Key Invariants in the Evolution of Sociality Across Taxa
Simple Summary
Abstract
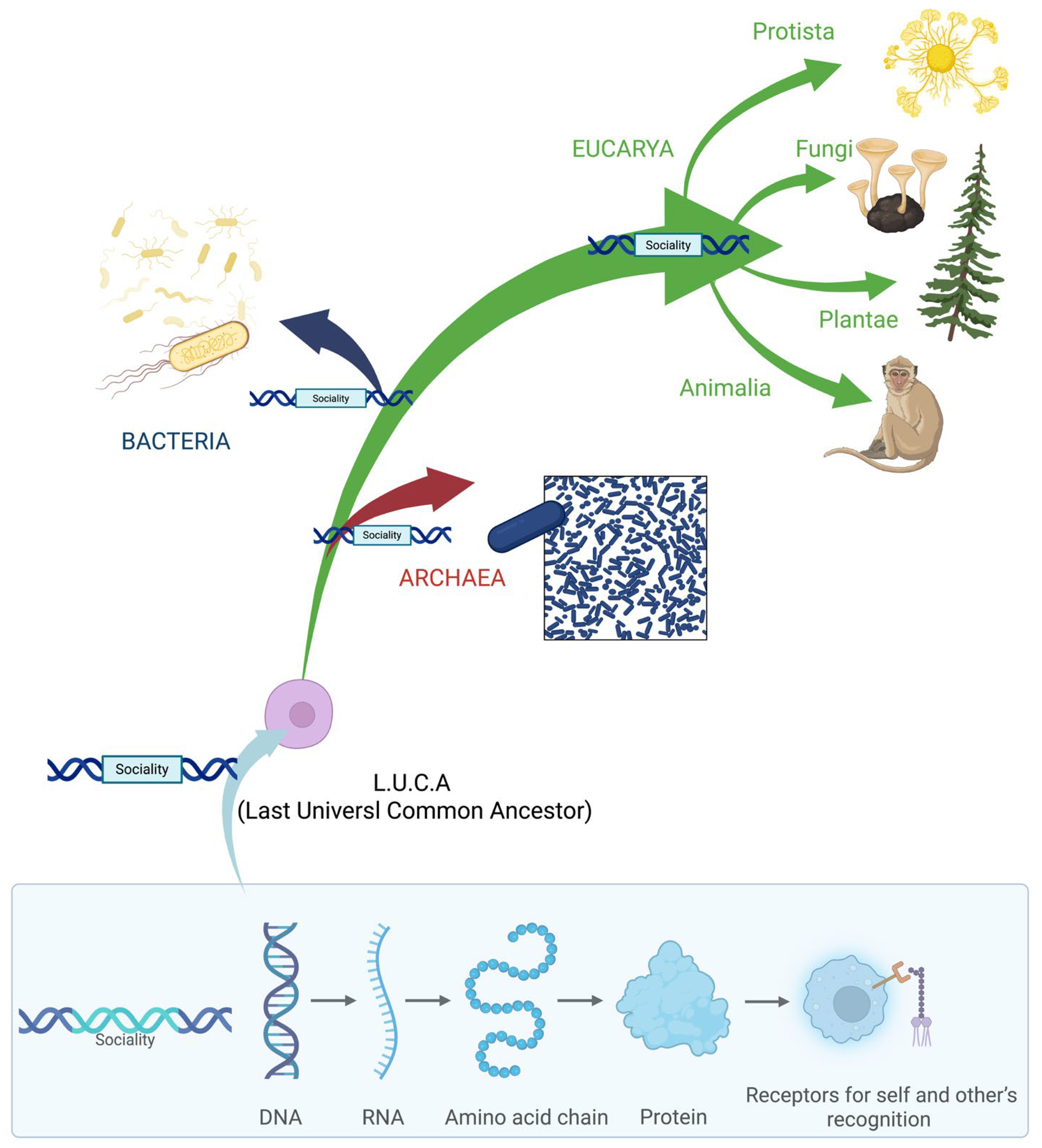
1. Introduction
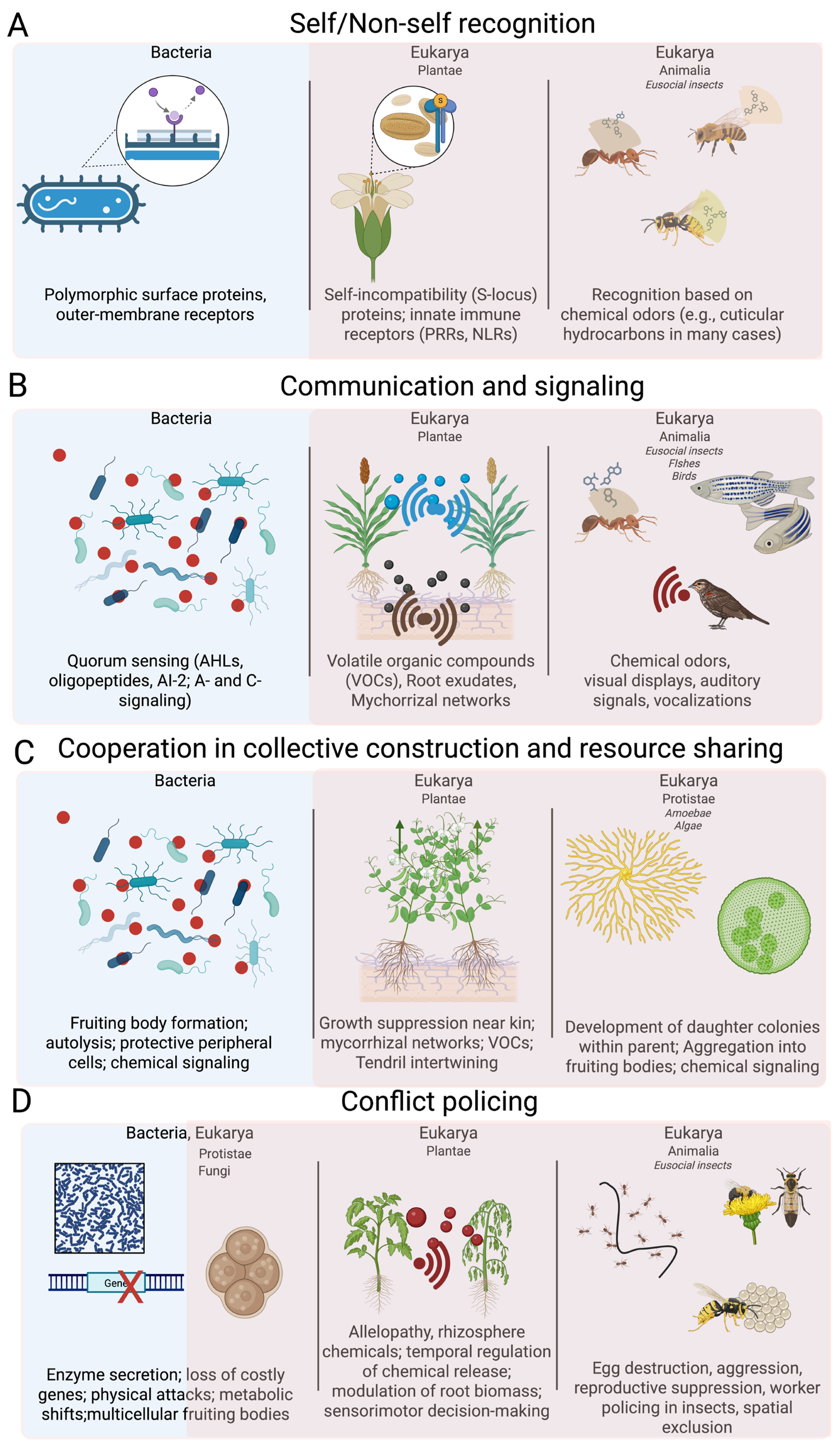
2. Self/Non-Self Recognition
3. Communication and Signaling
3.1. Communication and Signaling in Microbial Domain
3.2. Communication and Signaling in Plants
3.3. Communication and Signaling in Animals
4. Cooperation in Collective Construction & Resource Sharing
4.1. Cooperation in Microbial Domain
4.2. Cooperation in Plants
5. Conflict Policing
5.1. Conflict Policing in Eusocial Insects
5.2. Conflict Policing in Microbial Domain
5.3. Conflict Policing in Plants
6. From Genes to Selection Gradients
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Literature-Search Criteria & Inclusion Thresholds
References
- Toth, A.L.; Robinson, G.E. Evo-devo and the evolution of social behavior. Trends Genet. 2007, 23, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Cahan, S.H.; Blumstein, D.T.; Sundström, L.; Liebig, J.; Griffin, A. Social trajectories and the evolution of social behavior. Oikos 2002, 96, 206–216. [Google Scholar] [CrossRef]
- Brothers, L.; Ring, B.; Kling, A. Response of neurons in the macaque amygdala to complex social stimuli. Behav. Brain Res. 1990, 41, 199–213. [Google Scholar] [CrossRef]
- Kling, A.S.; Brothers, L.A. The amygdala and social behavior. In The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction; Aggleton, J.P., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1992; pp. 353–377. [Google Scholar]
- Raleigh, M.J.; Steklis, H.D. Effects of orbitofrontal and temporal neocortical lesions on the affiliative behavior of vervet monkeys (Cercopithecus aethiops sabaeus). Exp. Neurol. 1981, 73, 378–389. [Google Scholar] [CrossRef]
- Gallese, V.; Fadiga, L.; Fogassi, L.; Rizzolatti, G. Action recognition in the premotor cortex. Brain 1996, 119, 593–609. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Fogassi, L.; Gallese, V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001, 2, 661–670. [Google Scholar] [CrossRef]
- Perrett, D.I.; Hietanen, J.K.; Oram, M.W.; Benson, P.J. Organization and functions of cells responsive to faces in the temporal cortex. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1992, 335, 23–30. [Google Scholar]
- Adolphs, R. Investigating the cognitive neuroscience of social behavior. Neuropsychologia 2003, 41, 119–126. [Google Scholar] [CrossRef]
- Amodio, D.M.; Frith, C.D. Meeting of minds: The medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006, 7, 268–277. [Google Scholar] [CrossRef]
- Costa, J.T. The Other Insect Societies; Harvard University Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Wright, D.; Ward, A.J.W.; Croft, D.P.; Krause, J. Social organization, grouping, and domestication in fish. Zebrafish 2006, 3, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Pollen, A.A.; Dobberfuhl, A.P.; Scace, J.; Igulu, M.M.; Renn, S.C.; Shumway, C.A.; Hofmann, H.A. Environmental complexity and social organization sculpt the brain in Lake Tanganyikan cichlid fish. Brain Behav. Evol. 2007, 70, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Lahti, K. Social dominance and survival in flocking passerine birds: A review with an emphasis on the willow tit Parus montanus. Ornis Fenn. 1998, 75, 1–17. [Google Scholar]
- Riters, L.V.; Kelm-Nelson, C.A.; Spool, J.A. Why do birds flock? A role for opioids in the reinforcement of gregarious social interactions. Front. Physiol. 2019, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Castiello, U.; Becchio, C.; Zoia, S.; Nelini, C.; Sartori, L.; Blason, L.; Gallese, V. Wired to be social: The ontogeny of human interaction. PLoS ONE 2010, 5, e13199. [Google Scholar] [CrossRef]
- Moulia, B.; Bastien, R.; Chauvet-Thiry, H.; Leblanc-Fournier, N. Posture control in land plants: Growth, position sensing, proprioception, balance, and elasticity. J. Exp. Bot. 2019, 70, 3467–3494. [Google Scholar] [CrossRef]
- Moulia, B.; Douady, S.; Hamant, O. Fluctuations shape plants through proprioception. Science 2021, 372, eabc6868. [Google Scholar] [CrossRef]
- Baluška, F.; Mancuso, S. Individuality, self and sociality of vascular plants. Philos. Trans. R. Soc. B 2021, 376, 20190760. [Google Scholar] [CrossRef]
