A Simple Three-Dimensional Compartmentalized Co-Culture Model for Basal Forebrain and Hippocampal Neurons
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Hippocampal and Basal Forebrain Cell Suspensions
2.2. Model Construction
2.3. Live-Cell Imaging
2.4. Immunofluorescence Staining
2.5. Image Processing and Quantification
2.6. Analysis of Neurite Outgrowth and Complexity
2.7. Calcium Imaging
2.8. Data Analysis
3. Results
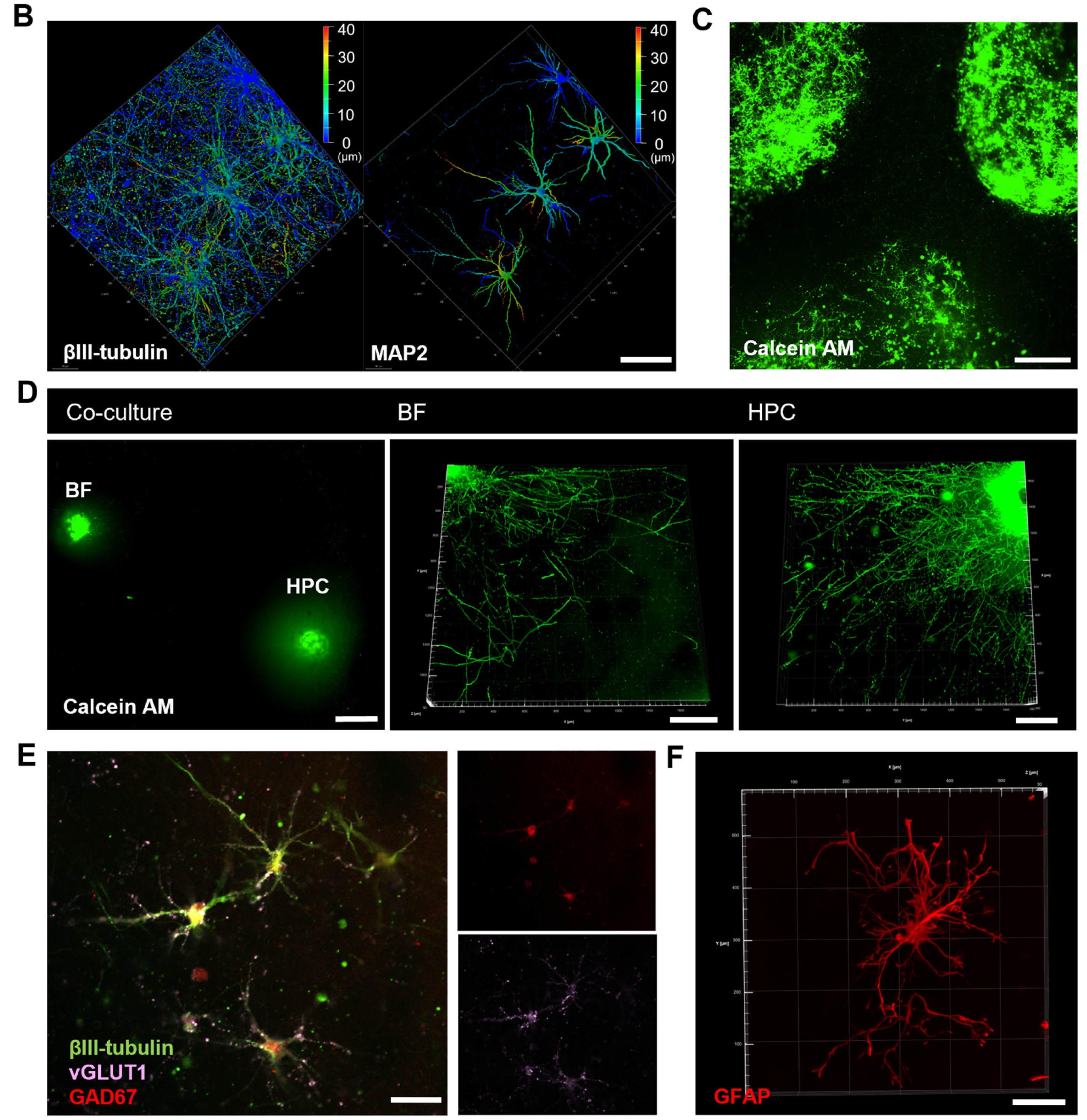
3.1. Characterization of the Compartmentalized Co-Culture System
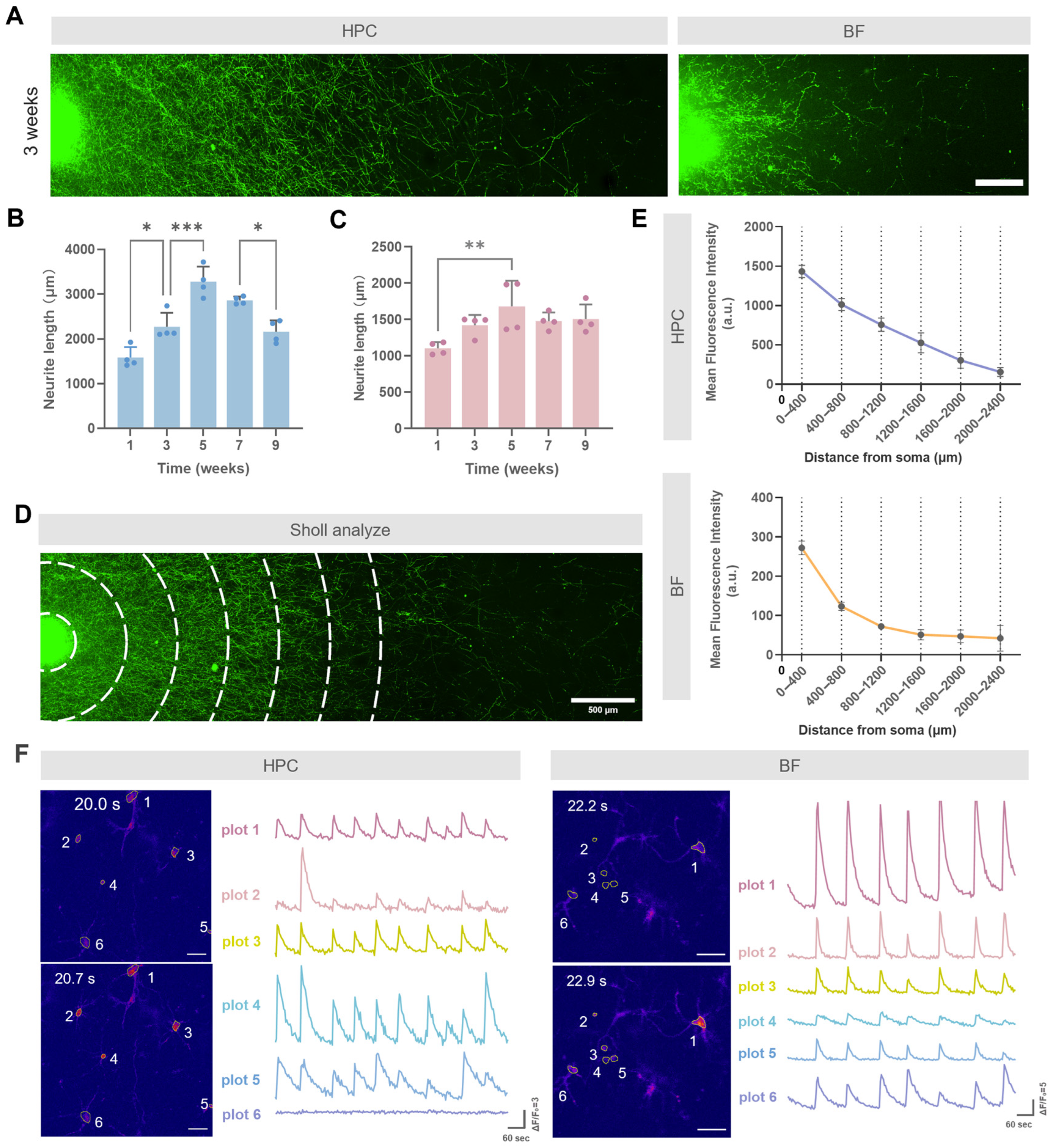
3.2. Co-Culturing Promotes Polarized Development of Basal Forebrain Neurons
3.3. Dynamics of Neuronal Maturation and Degeneration in Long-Term Co-Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, J.; Huang, S.; Wang, X.-Y.; Chen, X.; Liu, G.-H.; Ye, K.; Song, W.; Masters, C.L.; Wang, J.; et al. Antiageing Strategy for Neurodegenerative Diseases: From Mechanisms to Clinical Advances. Sig. Transduct. Target. Ther. 2025, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.Y.; Hansen, H.D.; Wey, H.-Y. Advances in Simultaneous PET/MR for Imaging Neuroreceptor Function. J. Cereb. Blood Flow. Metab. 2020, 40, 1148–1166. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Renslo, J.; Wong, K.; Clifford, T.G.; Beutler, B.D.; Kim, P.E.; Gholamrezanezhad, A. Current Trends and Applications of PET/MRI Hybrid Imaging in Neurodegenerative Diseases and Normal Aging. Diagnostics 2024, 14, 585. [Google Scholar] [CrossRef]
- Yao, M.; Tudi, A.; Jiang, T.; An, X.; Jia, X.; Li, A.; Huang, Z.J.; Gong, H.; Li, X.; Luo, Q. From Individual to Population: Circuit Organization of Pyramidal Tract and Intratelencephalic Neurons in Mouse Sensorimotor Cortex. Research 2024, 7, 0470. [Google Scholar] [CrossRef]
