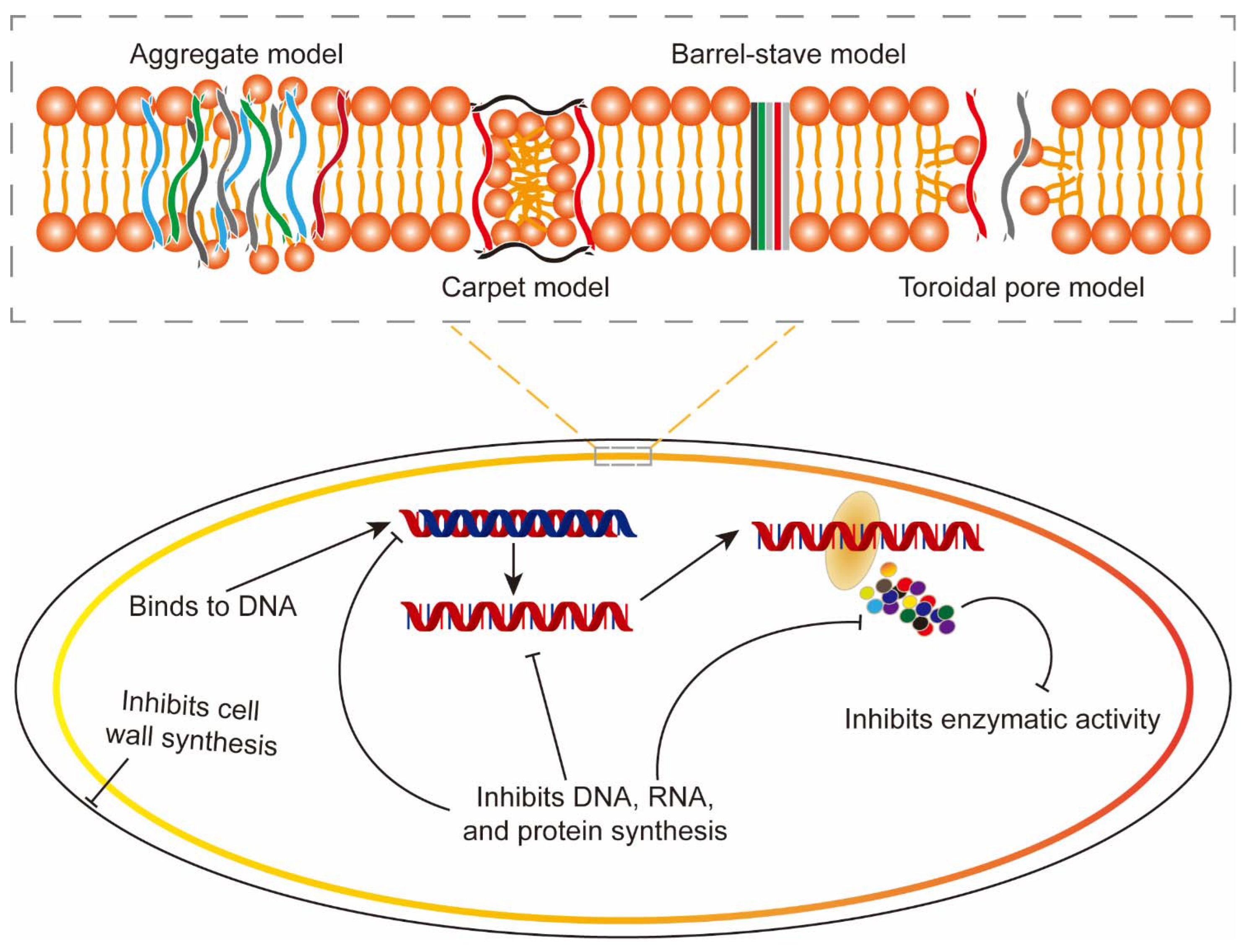
2.2. Mechanisms of Action
3. Intestinal Microbiota
4. Interaction Between HDPs and Intestinal Microbiota
4.1. HDPs-Mediated Intestinal Microbiota
4.2. Feedback Regulation of HDPs Expression by Intestinal Microbiota
4.3. Dynamic Homeostasis in Microbiota and HDP Crosstalk
4.4. Non-Bacterial Intestinal Communities and Knowledge Gaps
5. Applications of the Crosstalk Mechanism in Fish
5.1. Disease Prevention and Control Through Microbiota–HDP Synergy
5.2. Mitigating Environmental Stress
5.3. Precision Regulation Through Targeted Microbiota–HDPs Interventions
5.4. Effects on Growth Performance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; Towards Blue Transformation: Rome, Italy, 2024. [Google Scholar]
- Long, L.N.; Zhang, H.G.; Ni, Q.; Liu, H.; Wu, F.; Wang, X.D. Effects of stocking density on growth, stress, and immune responses of juvenile Chinese sturgeon (Acipenser sinensis) in a recirculating aquaculture system. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2019, 219, 25–34. [Google Scholar] [CrossRef]
- Li, W.H.; Li, D.P.; Yang, Q.S.; Liu, L.; Liu, J.Y.; Lu, J.M.; Wang, Y.; Tang, R.; Li, L.; Zhang, X. Long-term crowding stress induces chronic inflammatory response and declines the immunity of grass carp (Ctenopharyngodon idella). Aquaculture 2023, 577, 739976. [Google Scholar] [CrossRef]
- Seo, J.; Park, J. Does stocking density affect growth performance and hematological parameters of juvenile olive flounder Paralichthys olivaceus in a recirculating aquaculture system? Animals 2023, 13, 44. [Google Scholar] [CrossRef]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: Impact on immune system development and atopic disease. Nat. Rev. Immunol. 2023, 23, 735–748. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Ding, L.G.; Huang, Z.Y.; Xu, H.Y.; Xu, Z. Commensal bacteria-immunity crosstalk shapes mucosal homeostasis in teleost fish. Rev. Aquac. 2021, 13, 2322–2343. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Yang, H.W.; Teame, T.; Ran, C.; Yang, Y.L.; Yao, Y.Y.; Ding, Q.W.; Liu, S.B.; Li, S.K.; Zhang, Z.; et al. Immune disorders induced by improper use of dietary immunostimulants in aquatic animals: Research progress and prospective solutions by targeting gut microbiota. Rev. Aquac. 2023, 16, 608–621. [Google Scholar] [CrossRef]
- Gómez, G.D.; Bálcazar, J.L. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol. Med. Microbiol. 2008, 52, 145–154. [Google Scholar] [CrossRef]
- Wu, S.G.; Pan, M.J.; Zan, Z.Y.; Jakovlic, I.; Zhao, W.S.; Zou, H.; Ringo, E.; Wang, G.T. Regulation of lipid metabolism by gut microbiota in aquatic animals. Rev. Aquac. 2023, 13, 12819. [Google Scholar] [CrossRef]
- Xavier, R.; Severino, R.; Silva, S.M. Signatures of dysbiosis in fish microbiomes in the context of aquaculture. Rev. Aquac. 2023, 26, 12862. [Google Scholar] [CrossRef]
- Law, I.K.M.; Cheng, M.W.; Shih, D.Q.; McGovern, D.P.B.; Koon, H.W. Chapter 3—The Roles of Antimicrobial Peptides in the Regulation of Gastrointestinal Microbiota and Innate Immunity. In Antimicrobial Peptides in Gastrointestinal Diseases; Cho, C.H., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 35–60. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Xia, H.Q.; Yang, H.L.; Wang, Y.L.; Zou, W.C. TLR2 signaling may play a key role in the probiotic modulation of intestinal microbiota in grouper Epinephelus coioides. Aquaculture 2014, 430, 50–56. [Google Scholar] [CrossRef]
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef]
- Puertolas-Balint, F.; Schroeder, B.O. Intestinal α-defensins play a minor role in modulating the small intestinal microbiota composition as compared to diet. Microbiol. Spectr. 2023, 11, 19. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Tonetti, F.R.; Eguileor, A.; Llorente, C. Goblet cells: Guardians of gut immunity and their role in gastrointestinal diseases. eGastroenterology 2024, 2, e100098. [Google Scholar] [CrossRef]
- Gierynska, M.; Szulc-Dabrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota-A Mutual Relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Yang, H.L.; Cai, G.H.; Li, S.; Ye, J.D.; Zhang, C.X.; Sun, Y.Z. LTA and PGN from Bacillus siamensis can alleviate soybean meal-induced enteritis and microbiota dysbiosis in Lateolabrax maculatus. Fish Shellfish Immunol. 2024, 149, 109618. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.G.; Xiao, W.; Zhou, C.M.; Pu, Q.Q.; Deng, X.; Lan, L.F.; Liang, H.H.; Song, X.R.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Duan, Z.X.; Song, J.Q.; Zhang, M.R.; Zhang, Z.; Li, N.; Fu, Y.Q.; Sun, Z.; Lu, T.C.; Li, S.Y.; Cao, M.Y.; et al. Effects of Yersinia pseudotuberculosis outer membrane vesicles on Pseudomonas aeruginosa antigens immune response. PLoS ONE 2024, 19, e0310652. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Bhagwat, P.; Singh, S.; Pillai, S. A review on the diversity of antimicrobial peptides and genome mining strategies for their prediction. Biochimie 2024, 227, 99–115. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Z.Y.; Xia, B.B.; Zhang, Y.W.; Liu, X.L.; Yu, Y.; Tang, N.; Tong, X.M.; Wang, M.; Ye, X.; et al. Identification of antimicrobial peptides from the human gut microbiome using deep learning. Nat. Biotechnol. 2022, 40, 921–931. [Google Scholar] [CrossRef]
