First Record of Clonostachys rosea as an Entomopathogenic Fungus of the Cephus fumipennis (Hymenoptera: Cephidae) in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolation and Purification
2.2. Strain Isolation and Purification
2.3. Morphological Characterization
2.4. Molecular Identification
2.5. Larval Bioassay with Fungal Strains
2.6. Optimization of the Culture Conditions of the Isolated Fungus
2.6.1. Influence of Medium on Fungal Growth and Sporulation
2.6.2. Optimizing the Carbon and Nitrogen Sources for Fungal Growth and Sporulation
2.6.3. Effect of Temperature on Fungal Growth and Sporulation
2.6.4. Optimization of Photoperiod for Fungal Growth and Sporulation
2.7. Statistical Analysis
3. Results
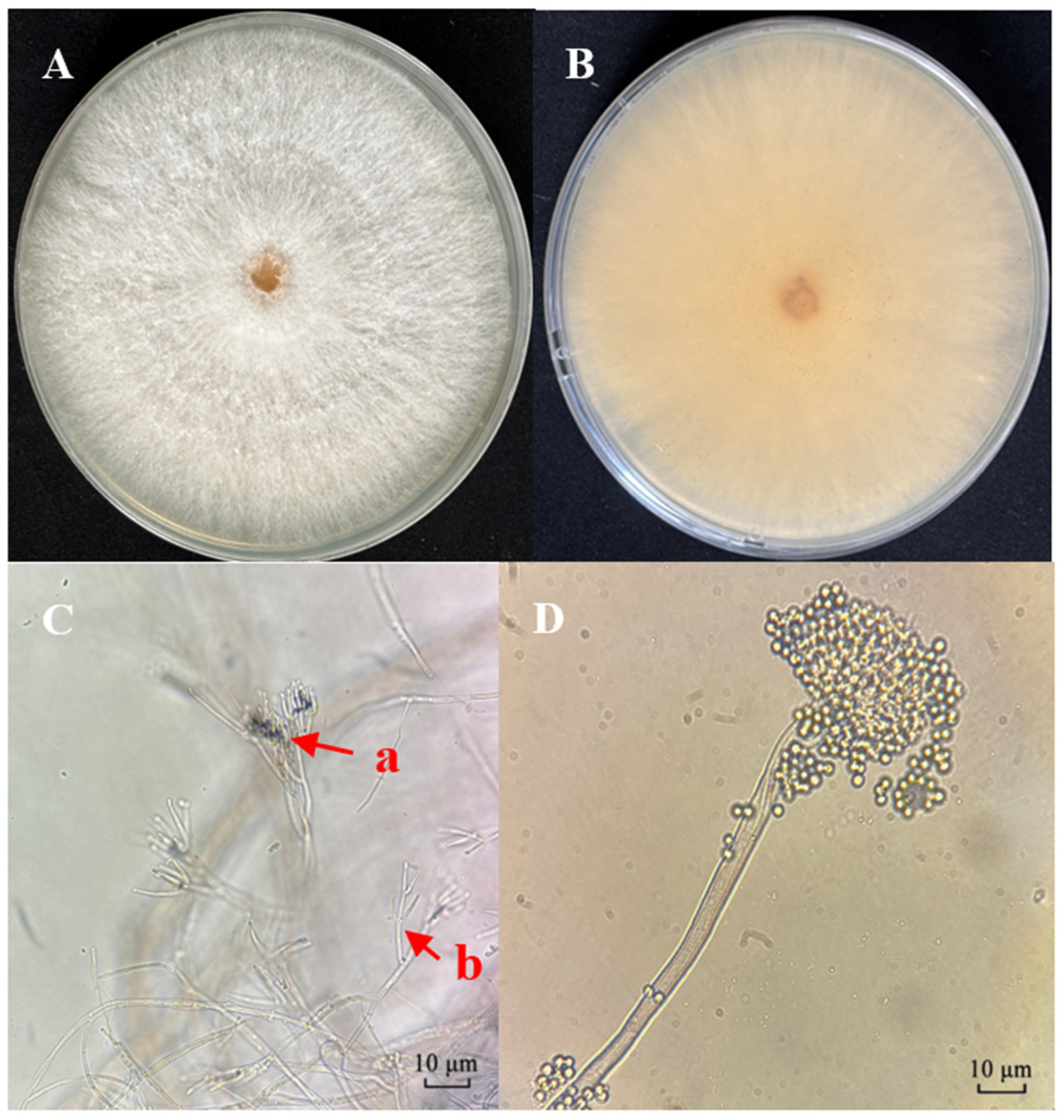
3.1. Morphological Identification of Strains
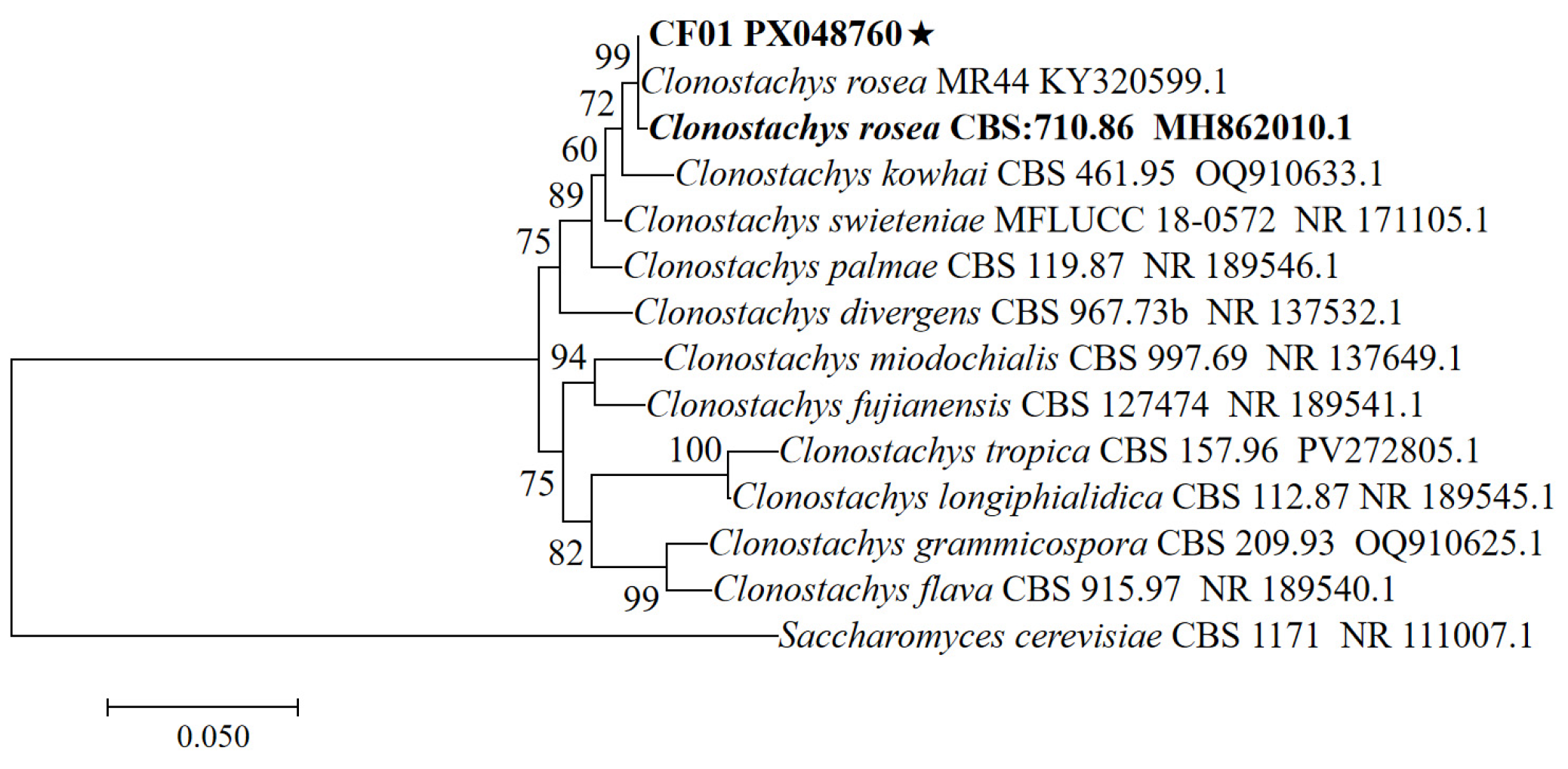
3.2. Molecular Biological Identification of the Strain
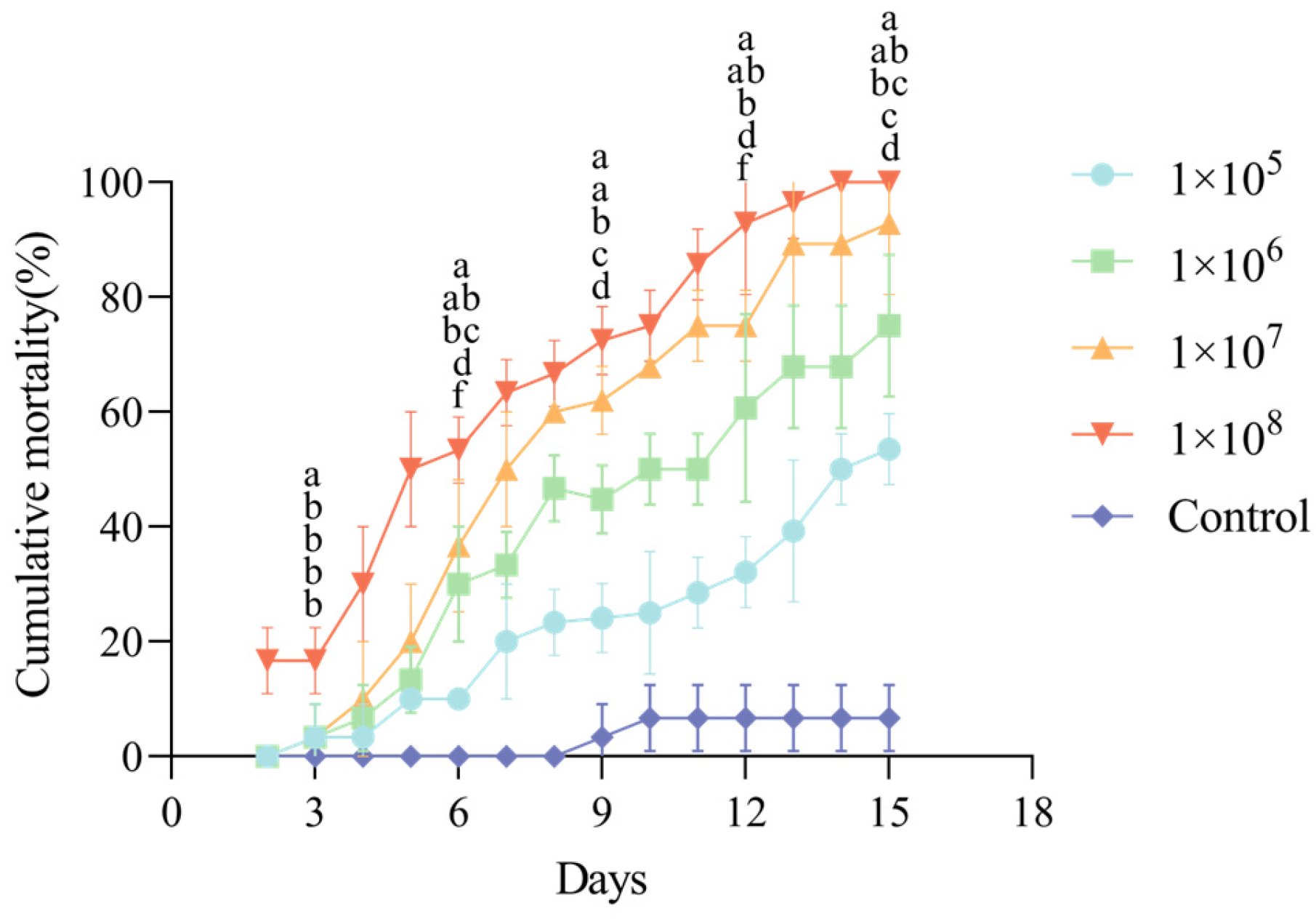
3.3. Pathogenicity of C. rosea CF01 Against C. fumipennis Larvae
3.4. Optimization of the Culture Conditions of the Isolated Fungus
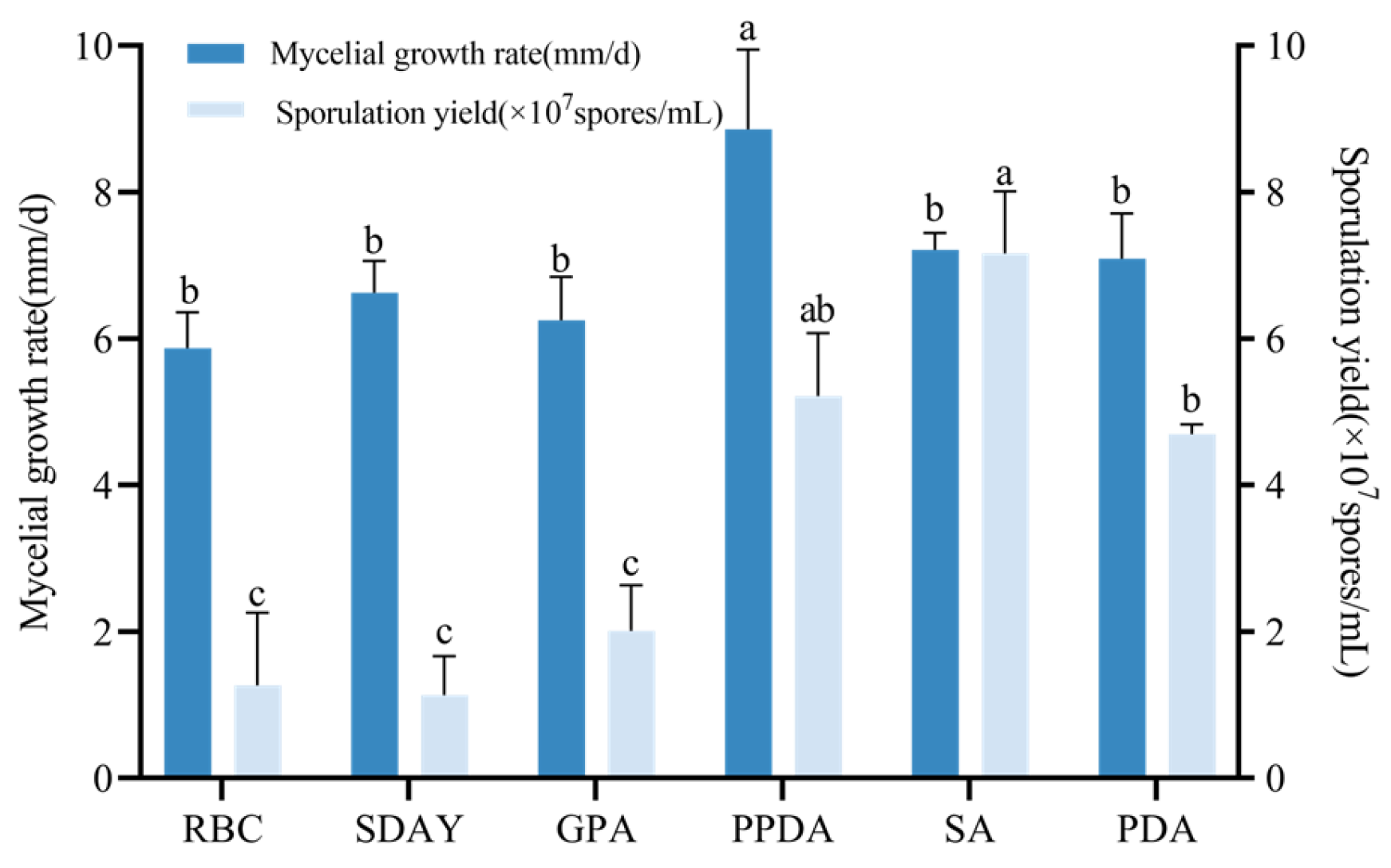
3.4.1. Optimization of Culture Medium for Strain CF01
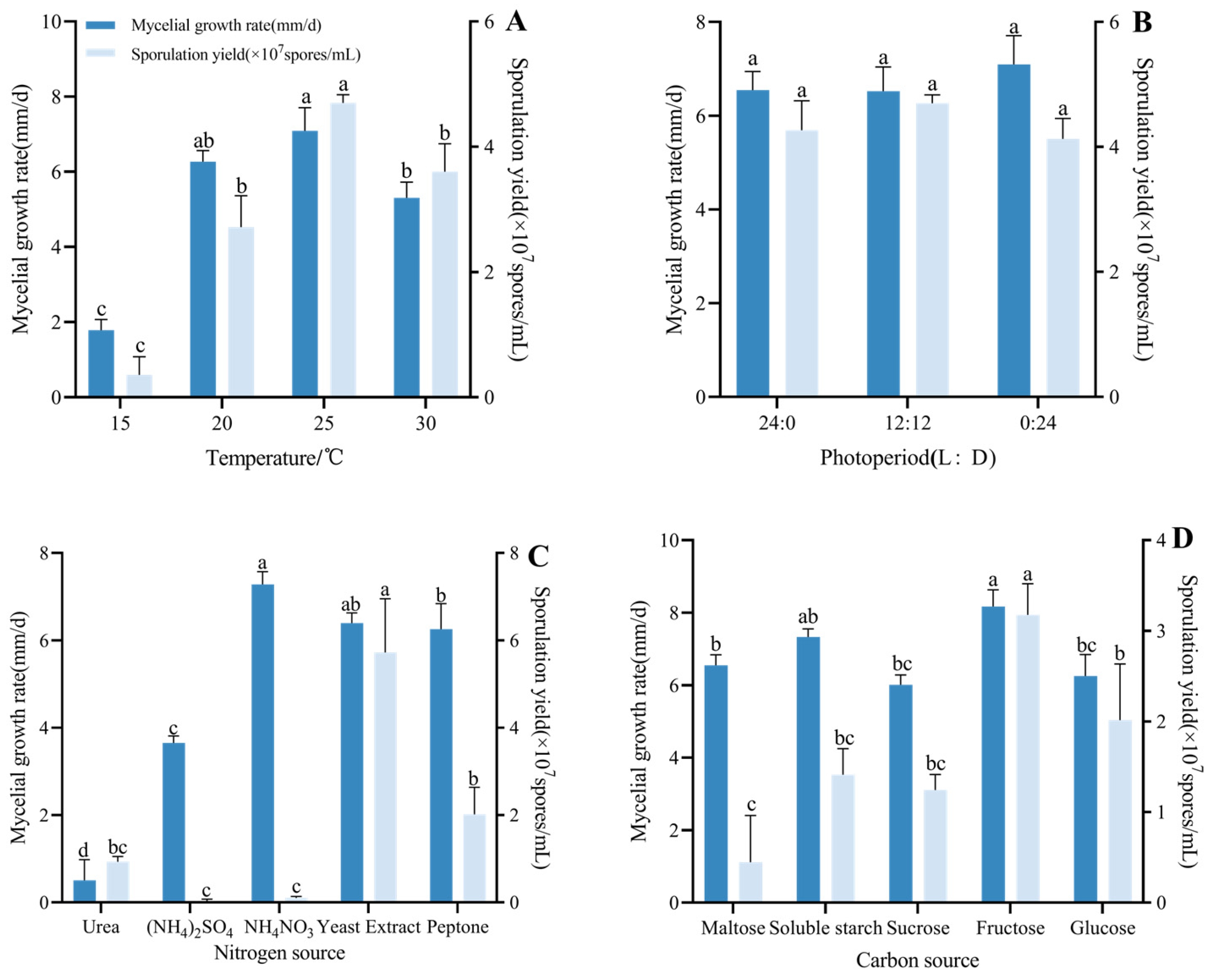
3.4.2. Effects of Temperature, Photoperiod, Carbon, and Nitrogen Sources on Growth and Sporulation of Strain CF01
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naito, T.; Smith, D.R.; Huang, F.S. Records of Cephidae (Hymenoptera) from China and Southeastern Asia with two new species of Janus Stephens. Jpn. J. Syst. Entomol. Matsuyama 1998, 4, 237–242. [Google Scholar]
- Hoelmer, K.A.; Shanower, T.G. Foreign exploration for natural enemies of cephid sawflies. J. Agric. Urban Entomol. 2004, 21, 223–238. [Google Scholar]
- Zhao, L.M. Morphology of Cephus fumipennis (Hymenoptera: Cephidae) andComparison with Other Three Cephids. Northwest J. Agric. 2011, 20, 167–173. [Google Scholar]