- Bothwell, M.N.G.F. Ngf, Bdnf, Nt3, and Nt4. Neurotrophic Factors 2014, 220, 3–15. [Google Scholar]
- Emerit, M.B.; Segovia, J.; Alho, H.; Mastrangelo, M.J.; Wise, B.C. Hippocampal Membranes Contain a Neurotrophic Activity That Stimulates Cholinergic Properties of Fetal Rat Septal Neurons Cultured under Serum-Free Conditions. J. Neurochem. 1989, 52, 952–961. [Google Scholar] [CrossRef]
- Wainer, B.H.; Lee, H.J.; Roback, J.D.; Hammond, D.N. In Vitro Cell Cultures as a Model of the Basal Forebrain. In The Basal Forebrain: Anatomy to Function; Springer: Boston, MA, USA, 1991; pp. 415–437. [Google Scholar]
- Hsiang, J.; Price, S.; Heller, A.; Hoffmann, P.; Wainer, B. Ultrastructural Evidence for Hippocampal Target Cell-Mediated Trophic Effects on Septal Cholinergic Neurons in Reaggregating Cell Cultures. Neuroscience 1988, 26, 417–431. [Google Scholar] [CrossRef]
- Xu, W.; Wu, C. Isolation and Culturing of Rat Primary Embryonic Basal Forebrain Cholinergic Neurons (BFCNs). Bio-Protocol 2017, 7, e2413. [Google Scholar] [CrossRef]
- Kropf, E.; Shekari, A.; Jaberi, S.; Puri, A.; Wu, C.; Fahnestock, M. Age-Induced Nitrative Stress Decreases Retrograde Transport of proNGF via TrkA and Increases proNGF Retrograde Transport and Neurodegeneration via p75NTR. Front. Mol. Neurosci. 2023, 16, 1241420. [Google Scholar] [CrossRef]
- Shekari, A.; Fahnestock, M. Retrograde Axonal Transport of BDNF and proNGF Diminishes with Age in Basal Forebrain Cholinergic Neurons. Neurobiol. Aging 2019, 84, 131–140. [Google Scholar] [CrossRef]