- Lin, S.H.; Chen, X.; Chen, H.M.; Cai, X.X.; Chen, X.; Wang, S.Y. The Bioprospecting of Microbial-Derived Antimicrobial Peptides for Sustainable Agriculture. Engineering 2023, 27, 222–233. [Google Scholar] [CrossRef]
- Thuluz, M.M.; Paul, P.J.; Alberto, S.M.; Dolores, H.R.; Eliel, R.M.; Guiomar, M.L. Antimicrobial Activity and Peptidomic Analysis of Halotolerant Lactiplantibacillus plantarum CH. Probiotics Antimicrob. Proteins 2025, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Yang, H.L.; Wei, C.Y.; Cai, G.H.; Ye, J.D.; Zhang, C.X.; Sun, Y.Z. Commensal Bacillus siamensis LF4 induces antimicrobial peptides expression via TLRs and NLRs signaling pathways in intestinal epithelial cells of Lateolabrax maculatus. Fish Shellfish Immunol. 2023, 134, 108634. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yang, H.L.; Li, S.; Cai, G.H.; Ye, J.D.; Zhang, C.X.; Sun, Y.Z. Paraprobiotic and postbiotic forms of Bacillus siamensis improved growth, immunity, liver and intestinal health in Lateolabrax maculatus fed soybean meal diet. Fish Shellfish Immunol. 2024, 145, 109370. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.Y.; Li, M.; Zhou, W.; Yao, Y.Y.; Yang, Y.L.; Zhang, Z.; Ringo, E.; Olsen, R.E.; Clarke, J.L.; Xie, S.Q.; et al. The Fish Microbiota: Research Progress and Potential Applications. Engineering 2023, 29, 137–146. [Google Scholar] [CrossRef]
- Gupta, S.; de Leon, A.V.P.; Kodama, M.; Hoetzinger, M.; Clausen, C.G.; Pless, L.; Verissimo, A.R.A.; Stengel, B.; Calabuig, V.; Kvingedal, R.; et al. The need for high-resolution gut microbiome characterization to design efficient strategies for sustainable aquaculture production. Commun. Biol. 2024, 7, 1391. [Google Scholar] [CrossRef]
- Medina-Félix, D.; Garibay-Valdez, E.; Vargas-Albores, F.; Olivas-Bernal, C.A.; Mendez-Martínez, Y.; Martínez-Córdova, L.R.; Martínez-Porchas, M. Fish as biological models: Implications for gut microbiota research in biomedical and aquaculture sciences. Aquac. Int. 2025, 33, 310. [Google Scholar] [CrossRef]
- Wang, S.C.; Fan, L.M.; Pan, H.Y.; Li, Y.Y.; Qiu, Y.; Lu, Y.M. Antimicrobial peptides from marine animals: Sources, structures, mechanisms and the potential for drug development. Front. Mar. Sci. 2023, 9, 1112595. [Google Scholar] [CrossRef]
- Lee, L.T.; Ang, A.; Mahmood, I.; Najm, A.A.; Adnan, A.M.; Fazry, S.; Law, D. A Systematic Review of Aquatic Organism Antimicrobial Peptides. Pertanika J. Sci. Technol. 2024, 32, 2847–2872. [Google Scholar] [CrossRef]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef]
- Arthington, A.H.; Dulvy, N.K.; Gladstone, W.; Winfield, I.J. Fish conservation in freshwater and marine realms: Status, threats and management. Aquat. Conserv.-Mar. Freshw. Ecosyst. 2016, 26, 838–857. [Google Scholar] [CrossRef]
- Valero, Y.; Saraiva-Fraga, M.; Costas, B.; Guardiola, F.A. Antimicrobial peptides from fish: Beyond the fight against pathogens. Rev. Aquac. 2020, 12, 224–253. [Google Scholar] [CrossRef]
- Kumar, S.; Paul, T.; Sarkar, P.; Kumar, K. Environmental Factors Affecting Aquatic Animal Health. In Management of Fish Diseases; Mallik, S.K., Shahi, N., Pandey, P.K., Eds.; Springer Nature: Singapore, 2025; pp. 171–188. [Google Scholar] [CrossRef]
- Sures, B.; Nachev, M.; Schwelm, J.; Grabner, D.; Selbach, C. Environmental parasitology: Stressor effects on aquatic parasites. Trends Parasitol. 2023, 39, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Novoslavskij, A.; Terentjeva, M.; Eizenberga, I.; Valcina, O.; Bartkevics, V.; Berzins, A. Major foodborne pathogens in fish and fish products: A review. Ann. Microbiol. 2016, 66, 1–15. [Google Scholar] [CrossRef]
- Mbanga, J.; Abia, A.L.K.; Amoako, D.G.; Essack, S.Y. Quantitative microbial risk assessment for waterborne pathogens in a wastewater treatment plant and its receiving surface water body. BMC Microbiol. 2020, 20, 346. [Google Scholar] [CrossRef] [PubMed]
- Bin Hafeez, A.; Jiang, X.K.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Phan-Aram, P.; Mahasri, G.; Kayansamruaj, P.; Amparyup, P.; Srisapoome, P. Immune Regulation, but Not Antibacterial Activity, Is a Crucial Function of Hepcidins in Resistance against Pathogenic Bacteria in Nile Tilapia (Oreochromis niloticus Linn.). Biomolecules 2020, 10, 1132. [Google Scholar] [CrossRef]
- Falzacappa, M.V.V.; Muckenthaler, M.U. Hepcidin: Iron-hormone and anti-microbial peptide. Gene 2005, 364, 37–44. [Google Scholar] [CrossRef]
- Lu, X.; Han, Y.C.; Shepherd, B.S.; Xiang, Y.; Deng, D.F.; Vinyard, B.T. Molecular Analysis and Sex-specific Response of the Hepcidin Gene in Yellow Perch (Perca Flavescens) Following Lipopolysaccharide Challenge. Probiotics Antimicrob. Proteins 2023, 15, 215–225. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C.Y.; Zhang, M.Z.; Zhang, S.P. Beta-defensin derived cationic antimicrobial peptides with potent killing activity against gram negative and gram positive bacteria. BMC Microbiol. 2018, 18, 54. [Google Scholar] [CrossRef]
- Fu, J.; Zong, X.; Jin, M.L.; Min, J.X.; Wang, F.D.; Wang, Y.Z. Mechanisms and regulation of defensins in host defense. Signal Transduct. Target. Ther. 2023, 8, 3985–4014. [Google Scholar] [CrossRef]
- Raju, S.V.; Sarkar, P.; Kumar, P.; Arockiaraj, J. Piscidin, Fish Antimicrobial Peptide: Structure, Classification, Properties, Mechanism, Gene Regulation and Therapeutical Importance. Int. J. Pept. Res. Ther. 2021, 27, 91–107. [Google Scholar] [CrossRef]