- Bilas, R.D.; Bretman, A.; Bennett, T. Friends, neighbours and enemies: An overview of the communal and social biology of plants. In Plant, Cell & Environment; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 102–138. [Google Scholar]
- Bonato, B.; Peressotti, F.; Guerra, S.; Wang, Q.; Castiello, U. Cracking the code: A comparative approach to plant communication. Commun. Integr. Biol. 2021, 14, 176–185. [Google Scholar] [CrossRef]
- Bonato, B.; Simonetti, V.; Bulgheroni, M.; Wang, Q.; Guerra, S.; Quaggiotti, S.; Castiello, U. Evidence of motor intentions in plants: A kinematical study. J. Comp. Psychol. 2023, 137, 228–237. [Google Scholar] [CrossRef]
- Bonato, B.; Wang, Q.; Guerra, S.; Simonetti, V.; Bulgheroni, M.; Quaggiotti, S.; Ruperti, B.; Castiello, U. ‘United we stand, divided we fall’: Intertwining as evidence of joint actions in pea plants. AoB Plants 2024, 16, plad088. [Google Scholar] [CrossRef]
- Grosberg, R.K. The evolution of allorecognition specificity in clonal invertebrates. Q. Rev. Biol. 1988, 63, 377–412. [Google Scholar] [CrossRef]
- Tsutsui, N.D. Scents of self: The expression component of self/non-self recognition systems. In Annales Zoologici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2004; pp. 713–727. [Google Scholar]
- Breed, M.D.; Bennett, B. Kin recognition in highly eusocial insects. In Kin Recognition in Animals; Fletcher, D.J.C., Michener, C.D., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 1987; pp. 243–285. [Google Scholar]
- Holmes, W.G.; Sherman, P.W. Kin recognition in animals: The prevalence of nepotism among animals raises basic questions about how and why they distinguish relatives from unrelated individuals. Am. Sci. 1983, 71, 46–55. [Google Scholar]
- Takayama, S.; Isogai, A. Self-incompatibility in plants. Annu. Rev. Plant Biol. 2005, 56, 467–489. [Google Scholar] [CrossRef] [PubMed]
- Dudley, S.A.; File, A.L. Kin recognition in an annual plant. Biol. Lett. 2007, 3, 435–438. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Bull, J.J. Evolution of Sex Determining Mechanisms; John Wiley & Sons: Hoboken, NJ, USA, 1983; pp. xx + 316. [Google Scholar]
- Charlesworth, D. Effects of inbreeding on the genetic diversity of populations. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 1051–1070. [Google Scholar] [CrossRef]
- Casselton, L.A. Mate recognition in fungi. Heredity 2002, 88, 142–147. [Google Scholar] [CrossRef]
- Gamboa, H.; Fred, A. A behavioral biometric system based on human-computer interaction. In Biometric Technology for Human Identification; SPIE: Bellingham, WA, USA, 2004; Volume 5404, pp. 381–392. [Google Scholar]
- Singer, T.L. Roles of hydrocarbons in the recognition systems of insects. Am. Zool. 1998, 38, 394–405. [Google Scholar] [CrossRef]
- Keller, L.; Surette, M.G. Communication in bacteria: An ecological and evolutionary perspective. Nat. Rev. Microbiol. 2006, 4, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Endler, J.A. Some general comments on the evolution and design of animal communication systems. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1993, 340, 215–225. [Google Scholar]
- Choe, J.C.; Crespi, B.J. (Eds.) The Evolution of Social Behavior in Insects and Arachnids; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Griffin, A.S.; West, S.A.; Buckling, A. Cooperation and competition in pathogenic bacteria. Nature 2004, 430, 1024–1027. [Google Scholar] [CrossRef]
- Fiegna, F.; Velicer, G.J. Exploitative and hierarchical antagonism in a cooperative bacterium. PLoS Biol. 2005, 3, e370. [Google Scholar] [CrossRef]
- Escalante, R.; Vicente, J.J. Dictyostelium discoideum: A model system for differentiation and patterning. Int. J. Dev. Biol. 2000, 44, 819–836. [Google Scholar] [PubMed]
- Kaiser, D. Signaling in myxobacteria. Annu. Rev. Microbiol. 2004, 58, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Weijer, C.J. Dictyostelium morphogenesis. Curr. Opin. Genet. Dev. 2004, 14, 392–398. [Google Scholar] [CrossRef]
- Shimkets, L.J. Intercellular signaling during fruiting-body development of Myxococcus xanthus. Annu. Rev. Microbiol. 1999, 53, 525–549. [Google Scholar] [CrossRef] [PubMed]