- Zhao, L.M.; Zhang, H.L. Economic-Injury Level and Economic Threshold of Cephus fumipennis (Hymenoptera: Cephidae). Northwest J. Agric. 2008, 17, 65–69. [Google Scholar]
- Chen, S.; Hoelmer, K.A.; Chen, H.; Liu, A. A Review of Wheat Stem Sawfly (Hymenoptera: Cephidae). J. Agric. Urban Entomol 2004, 21, 249–256. [Google Scholar]
- Rand, T.A.; Morrill, W.L.; Runyon, J.B.; Hoelmer, K.A.; Shanower, T.G.; Littlefield, J.L.; Weaver, D.K. Assessing phenological synchrony between the Chinese sawfly, Cephus fumipennis (Hymenoptera: Cephidae), its egg-larval parasitoid, Collyria catoptron (Hymenoptera: Ichneumonidae), and the North American sawfly, Cephus cinctus: Implications for biological control. Can. Entomol. 2016, 148, 482–492. [Google Scholar]
- Wu, X.H.; Héctor, C.A.; Pang, B.P. Progress in wheat stem wasp research. Plant Prot. 2016, 42, 18–26. [Google Scholar]
- Limin, Z. Identification of the natural resistance of several spring wheat varieties to Cephus fumipennis Eversmann. Acta Agric. Boreali-Occident. Sin. 2000, 9, 39–40. [Google Scholar]
- Wahl, D.B.; Shanower, T.G.; Hoelmer, K.A. A new species of Collyria Schiødte (Hymenoptera: Ichneumonidae: Collyriinae), a parasitoid of Cephus fumipennis (Hymenoptera: Cephidae) in China, and potential biological control agent for Cephus cinctus in North America. J. Kans. Entomol. Soc. 2007, 80, 43–50. [Google Scholar] [CrossRef]
- Runyon, J.; Hurley, R.; Morrill, W.; Weaver, D. Distinguishing adults of Bracon cephi and Bracon lissogaster (Hymenoptera: Braconidae), parasitoids of the wheat stem sawfly (Hymenoptera: Cephidae). Can. Entomol. 2001, 133, 215–217. [Google Scholar] [CrossRef]
- Nelson, W.; Farstad, C. Biology of Bracon cephi (Gahan) (Hymenoptera: Braconidae), An Important Native Parasite of the Wheat Stem Sawfly, Cephus cinctus Nort. (Hymenoptera: Cephidae), in Western Canada. Can. Entomol. 1953, 85, 103–107. [Google Scholar] [CrossRef]