- Dzurová, L.; Holásková, E.; Pospísilová, H.; Rauber, G.S.; Frébortová, J. Cathelicidins: Opportunities and Challenges in Skin Therapeutics and Clinical Translation. Antibiotics 2025, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Gunne, H.; Agerberth, B.; Boman, A.; Bergman, T.; Olsson, B.; Dagerlind, A.; Wigzell, H.; Boman, H.G.; Gudmundsson, G.H. NK-lysin, structure and function of a novel effector molecule of porcine T and NK cells. Vet. Immunol. Immunopathol. 1996, 54, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, P.; Varela, M.; Diaz-Rosales, P.; Romero, A.; Dios, S.; Figueras, A.; Novoa, B. Zebrafish Nk-lysins: First insights about their cellular and functional diversification. Dev. Comp. Immunol. 2015, 51, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ortjohann, M.; Leippe, M. Characterization of NK-lysin A, a potent antimicrobial peptide from the zebrafish Danio rerio. Dev. Comp. Immunol. 2025, 162, 105266. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Chen, F.Y.; Hao, H.; Wang, K.J. A novel antimicrobial peptide Larimicin78-102 from large yellow croaker (Larimichthys crocea) shows potent antibacterial activity in vitro and enhances resistance to vibrio fluvialis infection in vivo. Fish Shellfish Immunol. 2025, 161, 110279. [Google Scholar] [CrossRef]
- Wang, D.J.; Shi, J.R.; Chen, C.; Wang, Z.Q.; Liu, Y. Truncated Pleurocidin Derivative with High Pepsin Hydrolysis Resistance to Combat Multidrug-Resistant Pathogens. Pharmaceutics 2022, 14, 2025. [Google Scholar] [CrossRef]
- Souza, A.L.A.; Díaz-Dellavalle, P.; Cabrera, A.; Larrañaga, P.; Dalla-Rizza, M.; De-Simone, S.G. Antimicrobial activity of pleurocidin is retained in Plc-2, a C-terminal 12-amino acid fragment. Peptides 2013, 45, 78–84. [Google Scholar] [CrossRef]
- Kanaujia, K.A.; Wagh, S.; Pandey, G.; Phatale, V.; Khairnar, P.; Kolipaka, T.; Rajinikanth, P.S.; Saraf, S.A.; Srivastava, S.; Kumar, S. Harnessing marine antimicrobial peptides for novel therapeutics: A deep dive into ocean-derived bioactives. Int. J. Biol. Macromol. 2025, 307, 142158. [Google Scholar] [CrossRef] [PubMed]
- Serna-Duque, J.A.; Ruiz, C.E.; Lopez, S.M.; Sánchez-Ferrer, A.; Esteban, M.A. Immunometabolic involvement of hepcidin genes in iron homeostasis, storage, and regulation in gilthead seabream (Sparus aurata). Front. Mar. Sci. 2022, 9, 1073060. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, E.J.; Nam, Y.K. Chondrostean sturgeon hepcidin: An evolutionary link between teleost and tetrapod hepcidins. Fish Shellfish Immunol. 2019, 88, 117–125. [Google Scholar] [CrossRef]
- Soofiyani, S.R.; Soltani, E.; Nejad-Khelejani, M.K.; Ghanbari, R.; Memar, M.Y. Hepcidin: A potent antimicrobial peptide involved in iron homeostasis. Gene Rep. 2024, 37, 102082. [Google Scholar] [CrossRef]
- Luo, S.W.; Luo, Z.Y.; Yan, T.; Luo, K.K.; Feng, P.H.; Liu, S.J. Antibacterial and immunoregulatory activity of a novel hepcidin homologue in diploid hybrid fish (Carassius auratus cuvieri ♀ × Carassius auratus red var ♂). Fish Shellfish Immunol. 2020, 98, 551–563. [Google Scholar] [CrossRef]
- Liu, Z.M.; Chen, J.; Lv, Y.P.; Hu, Z.H.; Dai, Q.M.; Fan, X.L. Molecular characterization of a hepcidin homologue in starry flounder (Platichthys stellatus) and its synergistic interaction with antibiotics. Fish Shellfish Immunol. 2018, 83, 45–51. [Google Scholar] [CrossRef]
- Hilton, K.B.; Lambert, L.A. Molecular evolution and characterization of hepcidin gene products in vertebrates. Gene 2008, 415, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, W.; Xu, Y.W.; Chen, R.Y.; Xu, Q. Sequence analysis of hepcidin in barbel steed (Hemibarbus labeo): QSHLS motif confers hepcidin iron-regulatory activity but limits its antibacterial activity. Dev. Comp. Immunol. 2021, 114, 103845. [Google Scholar] [CrossRef]
- Chen, J.; Nie, L.; Chen, J. Mudskipper (Boleophthalmus pectinirostris) Hepcidin-1 and Hepcidin-2 Present Different Gene Expression Profile and Antibacterial Activity and Possess Distinct Protective Effect against Edwardsiella tarda Infection. Probiotics Antimicrob. Proteins 2018, 10, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Dixit, A.; Garg, L.C.; Sahoo, P.K. Hepcidin gene of Indian major carp, Labeo rohita: Molecular, structural and functional characterization, and antibacterial activity of recombinant hepcidin. Aquaculture 2019, 511, 734218. [Google Scholar] [CrossRef]
- Anju, M.V.; Archana, K.; Musthafa, S.M.; Anooja, V.V.; Athira, P.P.; Neelima, S.; Dhaneesha, M.; Sajeevan, T.P.; Singh, I.S.B.; Philip, R. A Novel Hepcidin Isoform Jd-Hep from the Sin Croaker Johnius dussumieri (Cuvier, 1830): Recombinant Expression and Insights into the Antibacterial Property and Modes of Action. Mar. Biotechnol. 2025, 27, 52. [Google Scholar] [CrossRef] [PubMed]
- Vela, D.; Vela-Gaxha, Z. Differential regulation of hepcidin in cancer and non-cancer tissues and its clinical implications. Exp. Mol. Med. 2018, 50, e436. [Google Scholar] [CrossRef] [PubMed]
- Cervera, L.; Arizcun, M.; Mercado, L.; Chaves-Pozo, E.; Cuesta, A. Hepcidin and dicentracin peptides show preventive antiviral applications against NNV infection in European sea bass through immunomodulatory roles. Aquaculture 2024, 583, 740592. [Google Scholar] [CrossRef]