- Strassmann, J.E.; Zhu, Y.; Queller, D.C. Altruism and social cheating in the social amoeba Dictyostelium discoideum. Nature 2000, 408, 965–967. [Google Scholar] [CrossRef]
- Simard, S.W.; Perry, D.A.; Jones, M.D.; Myrold, D.D.; Durall, D.M.; Molina, R. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 1997, 388, 579–582. [Google Scholar] [CrossRef]
- Heil, M. Herbivore-induced plant volatiles: Targets, perception and unanswered questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Gagliano, M.; Grimonprez, M. Breaking the silence—Language and the making of meaning in plants. Ecopsychology 2015, 7, 145–152. [Google Scholar] [CrossRef]
- Karban, R.; Shiojiri, K.; Ishizaki, S. Plant communication–why should plants emit volatile cues? J. Plant Interact. 2011, 6, 81–84. [Google Scholar] [CrossRef]
- Trewavas, A. Plant Behaviour and Intelligence; OUP Oxford: Oxford, UK, 2014. [Google Scholar]
- Dicke, M.; Sabelis, M.W. How plants obtain predatory mites as bodyguards. Neth. J. Zool. 1988, 38, 148–165. [Google Scholar] [CrossRef]
- Turlings, T.C.; Tumlinson, J.H.; Lewis, W.J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–634. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; Von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions:“Talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Shiojiri, K.; Ishizaki, S.; Wetzel, W.C.; Evans, R.Y. Kin recognition affects plant communication and defence. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123062. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef]
- Karban, R.; Shiojiri, K.; Huntzinger, M.; McCall, A.C. Damage-induced resistance in sagebrush: Volatiles are key to intra-and interplant communication. Ecology 2006, 87, 922–930. [Google Scholar] [CrossRef]
- Heil, M.; Ton, J. Long-distance signalling in plant defence. Trends Plant Sci. 2008, 13, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. The chemical basis of plant defense. In Plant Defenses Against Mammalian Herbivory; CRC Press: Boca Raton, FL, USA, 1991; Volume 45. [Google Scholar]
- Holopainen, J.K. Multiple functions of inducible plant volatiles. Trends Plant Sci. 2004, 9, 529–533. [Google Scholar] [CrossRef]
- Vet, L.E.; Dicke, M. Ecology of infochemical by natural enemies in a tritrophic context. Annu. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- Dicke, M.; Sabelis, M.W.; Takabayashi, J.; Bruin, J.; Posthumus, M.A. Plant strategies of manipulating predatorprey interactions through allelochemicals: Prospects for application in pest control. J. Chem. Ecol. 1990, 16, 3091–3118. [Google Scholar] [CrossRef]
- Takabayashi, J. Herbivory-induced plant volatiles mediate multitrophic relationships in ecosystems. Plant Cell Physiol. 2022, 63, 1344–1355. [Google Scholar] [CrossRef]
- Karban, R.; Shiojiri, K. Self-recognition affects plant communication and defense. Ecol. Lett. 2008, 12, 502–506. [Google Scholar] [CrossRef]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.P.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef]
- Wheeldon, C.D.; Hamon-Josse, M.; Lund, H.; Yoneyama, K.; Bennett, T. Environmental strigolactone drives early growth responses to neighboring plants and soil volume in pea. Curr. Biol. 2022, 32, 3593–3600. [Google Scholar] [CrossRef]
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant 2013, 6, 18–28. [Google Scholar] [CrossRef]
- Bonato, B.; Bennett, T.; Guerra, S.; Avesani, S.; Castiello, U. Do strigolactones play a role in the ascent and attachment behavior of Pisum sativum? Plant Signal. Behav. 2025, 20, 2447455. [Google Scholar] [CrossRef]
- DiFrisco, J.; Wagner, G.P.; Love, A.C. Reframing research on evolutionary novelty and co-option: Character identity mechanisms versus deep homology. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2023; Volume 145, pp. 3–12. [Google Scholar]
- Jones Jr, S.K.; Bennett, R.J. Fungal mating pheromones: Choreographing the dating game. Fungal Genet. Biol. 2011, 48, 668–676. [Google Scholar] [CrossRef]
- Ladich, F. Ecology of sound communication in fishes. Fish. Fish. 2019, 20, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Bass, A.H.; McKibben, J.R. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog. Neurobiol. 2003, 69, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.N. Semantic communication in birds: Evidence from field research over the past two decades. Ecol. Res. 2016, 31, 307–319. [Google Scholar] [CrossRef]