- Morrill, W.L.; Weaver, D.K.; Irish, N.J.; Barr, W.F. Phyllobaenus dubius (Wolcott) (Coleoptera: Cleridae), a new record of a predator of the wheat stem sawfly (Hymenoptera: Cephidae). J. Kans. Entomol. Soc. 2001, 73, 181–183. [Google Scholar]
- Portman, S.L.; Krishnankutty, S.M.; Reddy, G.V. Entomopathogenic nematodes combined with adjuvants presents a new potential biological control method for managing the wheat stem sawfly, Cephus cinctus (Hymenoptera: Cephidae). PLoS ONE 2016, 11, e0169022. [Google Scholar] [CrossRef] [PubMed]
- Wenda-Piesik, A.; Sun, Z.; Grey, W.E.; Weaver, D.K.; Morrill, W.L. Mycoses of wheat stem sawfly (Hymenoptera: Cephidae) larvae by Fusarium spp. isolates. Environ. Entomol. 2009, 38, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Piesik, D.; Wenda-Piesik, A.; Weaver, D.K.; Macedo, T.B.; Morrill, W.L. Influence of Fusarium and wheat stem sawfly infestation on volatile compounds production by wheat plants. J. Plant Prot. Res. 2009, 49, 167–174. [Google Scholar] [CrossRef]
- Sun, Z. The Pathogenicity of Fusarium spp. to Wheat Stem Sawfly, Cephus Cinctus Norton (Hymenoptera: Cephidae); Montana State University: Bozeman, MT, USA, 2008. [Google Scholar]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Singh, D.; Raina, T.K.; Singh, J. Entomopathogenic fungi: An effective biocontrol agent for management of insect populations naturally. J. Pharm. Sci. Res. 2017, 9, 833. [Google Scholar]
- Maina, U.; Galadima, I.; Gambo, F.; Zakaria, D. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J. Entomol. Zool. Stud. 2018, 6, 27–32. [Google Scholar]
- Koul, O. Biorationals and Biopesticides: Pest Management; Springer International Publishing: Berlin, Germany, 2023. [Google Scholar]