- Taheri, B.; Mohammadi, M.; Momenzadeh, N.; Farshadzadeh, Z.; Roozbehani, M.; Dehghani, P.; Hajian, S.; Darvishi, S.; Shamseddin, J. Substitution of lysine for isoleucine at the center of the nonpolar face of the antimicrobial peptide, piscidin-1, leads to an increase in the rapidity of bactericidal activity and a reduction in toxicity. Infect. Drug Resist. 2019, 12, 1629–1647. [Google Scholar] [CrossRef]
- Barroso, C.; Carvalho, P.; Carvalho, C.; Santarém, N.; Gonçalves, J.F.M.; Rodrigues, P.N.S.; Neves, J.V. The Diverse Piscidin Repertoire of the European Sea Bass (Dicentrarchus labrax): Molecular Characterization and Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 4613. [Google Scholar] [CrossRef]
- Milne, D.J.; Fernández-Montero, A.; Gundappa, M.K.; Wang, T.H.; Acosta, F.; Torrecillas, S.; Montero, D.; Zou, J.; Sweetman, J.; Secombes, C.J. An insight into piscidins: The discovery, modulation and bioactivity of greater amberjack, Seriola dumerili, piscidin. Mol. Immunol. 2019, 114, 378–388. [Google Scholar] [CrossRef]
- Chen, P.Y.; Ye, T.; Li, C.Y.; Praveen, P.; Hu, Z.L.; Li, W.Y.; Shang, C.J. Embracing the era of antimicrobial peptides with marine organisms. Nat. Prod. Rep. 2024, 41, 331–346. [Google Scholar] [CrossRef]
- Campagna, S.; Saint, N.; Molle, G.; Aumelas, A. Structure and mechanism of action of the antimicrobial peptide piscidin. Biochemistry 2007, 46, 1771–1778. [Google Scholar] [CrossRef]
- Asensio-Calavia, P.; González-Acosta, S.; Otazo-Pérez, A.; López, M.R.; Morales-delaNuez, A.; de la Lastra, J.M.P. Teleost Piscidins-In Silico Perspective of Natural Peptide Antibiotics from Marine Sources. Antibiotics 2023, 12, 855. [Google Scholar] [CrossRef]
- Mahrous, K.F.; Mabrouk, D.M.; Aboelenin, M.M.; Abd El-Kader, H.A.M.; Gaafar, A.Y.; Younes, A.M.; Mahmoud, M.A.; Khalil, W.K.B.; Hassanane, M.S. Molecular characterization and immunohistochemical localization of tilapia piscidin 3 in response to Aeromonas hydrophila infection in Nile tilapia. J. Pept. Sci. 2020, 26, e3280. [Google Scholar] [CrossRef]
- Prior, B.S.; Lange, M.D.; Salger, S.A.; Reading, B.J.; Peatman, E.; Beck, B.H. The effect of piscidin antimicrobial peptides on the formation of Gram-negative bacterial biofilms. J. Fish Dis. 2022, 45, 99–105. [Google Scholar] [CrossRef]
- Sun, H.R.; Zhang, J.G.; Li, X.L.; Gao, H.L.; Liao, C.S.; Wang, R.B.; Wen, B.; Luo, W.Y.; Zhang, W.; Zhu, C.L.; et al. Fish-derived cationic antimicrobial peptide piscidin 1 exerts broad-spectrum bactericidal activities against multidrug-resistant Gram-negative pathogens. LWT-Food Sci. Technol. 2025, 215, 117189. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Bhat, R.A.H.; Pande, A. Antimicrobial peptides of fish: Innocuous alternatives to antibiotics. Rev. Aquac. 2020, 12, 85–106. [Google Scholar] [CrossRef]
- Garcia, A.E.; Ösapay, G.; Tran, P.A.; Yuan, J.; Selsted, M.E. Isolation, Synthesis, and Antimicrobial Activities of Naturally Occurring θ-Defensin Isoforms from Baboon Leukocytes. Infect. Immun. 2008, 76, 5883–5891. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.; Shirdel, I.; Braun, M.S.; Wink, M. Defensins: Transcriptional regulation and function beyond antimicrobial activity. Dev. Comp. Immunol. 2020, 104, 103556. [Google Scholar] [CrossRef] [PubMed]
- Akhavan-Bahabadi, M.; Shekarbi, S.P.H.; Sharifinia, M.; Khanjani, M.H. Exploring Fish Antimicrobial Peptides (Amps): Classification, Biological Activities, and Mechanisms of Action. Int. J. Pept. Res. Ther. 2024, 30, 63. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Zhao, H.; Zhang, X.S.; Luo, H.Y.; Xue, X.L.; Li, Z.Y.; Yao, B. Identification, expression and bioactivity of Paramisgurnus dabryanus β-defensin that might be involved in immune defense against bacterial infection. Fish Shellfish Immunol. 2013, 35, 399–406. [Google Scholar] [CrossRef]
- Shinnar, A.E.; Butler, K.L.; Park, H.J. Cathelicidin family of antimicrobial peptides: Proteolytic processing and protease resistance. Bioorganic Chem. 2003, 31, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Uzzell, T.; Stolzenberg, E.D.; Shinnar, A.E.; Zasloff, M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides 2003, 24, 1655–1667. [Google Scholar] [CrossRef]
- Maier, V.H.; Dorn, K.V.; Gudmundsdottir, B.K.; Gudmundsson, G.H. Characteirisation of cathelicidin gene family members in divergent fish species. Mol. Immunol. 2008, 45, 3723–3730. [Google Scholar] [CrossRef] [PubMed]
- Bridle, A.; Nosworthy, E.; Polinski, M.; Nowak, B. Evidence of an Antimicrobial-Immunomodulatory Role of Atlantic Salmon Cathelicidins during Infection with Yersinia ruckeri. PLoS ONE 2011, 6, e23417. [Google Scholar] [CrossRef]
- Hernández-Arvizu, E.E.; Silis-Moreno, T.M.; García-Arredondo, J.A.; Rodríguez-Torres, A.; Cervantes-Chávez, J.A.; Mosqueda, J. Aquiluscidin, a Cathelicidin from Crotalus aquilus, and the Vcn-23 Derivative Peptide, Have Anti-Microbial Activity against Gram-Negative and Gram-Positive Bacteria. Microorganisms 2023, 11, 2778. [Google Scholar] [CrossRef]
- de Bruijn, I.; Belmonte, R.; Anderson, V.L.; Saraiva, M.; Wang, T.H.; van West, P.; Secombes, C.J. Immune gene expression in trout cell lines infected with the fish pathogenic oomycete Saprolegnia parasitica. Dev. Comp. Immunol. 2012, 38, 44–54. [Google Scholar] [CrossRef]