- Kroodsma, D.E.; Miller, E.H. (Eds.) Ecology and Evolution of Acoustic Communication in Birds; Cornell University Press: Ithaca, NY, USA, 2020. [Google Scholar]
- Grieves, L.A.; Gilles, M.; Cuthill, I.C.; Székely, T.; MacDougall-Shackleton, E.A.; Caspers, B.A. Olfactory camouflage and communication in birds. Biol. Rev. 2022, 97, 1193–1209. [Google Scholar] [CrossRef] [PubMed]
- Witzany, G. Why biocommunication of animals? In Biocommunication of Animals; Springer: Dordrecht, The Netherlands, 2013; pp. 1–6. [Google Scholar]
- Connor, R.C. The benefits of mutualism: A conceptual framework. Biol. Rev. 1995, 70, 427–457. [Google Scholar] [CrossRef]
- Frank, S.A. Repression of competition and the evolution of cooperation. Evolution 2003, 57, 693–705. [Google Scholar] [CrossRef]
- Crespi, B.J. The evolution of social behavior in microorganisms. Trends Ecol. Evol. 2001, 16, 178–183. [Google Scholar] [CrossRef]
- Tarnita, C.E. The ecology and evolution of social behavior in microbes. J. Exp. Biol. 2017, 220, 18–24. [Google Scholar] [CrossRef]
- Bonner, J.T. The social amoebae: The biology of cellular slime molds. In The Social Amoebae; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Strassmann, J.E.; Queller, D.C. Evolution of cooperation and control of cheating in a social microbe. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 2), 10855–10862. [Google Scholar] [CrossRef]
- Fortunato, A.; Strassmann, J.E.; Santorelli, L.; Queller, D.C. Co-occurrence in nature of different clones of the social amoeba, Dictyostelium discoideum. Mol. Ecol. 2003, 12, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Velicer, G.J.; Vos, M. Sociobiology of the myxobacteria. Annu. Rev. Microbiol. 2009, 63, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Shimkets, L.J. Social and developmental biology of the myxobacteria. Microbiol. Rev. 1990, 54, 473–501. [Google Scholar] [CrossRef]
- Dworkin, M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 1996, 60, 70–102. [Google Scholar] [CrossRef] [PubMed]
- Gadagkar, R.; Bonner, J. Social insects and social amoebae. J. Biosci. 1994, 19, 219–245. [Google Scholar] [CrossRef]
- Sharma, A.; Sahgal, M.; Johri, B.N. Microbial communication in the rhizosphere: Operation of quorum sensing. Curr. Sci. 2003, 85, 1164–1172. [Google Scholar]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef]
- Semchenko, M.; Zobel, K.; Hutchings, M.J. To compete or not to compete: An experimental study of interactions between plant species with contrasting root behaviour. Evol. Ecol. 2010, 24, 1433–1445. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- Dudley, S.A. Plant cooperation. AoB Plants 2015, 7, plv113. [Google Scholar] [CrossRef]
- Kochert, G. Differentiation of reproductive cells in Volvox carteri. J. Protozool. 1968, 15, 438–452. [Google Scholar] [CrossRef]
- Rowe, N.P.; Speck, T. Stem biomechanics, strength of attachment, and developmental plasticity of vines and lianas. In Ecology of Lianas; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 323–342. [Google Scholar]
- Simard, S.W. The foundational role of mycorrhizal networks in self-organization of interior Douglas-fir forests. For. Ecol. Manag. 2009, 258, S95–S107. [Google Scholar] [CrossRef]
- Beiler, K.J.; Durall, D.M.; Simard, S.W.; Maxwell, S.A.; Kretzer, A.M. Architecture of the wood-wide web: Rhizopogon spp. genets link multiple Douglas-fir cohorts. New Phytol. 2010, 185, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Castiello, U. (Re) claiming plants in comparative psychology. J. Comp. Psychol. 2021, 135, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.F.; Leuzinger, S. Hydraulic coupling of a leafless kauri tree remnant to conspecific hosts. iScience 2019, 19, 1238–1247. [Google Scholar] [CrossRef]
- van Der Heijden, M.G.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Monat, J.P. The Self-Awareness of the Forest. Futures 2024, 163, 103429. [Google Scholar] [CrossRef]
- Sen, R. Budgeting for the wood-wide web. New Phytol. 2000, 145, 161–163. [Google Scholar] [CrossRef]