- Shahid, A.A.; Rao, Q.A.; Bakhsh, A.; Husnain, T. Entomopathogenic fungi as biological controllers: New insights into their virulence and pathogenicity. Arch. Biol. Sci. 2012, 64, 21–42. [Google Scholar] [CrossRef]
- Wang, C.; Feng, M.-G. Advances in fundamental and applied studies in China of fungal biocontrol agents for use against arthropod pests. Biol. Control 2014, 68, 129–135. [Google Scholar] [CrossRef]
- Mora, M.A.E.; Castilho, A.M.C.; Fraga, M.E. Classification and infection mechanism of entomopathogenic fungi. Arq. Do Inst. Biológico 2017, 84, e0552015. [Google Scholar] [CrossRef]
- Sun, Z.-B.; Li, S.-D.; Ren, Q.; Xu, J.-L.; Lu, X.; Sun, M.-H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.V.; Virla, E.; Humber, R.A.; Paradell, S.L.; Lastra, C.L. First record of Clonostachys rosea (Ascomycota: Hypocreales) as an entomopathogenic fungus of Oncometopia tucumana and Sonesimia grossa (Hemiptera: Cicadellidae) in Argentina. J. Invertebr. Pathol. 2006, 92, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xia, J.; Zhuang, F.; Xu, R.; Zeng, T.; Li, Y. Identification and biological characterization of mycoparasitic fungus Clonostachys rosea isolated from sawfly Cephalcia chuxiongica. J. Plant Prot. 2018, 45, 622–631. [Google Scholar] [CrossRef]
- Wei, J.C. Fungi Identification Manual; Shanghai Scientific & Technical Publishers: Shanghai, China, 1979; pp. 58–90. [Google Scholar]
- Rodriguez, M.A.; Rothen, C.; Lo, T.E.; Cabrera, G.M.; Godeas, A.M. Suppressive soil against Sclerotinia sclerotiorum as a source of potential biocontrol agents: Selection and evaluation of Clonostachys rosea BAFC1646. Biocontrol Sci. Technol. 2015, 25, 1388–1409. [Google Scholar] [CrossRef]
- Dubey, M.K.; Jensen, D.F.; Karlsson, M. Hydrophobins are required for conidial hydrophobicity and plant root colonization in the fungal biocontrol agent Clonostachys rosea. BMC Microbiol. 2014, 14, 18. [Google Scholar] [CrossRef]