- Lu, X.J.; Chen, J.; Huang, Z.A.; Shi, Y.H.; Lü, J.N. Identification and characterization of a novel cathelicidin from ayu, Plecoglossus altivelis. Fish Shellfish Immunol. 2011, 31, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Pan, C.Y.; Rajanbabu, V.; Chan, Y.L.; Wu, C.J.; Chen, J.Y. Modulation of immune responses by the antimicrobial peptide, epinecidin (Epi)-1, and establishment of an Epi-1-based inactivated vaccine. Biomaterials 2011, 32, 3627–3636. [Google Scholar] [CrossRef]
- Valero, Y.; Chaves-Pozo, E.; Cuesta, A. NK-lysin is highly conserved in European sea bass and gilthead seabream but differentially modulated during the immune response. Fish Shellfish Immunol. 2020, 99, 435–441. [Google Scholar] [CrossRef]
- Ortega, L.; Carrera, C.; Muñoz-Flores, C.; Salazar, S.; Villegas, M.F.; Starck, M.F.; Valenzuela, A.; Agurto, N.; Montesino, R.; Astuya, A.; et al. New insight into the biological activity of Salmo salar NK-lysin antimicrobial peptides. Front. Immunol. 2024, 15, 1191966. [Google Scholar] [CrossRef]
- Wang, G.L.; Li, E.Z.; Li, D.H.; Wang, M.C.; Sun, S.S.; Xiong, R.Y.; Li, C.F.; Sun, B.J.; Xie, H.X. Identification, distribution, bactericidal and immunoregulatory activities of NK-lysins in grass carp (Ctenopharyngodon idella). Aquaculture 2024, 584, 740671. [Google Scholar] [CrossRef]
- Kim, W.H.; Lillehoj, H.S.; Min, W. Evaluation of the Immunomodulatory Activity of the Chicken NK-Lysin-Derived Peptide cNK-2. Sci. Rep. 2017, 7, 45099. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, D.; Ivica, J.; Separovic, F.; de Planque, M.R.R. Characterisation of cell membrane interaction mechanisms of antimicrobial peptides by electrical bilayer recording. Biophys. Chem. 2022, 281, 106721. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, A.L.; Zhang, F.; Zhang, M.H.; Yang, H.X.; Li, J.N.; Su, P.C.; Chen, Y.; Yu, H.N.; Wang, Y.P. The protective effect of fish-derived cathelicidins on bacterial infections in zebrafish, Danio rerio. Fish Shellfish Immunol. 2019, 92, 519–527. [Google Scholar] [CrossRef]
- Huang, Y.B.; Huang, J.F.; Chen, Y.X. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef]
- Bertelsen, M.; Lacey, M.M.; Nichol, T.; Miller, K. Mechanistic Insight into the Early Stages of Toroidal Pore Formation by the Antimicrobial Peptide Smp24. Pharmaceutics 2023, 15, 2399. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Cervera, L.; Arizcun, M.; Mercado, L.; Chaves-Pozo, E.; Cuesta, A. Synthetic antimicrobial Nkl and Dic peptides are immunomodulatory but only Dic peptide can be therapeutic against nodavirus infection. Fish Shellfish Immunol. 2024, 152, 109772. [Google Scholar] [CrossRef]
- Peng, C.; Liu, Y.; Shui, L.Y.; Zhao, Z.Y.; Mao, X.F.; Liu, Z.Y. Mechanisms of Action of the Antimicrobial Peptide Cecropin in the Killing of Candida albicans. Life 2022, 12, 1581. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.R. Non-membrane mechanisms of antimicrobial peptide P7 against Escherichia coli. Acta Microbiol. Sin. 2016, 56, 1737–1745. [Google Scholar] [CrossRef]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef] [PubMed]
- Keshri, A.K.; Rawat, S.S.; Chaudhary, A.; Sharma, S.; Kapoor, A.; Mehra, P.; Kaur, R.; Mishra, A.; Prasad, A. LL-37, the master antimicrobial peptide, its multifaceted role from combating infections to cancer immunity. Int. J. Antimicrob. Agents 2025, 65, 107398. [Google Scholar] [CrossRef]
- Turley, J.L.; Lavelle, E.C. Resolving adjuvant mode of action to enhance vaccine efficacy. Curr. Opin. Immunol. 2022, 77, 102229. [Google Scholar] [CrossRef]
- Palgen, J.L.; Feraoun, Y.; Dzangué-Tchoupou, G.; Joly, C.; Martinon, F.; Le Grand, R.; Beignon, A.S. Optimize Prime/Boost Vaccine Strategies: Trained Immunity as a New Player in the Game. Front. Immunol. 2021, 12, 612747. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Hoseini, S.M.; Mirghaed, A.T.; Ghelichpour, M.; Shirzad-Aski, H.; Van Doan, H.; El-Haroun, E.; Safari, R.; Khanzadeh, M. Comparison of the effects of host-associated (autochthonous) and commercial probiotics on immune responses, growth parameters and intestinal microbiota of Caspian whitefish (Rutilus frisii kutum) fry. Front. Mar. Sci. 2024, 11, 1446927. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yang, H.L.; Hu, L.H.; Yang, W.; Ai, C.X.; Sun, Y.Z. Dose-Dependent Effects of Histamine on Growth, Immunity and Intestinal Health in Juvenile Grouper (Epinephelus coioides). Front. Mar. Sci. 2021, 8, 685720. [Google Scholar] [CrossRef]
- Ding, X.Y.; Wei, C.Y.; Liu, Z.Y.; Yang, H.L.; Han, F.; Sun, Y.Z. Autochthonous Bacillus subtilis and Enterococcus faecalis improved liver health, immune response, mucosal microbiota and red-head disease resistance of yellow drum (Nibea albiflora). Fish Shellfish Immunol. 2023, 134, 108575. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yang, H.L.; Hu, L.H.; Yang, W.; Ai, C.X.; Sun, Y.Z. Autochthonous Probiotics Alleviate the Adverse Effects of Dietary Histamine in Juvenile Grouper (Epinephelus coioides). Front. Microbiol. 2021, 12, 792718. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Hong, Y.H.; Li, Y.L.; Cai, G.H.; Wang, P.; Zhu, C.Z.; Lu, K.L.; Yang, H.L.; Sun, Y.Z. Pomelo Peel and Soybean Meal Fermented Compound as Feedstuff for Large Yellow Croaker (Larimichthys crocea): A Study on Growth and Intestinal Health. Aquac. Nutr. 2025, 2025, 6556868. [Google Scholar] [CrossRef]
- Shao, J.C.; Li, Z.Q.; You, H.K.; Wang, D.J.; Zhang, J.N.; Wang, L.; Zhao, C.; Zhao, W. Soybean Glycinin Reduced Growth Performance and Antioxidant Capacity and Caused Intestinal Inflammation and Microbiome Changes in Large Yellow Croaker (Larimichthys crocea). Food Front. 2025, 6, 248–258. [Google Scholar] [CrossRef]