- Scott, J. Trend report social network analysis. Sociology 1988, 22, 109–127. [Google Scholar] [CrossRef]
- Jeong, J. Analysis of plant social network of vascular plants. In Environment, Development and Sustainability; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–20. [Google Scholar]
- Von Humboldt, A.; Bonpland, A. Ideen zu Einer Geographie der Pflanzen Nebst Einem Naturgemälde der Tropenländer: Auf Beobachtungen und Messungen Gegründet, Welche vom 10ten Grade Nördlicher bis Zum 10ten Grade Südlicher Breite, in den Jahren 1799, 1800, 1801, 1802 und 1803 Angestellt Worden Sind; Missouri Botanical Garden, Peter H. Raven Library: St. Louis, MO, USA, 1807; Volume 1. [Google Scholar]
- Miller, R.S. Pattern and process in competition. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 1967; Volume 4, pp. 1–74. [Google Scholar]
- Darwin, C. The Evidence of the Descent of Man and Selection in Relation to Sex; John Murray: London, UK, 1871. [Google Scholar]
- Huntingford, F.A. Animal Conflict; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Beekman, M.; Ratnieks, F.L. Power over reproduction in social Hymenoptera. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 1741–1753. [Google Scholar] [CrossRef]
- Ratnieks, F.L.; Foster, K.R.; Wenseleers, T. Conflict resolution in insect societies. Annu. Rev. Entomol. 2006, 51, 581–608. [Google Scholar] [CrossRef]
- Ghoul, M.; Mitri, S. The ecology and evolution of microbial competition. Trends Microbiol. 2016, 24, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Nadell, C.D.; Bassler, B.L. A fitness trade-off between local competition and dispersal in Vibrio cholerae biofilms. Proc. Natl. Acad. Sci. USA 2011, 108, 14181–14185. [Google Scholar] [CrossRef]
- Thiery, S.; Kaimer, C. The predation strategy of Myxococcus xanthus. Front. Microbiol. 2020, 11, 2. [Google Scholar] [CrossRef]
- Augustine, N.; Kumar, P.; Thomas, S. Inhibition of Vibrio cholerae biofilm by AiiA enzyme produced from Bacillus spp. Arch. Microbiol. 2010, 192, 1019–1022. [Google Scholar]
- Rendueles, O.; Ghigo, J.M. Multi-species biofilms: How to avoid unfriendly neighbors. FEMS Microbiol. Rev. 2012, 36, 972–989. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zamanizadehnajari, S.; Baldwin, I.T. Phytotoxic volatiles in the roots and shoots of Artemisia tridentata as detected by headspace solid-phase microextraction and gas chromatographic-mass spectrometry analysis. J. Chem. Ecol. 2010, 36, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, W. Allelopathic effects of volatiles from some Cruciferae species on lettuce, barnyard grass and wheat growth. Plant Soil 1987, 102, 271–273. [Google Scholar] [CrossRef]
- Effah, E.; Holopainen, J.K.; McCormick, A.C. Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 58–63. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Hasegawa, M.; Ino, T.; Ota, K.; Kujime, H. Contribution of momilactone A and B to rice allelopathy. J. Plant Physiol. 2010, 167, 787–791. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Yang, F.; Shao, P. Rhizosheaths stimulate short-term root decomposition in a semiarid grassland. Sci. Total Environ. 2018, 640, 1297–1301. [Google Scholar] [CrossRef]
- Roshchina, V.V.; Yashina, A.V.; Yashin, V.A.; Prizova, N.K. Models to study pollen allelopathy. Allelopath. J. 2009, 23, 3–24. [Google Scholar]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Gaofeng, X.; Shicai, S.; Fudou, Z.; Yun, Z.; Hisashi, K.N.; David, R.C. Relationship between allelopathic effects and functional traits of different allelopathic potential rice accessions at different growth stages. Rice Sci. 2018, 25, 32–41. [Google Scholar] [CrossRef]
- Fang, Y.; Jiao, X.; Xie, W.; Wang, S.; Wu, Q.; Shi, X.; Zhang, Y. Tomato yellow leaf curl virus alters the host preferences of its vector Bemisia tabaci. Sci. Rep. 2013, 3, 2876. [Google Scholar] [CrossRef]
- Falik, O.; de Kroon, H.; Novoplansky, A. Physiologically-mediated self/non-self root discrimination in Trifolium repens has mixed effects on plant performance. Plant Signal. Behav. 2006, 1, 116–121. [Google Scholar] [CrossRef]
- Boyden, S.; Binkley, D.; Stape, J.L. Competition among Eucalyptus trees depends on genetic variation and resource supply. Ecology 2008, 89, 2850–2859. [Google Scholar] [CrossRef] [PubMed]