- Roberti, R.; Veronesi, A.; Cesari, A.; Cascone, A.; Di Berardino, I.; Bertini, L.; Caruso, C. Induction of PR proteins and resistance by the biocontrol agent Clonostachys rosea in wheat plants infected with Fusarium culmorum. Plant Sci. 2008, 175, 339–347. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Borges, Á.V.; Saraiva, R.M.; Maffia, L.A. Biocontrol of gray mold in tomato plants by Clonostachys rosea. Trop. Plant Pathol. 2015, 40, 71–76. [Google Scholar] [CrossRef]
- Bienapfl, J.; Floyd, C.; Percich, J.; Malvick, D. First report of Clonostachys rosea causing root rot of soybean in the United States. Plant Dis. 2012, 96, 1700. [Google Scholar] [CrossRef]
- Salamone, A.L.; Gundersen, B.; Inglis, D.A. Clonostachys rosea, a potential biological control agent for Rhizoctonia solani AG-3 causing black scurf on potato. Biocontrol Sci. Technol. 2018, 28, 895–900. [Google Scholar] [CrossRef]
- Jensen, B.; Lübeck, P.S.; Jørgensen, H.J. Clonostachys rosea reduces spot blotch in barley by inhibiting prepenetration growth and sporulation of Bipolaris sorokiniana without inducing resistance. Pest Manag. Sci. 2016, 72, 2231–2239. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Niu, Q.; Zhao, X.; Ye, F.; Liang, L.; Zhang, K.-Q. Investigation on the infection mechanism of the fungus Clonostachys rosea against nematodes using the green fluorescent protein. Appl. Microbiol. Biotechnol. 2008, 78, 983–990. [Google Scholar] [CrossRef]
- Iqbal, M.; Dubey, M.; McEwan, K.; Menzel, U.; Franko, M.A.; Viketoft, M.; Jensen, D.F.; Karlsson, M. Evaluation of Clonostachys rosea for control of plant-parasitic nematodes in soil and in roots of carrot and wheat. Phytopathology 2018, 108, 52–59. [Google Scholar] [CrossRef]