- Rimoldi, S.; Montero, D.; Torrecillas, S.; Serradell, A.; Acosta, F.; Haffray, P.; Hostins, B.; Fontanillas, R.; Allal, F.; Bajek, A.; et al. Genetically superior European sea bass (Dicentrarchus labrax) and nutritional innovations: Effects of functional feeds on fish immune response, disease resistance, and gut microbiota. Aquacult. Rep. 2023, 33, 101747. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; Boutin, S.; Hoseinifar, S.H.; Derome, N. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 2014, 5, 207. [Google Scholar] [CrossRef]
- Singh, B.K.; Thakur, K.; Kumari, H.; Mahajan, D.; Sharma, D.; Sharma, A.K.; Kumar, S.; Singh, B.; Pankaj, P.P.; Kumar, R. A review on comparative analysis of marine and freshwater fish gut microbiomes: Insights into environmental impact on gut microbiota. FEMS Microbiol. Ecol. 2025, 101, fiae169. [Google Scholar] [CrossRef]
- Wang, A.R.; Ran, C.; Ringo, E.; Zhou, Z.G. Progress in fish gastrointestinal microbiota research. Rev. Aquac. 2018, 10, 626–640. [Google Scholar] [CrossRef]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Yang, H.L.; Liu, Z.Y.; Jin, Y.M.; Liu, Z.X.; Zhang, B.Y.; Yuan, Z.H.; Ye, J.D.; Sun, Y.Z. Preventive and reparative functions of host-associated probiotics against soybean meal induced growth, immune suppression and gut injury in Japanese seabass (Lateolabrax japonicus). Fish Shellfish Immunol. 2022, 128, 651–663. [Google Scholar] [CrossRef]
- Amillano-Cisneros, J.M.; Fuentes-Valencia, M.A.; Leyva-Morales, J.B.; Savín-Amador, M.; Márquez-Pacheco, H.; Bastidas-Bastidas, P.D.; Leyva-Camacho, L.; de la Torre-espinosa, Z.Y.; Badilla-Medina, C.N. Effects of Microorganisms in Fish Aquaculture from a Sustainable Approach: A Review. Microorganisms 2025, 13, 485. [Google Scholar] [CrossRef]
- Shija, V.M.; Chen, H.P.; Li, Y.; Ng’onga, L.; Amoah, K.; Yong, Z.; Chen, J.X.; Yu, D.P.; Mkuye, R.; Cai, J. Effects of dietary supplementation with fish-derived Bacillus amyloliquefaciens AV5 on growth status, immune response, microbiota, and intestinal health of Nile tilapia (Oreochromis niloticus). Aquacult. Rep. 2025, 41, 102658. [Google Scholar] [CrossRef]
- Bostick, J.W.; Zhou, L. Innate lymphoid cells in intestinal immunity and inflammation. Cell. Mol. Life Sci. 2016, 73, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Montalban-Arques, A.; De Schryver, P.; Bossier, P.; Gorkiewicz, G.; Mulero, V.; Gatlin, D.M.; Galindo-Villegas, J. Selective manipulation of the gut microbiota improves immune status in vertebrates. Front. Immunol. 2015, 6, 512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.A.; Chu, J.Y.; Feng, S.Y.; Guo, C.H.; Xue, B.G.; He, K.; Li, L.S. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef] [PubMed]
- Boltaña, S.; Roher, N.; Goetz, F.W.; MacKenzie, S.A. PAMPs, PRRs and the genomics of gram negative bacterial recognition in fish. Dev. Comp. Immunol. 2011, 35, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, B.P.; Deng, J.M.; Yang, Q.H.; Chi, S.Y.; Pang, A.B.; Xin, Y.; Liu, Y.; Zhang, H.T. PRR-Mediated Immune Response and Intestinal Flora Profile in Soybean Meal-Induced Enteritis of Pearl Gentian Groupers, Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂. Front. Immunol. 2022, 13, 814479. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, S.; Cai, G.H.; Zhang, C.X.; Sun, Y.Z.; Yang, H.L.; Ye, J.D. Probiotic components of Bacillus siamensis LF4 mitigated ß-conglycinin caused cell injury via modulating TLR2/MAPKs/NF-κB signaling in Lateolabrax maculatus. Fish Shellfish Immunol. 2023, 141, 109010. [Google Scholar] [CrossRef]
- Yang, H.L.; Sun, Y.Z.; Hu, X.; Ye, J.D.; Lu, K.L.; Hu, L.H.; Zhang, J.J. Bacillus pumilus SE5 originated PG and LTA tuned the intestinal TLRs/MyD88 signaling and microbiota in grouper (Epinephelus coioides). Fish Shellfish Immunol. 2019, 88, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, F.M.; Guo, H.Y.; Zhu, Y.Y.; Yuan, J.D.; Yang, G.W.; An, L.G. Molecular characterization of hepcidin gene in common carp (Cyprinus carpio L.) and its expression pattern responding to bacterial challenge. Fish Shellfish Immunol. 2013, 35, 1030–1038. [Google Scholar] [CrossRef]
- Sestok, A.E.; Linkous, R.O.; Smith, A.T. Toward a mechanistic understanding of Feo-mediated ferrous iron uptake. Metallomics 2018, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.; Layec, S.; Auger, S.; Juste, C.; Henry, C.; Charif, S.; Jaszczyszyn, Y.; Sokol, H.; Beney, L.; Langella, P.; et al. Faecalibacterium duncaniae A2-165 regulates the expression of butyrate synthesis, ferrous iron uptake, and stress-response genes based on acetate consumption. Sci. Rep. 2024, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.F.; Liu, Z.F.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. Coordination of Bactericidal and Iron Regulatory Functions of Hepcidin in Innate Antimicrobial Immunity in a Zebrafish Model. Sci. Rep. 2017, 7, 4265. [Google Scholar] [CrossRef]
- Naaz, T.; Lahiri, D.; Pandit, S.; Nag, M.; Gupta, P.K.; Al-Dayan, N.; Rai, N.; Chaubey, K.K.; Gupta, A.K. Antimicrobial Peptides Against Microbial Biofilms: Efficacy, Challenges, and Future Prospect. Int. J. Pept. Res. Ther. 2023, 29, 48. [Google Scholar] [CrossRef]