- Crutsinger, G.M.; Souza, L.; Sanders, N.J. Intraspecific diversity and dominant genotypes resist plant invasions. Ecol. Lett. 2008, 11, 16–23. [Google Scholar] [CrossRef]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.; Underwood, N.; Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef] [PubMed]
- West, S.A.; Pen, I.; Griffin, A.S. Cooperation and competition between relatives. Science 2002, 296, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, E.A.; Dale, J. Individual recognition: It is good to be different. Trends Ecol. Evol. 2007, 22, 529–537. [Google Scholar] [CrossRef]
- Callaway, R.M.; Mahall, B.E. Family roots. Nature 2007, 448, 145–146. [Google Scholar] [CrossRef]
- Johnstone, R.A. Eavesdropping and animal conflict. Proc. Natl. Acad. Sci. USA 2001, 98, 9177–9180. [Google Scholar] [CrossRef]
- Oliveira, P.S.; Pie, M.R. Interaction between ants and plants bearing extrafloral nectaries in cerrado vegetation. An. Soc. Entomológica Bras. 1998, 27, 161–176. [Google Scholar] [CrossRef]
- Arimura, G.I.; Ozawa, R.; Shimoda, T.; Nishioka, T.; Boland, W.; Takabayashi, J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 2000, 406, 512–515. [Google Scholar] [CrossRef]
- Dolch, R.; Tscharntke, T. Defoliation of alders (Alnus glutinosa) affects herbivory by leaf beetles on undamaged neighbours. Oecologia 2000, 125, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; van Poecke, R.M.; de Boer, J.G. Inducible indirect defence of plants: From mechanisms to ecological functions. Basic. Appl. Ecol. 2003, 4, 27–42. [Google Scholar] [CrossRef]
- Babikova, Z.; Gilbert, L.; Bruce, T.J.; Birkett, M.; Caulfield, J.C.; Woodcock, C.; Johnson, D. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecol. Lett. 2013, 16, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Ye, M.; Li, C.; He, X.; Zhu-Salzman, K.; Wang, R.L.; Zeng, R.S. Hijacking common mycorrhizal networks for herbivore-induced defence signal transfer between tomato plants. Sci. Rep. 2014, 4, 3915. [Google Scholar] [CrossRef]
- Runyon, J.B.; Mescher, M.C.; De Moraes, C.M. Volatile chemical cues guide host location and host selection by parasitic plants. Science 2006, 313, 1964–1967. [Google Scholar] [CrossRef]
- Crick, F.; Koch, C. Consciousness and neuroscience. Cereb. Cortex 1998, 8, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Crick, F.; Barnett, L.; Brenner, S.; Watts-Tobin, R.J. General nature of the genetic code for proteins. Nature 1961, 192, 1227–1232. [Google Scholar] [CrossRef]
- Nirenberg, M. Historical review: Deciphering the genetic code–a personal account. Trends Biochem. Sci. 2004, 29, 46–54. [Google Scholar] [CrossRef]
- Koonin, E.V.; Novozhilov, A.S. Origin and evolution of the genetic code: The universal enigma. IUBMB Life 2009, 61, 99–111. [Google Scholar] [CrossRef]
- Koonin, E.V.; Novozhilov, A.S. Origin and evolution of the universal genetic code. Annu. Rev. Genet. 2017, 51, 45–62. [Google Scholar] [CrossRef]
- Koonin, E.V. Comparative genomics, minimal gene-sets and the last universal common ancestor. Nat. Rev. Microbiol. 2003, 1, 127–136. [Google Scholar] [CrossRef]
- True, J.R.; Carroll, S.B. Gene co-option in physiological and morphological evolution. Annu. Rev. Cell Dev. Biol. 2002, 18, 53–80. [Google Scholar] [CrossRef] [PubMed]
- Rehan, S.M.; Toth, A.L. Climbing the social ladder: The molecular evolution of sociality. Trends Ecol. Evol. 2015, 30, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Belcher, L.J.; Dewar, A.E.; Ghoul, M.; West, S.A. Kin selection for cooperation in natural bacterial populations. Proc. Natl. Acad. Sci. USA 2022, 119, e2119070119. [Google Scholar] [CrossRef]
- de Oliveira, J.L.; Morales, A.C.; Stewart, B.; Gruenheit, N.; Engelmoer, J.; Brown, S.B.; de Brito, R.A.; Hurst, L.D.; Urrutia, A.O.; Thompson, C.R.L.; et al. Conditional expression explains molecular evolution of social genes in a microbe. Nat. Commun. 2019, 10, 3284. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.R.; Mikheyev, A.S.; Linksvayer, T.A. Genomic signature of kin selection in an ant with obligately sterile workers. Mol. Biol. Evol. 2017, 34, 1780–1787. [Google Scholar] [CrossRef]