- Anwar, W.; Ali, S.; Nawaz, K.; Iftikhar, S.; Javed, M.A.; Hashem, A.; Alqarawi, A.A.; Abd_Allah, E.F.; Akhter, A. Entomopathogenic fungus Clonostachys rosea as a biocontrol agent against whitefly (Bemisia tabaci). Biocontrol Sci. Technol. 2018, 28, 750–760. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Younus, A.S.; Ali, A.N. Efficacy of Clonostachys rosea, as a promising entomopathogenic fungus, against coleopteran stored product insect pests under laboratory conditions. Egypt. J. Biol. Pest Control 2021, 31, 55. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Lu, J.J.; Yang, M.G.; Ma, Y.Q.; Tian, Y.J.; Gou, C.J.; Liu, M. Virulence Determination and Optimization of Toxin Production Conditions of Biocontrol Strains of Plutella xylostella Larvae. Shandong Agric. Sci. 2024, 56, 132–137. [Google Scholar] [CrossRef]
- Hue, A.; Voldeng, H.; Savard, M.; Fedak, G.; Tian, X.; Hsiang, T. Biological control of fusarium head blight of wheat with Clonostachys rosea strain ACM941. Can. J. Plant Pathol. 2009, 31, 169–179. [Google Scholar] [CrossRef]
- Kulik, T.; Treder, K.; Rochoń, M.; Załuski, D.; Sulima, P.; Olszewski, J.; Bilska, K.; Elena, G.; Kowalski, T. Measurement of the effectiveness of Clonostachys rosea in reducing Fusarium biomass on wheat straw. J. Appl. Genet. 2024, 65, 937–947. [Google Scholar] [CrossRef]
- Demissie, Z.A.; Witte, T.; Robinson, K.A.; Sproule, A.; Foote, S.J.; Johnston, A.; Harris, L.J.; Overy, D.P.; Loewen, M.C. Transcriptomic and exometabolomic profiling reveals antagonistic and defensive modes of Clonostachys rosea action against Fusarium graminearum. Mol. Plant-Microbe Interact. 2020, 33, 842–858. [Google Scholar] [CrossRef]
- Braman, S.; Duncan, R.; Engelke, M.; Hanna, W.; Hignight, K.; Rush, D. Grass species and endophyte effects on survival and development of fall armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 2002, 95, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, M.E.; Rudgers, J.A. Endophyte-mediated resistance to herbivores depends on herbivore identity in the wild grass Festuca subverticillata. Environ. Entomol. 