- Ran, C.; Carrias, A.; Williams, M.A.; Capps, N.; Dan, B.C.T.; Newton, J.C.; Kloepper, J.W.; Ooi, E.L.; Browdy, C.L.; Terhune, J.S.; et al. Identification of Bacillus Strains for Biological Control of Catfish Pathogens. PLoS ONE 2012, 7, e45793. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Qian, Z.; Ding, Y.B.; Ji, J.; Ning, X.H.; Yin, S.W.; Zhang, K. Isolation and characterization of a strain Clostridium butyricum B3 from the intestine of Pelteobagrus fulvidraco and its potential probiotic roles. Aquaculture 2025, 595, 741590. [Google Scholar] [CrossRef]
- Wong, J.W.H.; Balskus, E.P. Small molecules as modulators of phage-bacteria interactions. Curr. Opin. Chem. Biol. 2025, 84, 102566. [Google Scholar] [CrossRef]
- de León, A.V.P.; Hensen, T.; Hoetzinger, M.; Gupta, S.; Weston, B.; Johnsen, S.M.; Rasmussen, J.A.; Clausen, C.G.; Pless, L.; Verissimo, A.R.A.; et al. Genomic and functional characterization of the Atlantic salmon gut microbiome in relation to nutrition and health. Nat. Microbiol 2024, 9, 3059–3074. [Google Scholar] [CrossRef] [PubMed]
- Krogdahl, Å.; Sundby, A.; Holm, H. Characteristics of digestive processes in Atlantic salmon (Salmo salar). Enzyme pH optima, chyme pH, and enzyme activities. Aquaculture 2015, 449, 27–36. [Google Scholar] [CrossRef]
- Mulder, I.E.; Wadsworth, S.; Secombes, C.J. Cytokine expression in the intestine of rainbow trout (Oncorhynchus mykiss) during infection with Aeromonas salmonicida. Fish Shellfish Immunol. 2007, 23, 747–759. [Google Scholar] [CrossRef]
- Kania, P.W.; Buchmann, K. Differential immune gene expression in rainbow trout, Oncorhynchus mykiss (walbaum), exposed to five pathogens: Aeromonas salmonicida, Flavobacterium psychrophilum, Vibrio anguillarum, Yersinia ruckeri and Ichthyophthirius multifiliis. Comp. Immunol. Rep. 2024, 7, 200166. [Google Scholar] [CrossRef]
- Sandlund, N.; Gjerset, B.; Bergh, O.; Modahl, I.; Olesen, N.J.; Johansen, R. Screening for Viral Hemorrhagic Septicemia Virus in Marine Fish along the Norwegian Coastal Line. PLoS ONE 2014, 9, e108529. [Google Scholar] [CrossRef][Green Version]
- Freundt, K.; Herzmann, C.; Biedziak, D.; Scheffzük, C.; Gaede, K.I.; Stamme, C. Surfactant Protein A Enhances the Degradation of LPS-Induced TLR4 in Primary Alveolar Macrophages Involving Rab7, β-arrestin2, and mTORC1. Infect. Immun. 2022, 90, e00250-21. [Google Scholar] [CrossRef]
- Leonard, J.N.; Skov, P.V. Capacity for thermal adaptation in Nile tilapia (Oreochromis niloticus): Effects on oxygen uptake and ventilation. J. Therm. Biol. 2022, 105, 103206. [Google Scholar] [CrossRef]
- Hou, Q.H.; Ye, L.L.; Liu, H.F.; Huang, L.L.; Yang, Q.; Turner, J.R.; Yu, Q.H. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018, 25, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Zang, R.X.; Zhou, R.; Li, Y.D.; Wu, H.H.; Lu, L.P.; Xu, H.W. The probiotic Lactobacillus plantarum alleviates colitis by modulating gut microflora to activate PPARγ and inhibit MAPKs/NF-κB. Eur. J. Nutr. 2025, 64, 32. [Google Scholar] [CrossRef]
- Tachibana, L.; Telli, G.S.; Dias, D.D.; Gonçalves, G.S.; Guimaraes, M.C.; Ishikawa, C.M.; Cavalcante, R.B.; Natori, M.M.; Alarcon, M.F.F.; Tapia-Paniagua, S.; et al. Bacillus subtilis and Bacillus licheniformis in diets for Nile tilapia (Oreochromis niloticus): Effects on growth performance, gut microbiota modulation and innate immunology. Aquac. Res. 2021, 52, 1630–1642. [Google Scholar] [CrossRef]
- D’Aes, J.; Fraiture, M.A.; Bogaerts, B.; De Keersmaecker, S.C.J.; Roosens, N.; Vanneste, K. Metagenomic Characterization of Multiple Genetically Modified Bacillus Contaminations in Commercial Microbial Fermentation Products. Life 2022, 12, 1971. [Google Scholar] [CrossRef]
- Tartor, Y.; Taha, M.; Mahboub, H.; El Ghamery, M. Yeast species associated with diseased fish: Occurrence, identification, experimental challenges and antifungal susceptibility testing. Aquaculture 2018, 488, 134–144. [Google Scholar] [CrossRef]
- Guirado-Flores, J.S.O.; Garibay-Valdez, E.; Medina-Félix, D.; Vargas-Albores, F.; Martínez-Córdova, L.R.; Mendez-Martínez, Y.; Martínez-Porchas, M. Intestinal Microeukaryotes in Fish: A Concise Review of an Underexplored Component of the Microbiota. Microbiol. Res. 2025, 16, 158. [Google Scholar] [CrossRef]
- Liu, R.Z.; Wang, S.; Huang, D.L.; Huang, Y.L.; He, T.L.; Chen, X.H. The probiotic roles of Lactiplantibacillus plantarum E2 as a dietary supplement in growth promotion and disease resistance of juvenile large yellow croaker (Larimichthys crocea). Aquaculture 2024, 578, 740082. [Google Scholar] [CrossRef]
- Long, J.Y.; Li, X.K.; Yao, C.Y.; Liu, X.L.; Li, N.; Zhou, Y.M.; Li, D.W.; Su, S.Q.; Wang, L.M.; Liu, H.; et al. The role of ZC3H12D-regulated TLR4-NF- κ B pathway in LPS-induced pro-inflammatory microglial activation. Neurosci. Lett. 2024, 832, 137800. [Google Scholar] [CrossRef]
- Caneos, W.G.; Shrivastava, J.; Ndugwa, M.; De Boeck, G. Physiological responses of European sea bass (Dicentrarchus labrax) exposed to increased carbon dioxide and reduced seawater salinities. Mol. Biol. Rep. 2024, 51, 496. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Pineda, J.; Kalinina, O.; Sperling, A.I.; Knight, K.L. Mechanism of TLR4-Mediated Anti-Inflammatory Response Induced by Exopolysaccharide from the Probiotic Bacillus subtilis. J. Immunol. 2023, 211, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Moniruzzaman, M.; Lee, S.; Bae, J.; Bai, S.C.; Min, T.; Lee, S. Evaluation of three fish-derived probiotic bacteria replacing antibiotics on growth, immunity, gut morphology and disease resistance in juvenile olive flounder Paralichthys olivaceus fed reduced fish meal diets. Front. Nutr. 2025, 12, 1519140. [Google Scholar] [CrossRef]