- Imrit, M.A.; Dogantzis, K.A.; Harpur, B.A.; Zayed, A. Eusociality influences the strength of negative selection on insect genomes. Proc. R. Soc. B 2020, 287, 20201512. [Google Scholar] [CrossRef]
- Rubenstein, D.R.; Ågren, J.A.; Carbone, L.; Elde, N.C.; Hoekstra, H.E.; Kapheim, K.M.; Hofmann, H.A. Coevolution of genome architecture and social behavior. Trends Ecol. Evol. 2019, 34, 844–855. [Google Scholar] [CrossRef]
- Saul, M.C.; Blatti, C.; Yang, W.; Bukhari, S.A.; Shpigler, H.Y.; Troy, J.M.; Seward, C.H.; Sloofman, L.; Chandrasekaran, S.; Bell, A.M.; et al. Cross-species systems analysis of evolutionary toolkits of neurogenomic response to social challenge. Genes Brain Behav. 2019, 18, e12502. [Google Scholar] [CrossRef]
- Mullon, C.; Keller, L.; Lehmann, L. Social polymorphism is favoured by the co-evolution of dispersal with social behaviour. Nat. Ecol. Evol. 2018, 2, 132–140. [Google Scholar] [CrossRef]
- Kay, C.; Peacock, L.; Williams, T.A.; Gibson, W. Signatures of hybridization in Trypanosoma brucei. PLoS Pathog. 2022, 18, e1010300. [Google Scholar] [CrossRef]
| Domain/ Examples | Mechanism/ Molecular Basis | Role in Social Behavior | Reference |
|---|---|---|---|
| Bacteria (Proteus mirabilis, Bacillus subtilis) | Polymorphic surface proteins, outer-membrane receptors | Kin recognition, territoriality, conflict resolution, restrict cooperation | Tsutsui, 2004 [25]; Breed & Bennett, 1987 [26] |
| Eukarya Plantae (Arabidopsis thaliana, Cakile Edentula, B. rapa, B. olerosa) | Self-incompatibility (S-locus) proteins; innate immune receptors (PRRs, NLRs) | Prevent self-fertilization, promote outcrossing; discriminate self vs. non-self roots; pathogen recognition | Takayama & Isogai, 2005 [28]; Dudley & File, 2007 [29]; Jones & Dangl, 2006 [30] |
| Eukarya Animalia (Apis mellifera, Camponotus vagus, Camponotus floridanus, Lassioglossum zephyrum, Polistes spp., Vespula spp.) | Recognition based on chemical odors (e.g., cuticular hydrocarbons in many cases) | Colony recognition, nestmate discrimination, caste/task signaling | Breed & Bennett, 1987 [26]; Gamboa & Fred, 2004 [34]; Tsutsui, 2004 [25] |
| Domain/ Examples | Mechanism/ Molecular Basis | Role in Social Behavior | Reference |
|---|---|---|---|
| Bacteria (Pseudomonas aeruginosa, Myxococcus xanthus) | Chemical signaling: quorum sensing (AHLs, oligopeptides, AI-2; A- and C- signaling in M. xanthus) | Kin recognition, cooperation, conflict resolution, aggregation, fruiting body formation, spore differentiation | Keller & Surette, 2006 [36]; Griffin et al., 2004 [39]; Fiegna & Velicer, 2005 [40]; Kaiser, 2004 [42]; Shimkets, 1999 [44]; Strassman, 2000 [45] |
| Eukarya Plantae (Phaseolus vulgaris, Zea Mays, Nicotiana tabacum, Oryza sativa, Gossypium hirstum, Pisum sativum, Artemisia Tridentata) | Volatile organic compounds (VOCs), Root exudates. | Herbivore defense, pollinator attraction, neighbor communication, pathogen defense, adaptation to stress | Dicke & Sabelis, 1988 [51]; Turlings et al., 1990 [52]; De Moraes et al., 2001 [53]; Dudareva & Pichersky, 2000 [55]; Karban et al., 2006 [57]; Gagliano & Grimonprez, 2015 [48]; Karban & Shiojiri, 2008 [49]. |
| Eukarya Animalia Insects (Camponotus vagus, Camponotus floridanus, Linepithema humile, Solenopsis invicta, Apis mellifera, Lasioglossum zephyrum, Polistes spp., Vespula spp.) Fish (Ostariophysi, Cichlidae, Gobidae, Pomacentridae, Porichthys notatu) Birds (Parus minor, Troglodytes aedon, Taeniopygia guttata, Parus spp., Junco hyemalis) Fungi (Saccharomyces cerevisiae) | Chemical odors, visual displays, auditory signals, vocalizations | Colony recognition, nestmate discrimination, social hierarchy maintenance, territoriality, mate choice | Breed & Bennett, 1987 [26]; Gamboa, 2004 [34]; Tsutsui, 2004 [25]; Ladich, 2019 [76]; Bass & McKibben, 2003 [77]; Suzuki, 2016 [78]; Kroodsma & Miller, 2020 [79]; Grieves et al., 2022 [80] |
| Domain/ Examples | Mechanism/ Molecular Basis | Role in Social Behavior | Reference |
|---|---|---|---|
| Bacteria (Myxococcus xanthus) Archea | Fruiting body formation; autolysis; protective peripheral cells; chemical signaling | Division of labor among spores, dying, and protective cells; cooperation enables survival under nutrient scarcity; social cheating can evolve | Velicer & Vos, 2009 [89] |