2009, 38, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Akello, J.; Dubois, T.; Coyne, D.; Kyamanywa, S. Endophytic Beauveria bassiana in banana (Musa spp.) reduces banana weevil (Cosmopolites sordidus) fitness and damage. Crop Prot. 2008, 27, 1437–1441. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef]
- Rodriguez, R.; White, J., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Hartley, S.E.; Eschen, R.; Horwood, J.M.; Gange, A.C.; Hill, E.M. Infection by a foliar endophyte elicits novel arabidopside-based plant defence reactions in its host, Cirsium arvense. New Phytol. 2015, 205, 816–827. [Google Scholar] [CrossRef]
- Anderson, C.M.; McGee, P.A.; Nehl, D.B.; Mensah, R.K. The fungus Lecanicillium lecanii colonises the plant Gossypium hirsutum and the aphid Aphis gossypii. Aust. Mycol. 2007, 26, 65–70. [Google Scholar]
- Portman, S.L.; Jaronski, S.T.; Weaver, D.K.; Reddy, G.V. Advancing biological control of the wheat stem sawfly: New strategies in a 100-yr struggle to manage a costly pest in the Northern Great Plains. Ann. Entomol. Soc. Am. 2018, 111, 85–91. [Google Scholar] [CrossRef]

| Medium | Medium Component |
|---|---|
| Potato Dextrose Agar (PDA) | Peeled potato 200 g/L, Dextrose 20 g/L, Agar 18 g/L, pH unadjusted |
| Starch Agar (SA) | soluble starch 40 g/L, yeast paste 5 g/L, Agar 20 g/L, pH unadjusted |
| Dextrose Peptone Agar (GPA) | Peptone 10 g/L, Dextrose 40 g/L, Agar 20 g/L, pH unadjusted |
| Rose Bengal Chloramphenicol Agar (RBC) | Peptone 5.0 g/L, Glucose 10.0 g/L, KH2PO4 1.0 g/L, MgSO4 0.5 g/L, 1/3000 Rose Bengal red 100 mL/L, Agar 18.0 g/L, pH unadjusted |
| Peptone Potato Dextrose Agar (PPDA) | Peeled potato 200 g/L, Glucose 20 g/L, Peptone 20 g/L, Agar 18 g/L, pH unadjusted |