- Santhi, J.J.; Guru, A.; Shaik, M.R.; Hussain, S.A.; Issac, P.K. Understanding the effects of perfluorobutane sulfonate in zebrafish larvae model (Danio rerio): Insights into potential ecotoxicological risks and human health. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2025, 287, 110069. [Google Scholar] [CrossRef]
- Chen, L.F.; Li, H.Y.; Li, J.Y.; Chen, Y.; Yang, Y.M. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis. Int. J. Mol. Med. 2019, 43, 1139–1148. [Google Scholar] [CrossRef]
- Satanwat, P.; Tapaneeyaworawong, P.; Wechprasit, P.; Boonprasertsakul, T.; Pungrasmi, W.; Sritunyalucksana, K.; Prachumwat, A.; Powtongsook, S. Total ammonia nitrogen removal and microbial community dynamics in an outdoor HDPE-lined shrimp pond with no water discharge. Aquaculture 2023, 577, 739898. [Google Scholar] [CrossRef]
- Lin, L.T.; Zhuo, H.B.; Zhang, Y.; Li, J.Y.; Zhou, X.X.; Wu, G.B.; Guo, C.A.; Liu, J.Y. Effects of ammonia exposure and post-exposure recovery in pacific white shrimp, Litopenaeus vannamei: Histological, physiological and molecular responses. Aquat. Toxicol. 2024, 277, 107133. [Google Scholar] [CrossRef]
- Tan, F.; Limbu, S.M.; Qian, Y.; Qiao, F.; Du, Z.Y.; Zhang, M.L. The Responses of Germ-Free Zebrafish (Danio rerio) to Varying Bacterial Concentrations, Colonization Time Points, and Exposure Duration. Front. Microbiol. 2019, 10, 2156. [Google Scholar] [CrossRef]
- Tian, H.Z.; Li, J.H.; Chen, X.P.; Ren, Z.N.; Pan, X.H.; Huang, W.N.; Bhatia, M.; Pan, L.L.; Sun, J. Oral Delivery of Mouse β-Defensin 14 (mBD14)-Producing Lactococcus lactis NZ9000 Attenuates Experimental Colitis in Mice. J. Agric. Food Chem. 2023, 71, 5185–5194. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Capillo, G.; Fernandes, J.M.O.; Kiron, V.; Lauriano, E.R.; Alesci, A.; Lo Cascio, P.; Guerrera, M.C.; Kuciel, M.; Zuwala, K.; et al. Expression of the Antimicrobial Peptide Piscidin 1 and Neuropeptides in Fish Gill and Skin: A Potential Participation in Neuro-Immune Interaction. Mar. Drugs 2022, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Abraham, T.J.; Sen, A.; Rajisha, R.; Nadella, R.K.; Chatterjee, N.S.; Patil, P.K. Impact of graded doses of enrofloxacin on the safety and biological responses of Nile tilapia Oreochromis niloticus. Drug Chem. Toxicol. 2024, 48, 784–796. [Google Scholar] [CrossRef]
- Wang, H.; de Carvalho, L.P.S. Metabolomic profiling reveals bacterial metabolic adaptation strategies and new metabolites. Curr. Opin. Chem. Biol. 2023, 74, 102287. [Google Scholar] [CrossRef]
- Baumgartner, S.; James, J.; Ellison, A. The supplementation of a prebiotic improves the microbial community in the gut and the skin of Atlantic salmon (Salmo salar). Aquacult. Rep. 2022, 25, 101204. [Google Scholar] [CrossRef] [PubMed]

| HDP Family | Typical Length/Structural Features | Representative Species | Primary Tissue Expression | Principal Functions |
|---|---|---|---|---|
| Hepcidins | 20–25 aa mature peptide; 8 conserved cysteines forming β-sheet | Zebrafish (Danio rerio), tilapia (Oreochromis niloticus), rainbow trout (Oncorhynchus mykiss) | Liver, intestine, gill, kidney | Iron homeostasis; broad-spectrum antimicrobial activity; immunomodulation |
| β-defensins | 30–45 aa; six conserved cysteines forming three disulfide bonds | Zebrafish, channel catfish (Ictalurus punctatus), grass carp (Ctenopharyngodon idella) | Intestine, gill, skin, spleen | Membrane disruption; chemotactic activity; regulation of adaptive immunity |
| Piscidins | 20–40 aa; amphipathic α-helical structure | Hybrid striped bass (Morone saxatilis × M. chrysops), European sea bass (Dicentrarchus labrax) | Mast cells, gill, intestine, skin | Potent antimicrobial activity (Gram+/Gram−, fungi, parasites); wound healing |
| Cathelicidins | 25–37 aa mature peptide; highly variable α-helical/β-sheet domains | Atlantic salmon (Salmo salar), rainbow trout | Head kidney, spleen, mucosal tissues | Direct antimicrobial activity; immune cell recruitment; modulation of inflammation |
| NK-lysins | 74–78 aa; saposin-like domain structure | Channel catfish, turbot (Scophthalmus maximus) | Head kidney, spleen, intestine, gill | Cytotoxicity against tumor and pathogen-infected cells; bacterial killing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.-Z.; Yu, Y.; Liu, Z.-Y. Crosstalk Between Intestinal Microbiota and Host Defense Peptides in Fish. Biology 2025, 14, 1243. https://doi.org/10.3390/biology14091243
Yu X-Z, Yu Y, Liu Z-Y. Crosstalk Between Intestinal Microbiota and Host Defense Peptides in Fish. Biology. 2025; 14(9):1243. https://doi.org/10.3390/biology14091243
Chicago/Turabian StyleYu, Xiao-Zheng, Yang Yu, and Zi-Yan Liu. 2025. "Crosstalk Between Intestinal Microbiota and Host Defense Peptides in Fish" Biology 14, no. 9: 1243. https://doi.org/10.3390/biology14091243
APA StyleYu, X.-Z., Yu, Y., & Liu, Z.-Y. (2025). Crosstalk Between Intestinal Microbiota and Host Defense Peptides in Fish. Biology, 14(9), 1243. https://doi.org/10.3390/biology14091243