| Eukarya Plantae (Pisum Sativum, Betula papyrifera, Pseudotsuga menziesii, Agathis australis) | Growth suppression near kin; mycorrhizal networks; VOCs; Tendril intertwining | Enhances collective fitness; resource and information sharing; hub trees support younger or stressed trees; “wood-wide web”; Joint action and mutual mechanical support; division of motor roles (handler vs. grasper) | Semchenko et al., 2010 [95]; Dudley, 2015 [97]; Wang et al. [6], 2020; Rowe & Speck, 2015 [99]; Castiello, 2021 [102]; Simard et al., 2009 [100]; Beiler et al., 2010 [101]; Bader & Leuzinger, 2019 [103]; van Der Hejiden et al., 2015 [104]; Jeong, 2024 [108]; Bonato et al., 2024 [23] |
| Eukarya Protistae (Dictyostelium discoideum, Volvox carteri) | Development of daughter colonies within parent; Aggregation into fruiting bodies; chemical signaling | Stalk cells sacrifice themselves to elevate spores; enables collective reproduction; cooperative division of labor even among unrelated individuals, Early forms of parental investment; kin-directed cooperation | Kocher, 1968 [98]; Castiello, 2021 [102]; Velicer & Vos, 2009 [89]; Strassman & Queller, 2011 [87]; Bonner, 2008 [86]; Fortunato et al., 2003 [88]. |
| Domain/ Examples | Mechanism/ Molecular Basis | Role in Social Behavior | Reference |
|---|---|---|---|
| Bacteria (Escherichia coli, Myxococcus xanthus, Bacillus subtilis, Vibrio cholerae) Protistae (Dictyostelium discoideum) Fungi (Saccharomyces cerevisiae) | Enzyme secretion; loss of costly genes; physical attacks; metabolic shifts; multicellular fruiting bodies | Limits competitor growth; maximizes resource acquisition; enhances survival under dense community conditions, Interference competition; inhibits biofilm formation of competitors | Ghoul & Mitri, 2016 [115]; Pfeiffer et al., 2001 [116]; Hibbing et al., 2010 [117]; Nadell & Bassler, 2011 [118]; Thiery & Kaimer, 2020 [119]. |
| Eukarya Plantae (Artemisia tridentata, Nicotiana attenuate, Lactuva sativa, Cakile edentula, Echinochloa crus-galli, Triticum Aestivum cv. Grana, Brassica nigra, Brassica juncea, Cuscuta pentagona) | Allelopathy via VOCs, rhizosphere chemicals; temporal regulation of chemical release; modulation of root biomass; sensorimotor decision-making | Inhibits germination and growth of neighbors; reduces metabolic cost while gaining early competitive advantage, Reduces intraspecific competition; promotes group-level fitness, Establishes dominance; competitive resource acquisition; resembles territoriality, Assesses competitor presence; primes defenses; adjusts growth; exploits neighbor information | Oleszek, 1987 [123]; Effah et al., 2019 [124]; Kato-Noguchi et al., 2010 [125]; Kong et al., 2018 [126]; Karban et al., 2013 [57]; Falik et al., 2006 [131]; Boyden et al., 2008 [132]; Crustinger et al., 2008 [133]; West et al., 2002 [135]; Tibbetts & Dale, 2007 [136]; Callaway, 2007 [137]; Wang et al., 2021 [96]; Bonato et al., 2025 [72]; Bonato et al., 2023 [22]; Dicke & Sabelis, 1988 [51]; Oliveira & Pie, 1998 [139]; Turlings & Tumlinson, 1990 [52]; Arimura et al., 2000 [140]; Dolch et al., 2000 [141]. |
| Eukarya Animalia (Formicidae, Vespidae, Apis Mellifera) | Egg destruction, aggression, reproductive suppression, worker policing in insects, spatial exclusion | Mantain social cohesion and cooperation, suppresses cheating, stabilizes group-level reproductive investment. | Ratnieks, 1988 [114]; Beekman, 2013 [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonato, B.; Dadda, M.; Castiello, U. Key Invariants in the Evolution of Sociality Across Taxa. Biology 2025, 14, 1239. https://doi.org/10.3390/biology14091239
Bonato B, Dadda M, Castiello U. Key Invariants in the Evolution of Sociality Across Taxa. Biology. 2025; 14(9):1239. https://doi.org/10.3390/biology14091239
Chicago/Turabian StyleBonato, Bianca, Marco Dadda, and Umberto Castiello. 2025. "Key Invariants in the Evolution of Sociality Across Taxa" Biology 14, no. 9: 1239. https://doi.org/10.3390/biology14091239
APA StyleBonato, B., Dadda, M., & Castiello, U. (2025). Key Invariants in the Evolution of Sociality Across Taxa. Biology, 14(9), 1239. https://doi.org/10.3390/biology14091239