| Sabouraud Dextrose Agar with Yeast Extract (SDAY) | Glucose 40 g/L, Yeast paste 10 g/L, Peptone 10 g/L, Agar 20 g/L, pH unadjusted |
| Concentration (Spores/mL) | Virulence Regression Equation | LT50 (Days) | R2 | 95%CI (Days) |
|---|---|---|---|---|
| 1 × 108 | y = 3.566x − 2.603 | 5.368 | 0.869 | 4.356–6.307 |
| 1 × 107 | y = 4.500x − 3.936 | 7.493 | 0.986 | 6.467–8.513 |
| 1 × 106 | y = 3.462x − 3.427 | 9.768 | 0.976 | 8.343–11.792 |
| 1 × 105 | y = 2.860x − 3.407 | 15.535 | 0.954 | 12.252–27.060 |
| Days | Virulence Regression Equation | LC50 (Days) | R2 | 95%CI (Days) |
|---|---|---|---|---|
| 7 | y = 0.397x − 2.812 | 1.217 × 107 | 0.998 | 7.178 × 105–5.285 × 1015 |
| 10 | y = 0.452x − 2.814 | 1.682 × 106 | 0.949 | 9.011 × 103–2.725 × 107 |
| 14 | y = 0.720x − 3.706 | 1.405 × 105 | 0.979 | 4.220 × 102–6.688 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, J.; An, Z.; Wang, S.; Lai, Y. First Record of Clonostachys rosea as an Entomopathogenic Fungus of the Cephus fumipennis (Hymenoptera: Cephidae) in China. Biology 2025, 14, 1240. https://doi.org/10.3390/biology14091240
Li M, Li J, An Z, Wang S, Lai Y. First Record of Clonostachys rosea as an Entomopathogenic Fungus of the Cephus fumipennis (Hymenoptera: Cephidae) in China. Biology. 2025; 14(9):1240. https://doi.org/10.3390/biology14091240
Chicago/Turabian StyleLi, Meiqi, Jingling Li, Zehao An, Shasha Wang, and Youpeng Lai. 2025. "First Record of Clonostachys rosea as an Entomopathogenic Fungus of the Cephus fumipennis (Hymenoptera: Cephidae) in China" Biology 14, no. 9: 1240. https://doi.org/10.3390/biology14091240
APA StyleLi, M., Li, J., An, Z., Wang, S., & Lai, Y. (2025). First Record of Clonostachys rosea as an Entomopathogenic Fungus of the Cephus fumipennis (Hymenoptera: Cephidae) in China. Biology, 14(9), 1240. https://doi.org/10.3390/biology14091240