- Joshua, M.; Lisberger, S.G. A Tale of Two Species: Neural Integration in Zebrafish and Monkeys. Neuroscience 2015, 296, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In Vitro Models of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Siddique, L.; Khalifey, H.T.; Mahereen, R.; Raziq, T.; Firdous, R.M.; Siddique, A.; Shakir, I.M.; Ahmed, Z.; Akbar, A.; et al. Brain Organoid Model Systems of Neurodegenerative Diseases: Recent Progress and Future Prospects. Front. Neurosci. 2025, 19, 1604435. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, P.-M.; Wu, J.; Luo, Z.-G. Advances and Applications of Brain Organoids. Neurosci. Bull. 2023, 39, 1703–1716. [Google Scholar] [CrossRef]
- Bang, S.; Na, S.; Jang, J.M.; Kim, J.; Jeon, N.L. Engineering-Aligned 3D Neural Circuit in Microfluidic Device. Adv. Healthc. Mater. 2016, 5, 159–166. [Google Scholar] [CrossRef]
- Habibey, R.; Rojo Arias, J.E.; Striebel, J.; Busskamp, V. Microfluidics for Neuronal Cell and Circuit Engineering. Chem. Rev. 2022, 122, 14842–14880. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Gao, Y.; Mathivanan, S.; Kong, L.; Tao, Y.; Dong, Y.; Li, X.; Bhattacharyya, A.; Zhao, X.; et al. 3D Bioprinting of Human Neural Tissues with Functional Connectivity. Cell Stem Cell 2024, 31, 260–274.e7. [Google Scholar] [CrossRef]
- Cadena, M.; Ning, L.; King, A.; Hwang, B.; Jin, L.; Serpooshan, V.; Sloan, S.A. Three Dimensional Bioprinting of Neural Tissues. Adv. Healthc. Mater. 2021, 10, e2001600. [Google Scholar] [CrossRef]
- Hopkins, A.M.; DeSimone, E.; Chwalek, K.; Kaplan, D.L. 3D in Vitro Modeling of the Central Nervous System. Progress. Neurobiol. 2015, 125, 1–25. [Google Scholar] [CrossRef]
- Dandia, H.; Makkad, K.; Tayalia, P. Glycated Collagen—A 3D Matrix System to Study Pathological Cell Behavior. Biomater. Sci. 2019, 7, 3480–3488. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-Dimensional Aligned Nanofibers-Hydrogel Scaffold for Controlled Non-Viral Drug/Gene Delivery to Direct Axon Regeneration in Spinal Cord Injury Treatment. Sci. Rep. 2017, 7, 42212. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A Complex Protein Mixture Required for Optimal Growth of Cell Culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Djemil, S.; Ressel, C.R.; Abdel-Ghani, M.; Schneeweis, A.K.; Pak, D.T.S. Central Cholinergic Synapse Formation in Optimized Primary Septal-Hippocampal Co-Cultures. Cell Mol. Neurobiol. 2021, 41, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Robertson, R.T.; Ribak, C.E.; Weiss, J.H. Cultured Basal Forebrain Cholinergic Neurons in Contact with Cortical Cells Display Synapses, Enhanced Morphological Features, and Decreased Dependence on Nerve Growth Factor. J. Comp. Neurol. 1996, 373, 451–465. [Google Scholar] [CrossRef]
- Carmo, S.D.; Kannel, B.; Cuello, A.C. The Nerve Growth Factor Metabolic Pathway Dysregulation as Cause of Alzheimer’s Cholinergic Atrophy. Cells 2021, 11, 16. [Google Scholar] [CrossRef]
- Odawara, A.; Gotoh, M.; Suzuki, I. A Three-Dimensional Neuronal Culture Technique That Controls the Direction of Neurite Elongation and the Position of Soma to Mimic the Layered Structure of the Brain. RSC Adv. 2013, 3, 23620–23630. [Google Scholar] [CrossRef]
- Geula, C.; Nagykery, N.; Nicholas, A.; Wu, C.-K. Cholinergic Neuronal and Axonal Abnormalities Are Present Early in Aging and in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2008, 67, 309–318. [Google Scholar] [CrossRef]
- Baskerville, K.A.; Kent, C.; Personett, D.; Lai, W.R.; Park, P.J.; Coleman, P.; McKinney, M. Aging Elevates Metabolic Gene Expression in Brain Cholinergic Neurons. Neurobiol. Aging 2008, 29, 1874–1893. [Google Scholar] [CrossRef]
- Kropf, E.; Fahnestock, M. Effects of Reactive Oxygen and Nitrogen Species on TrkA Expression and Signalling: Implications for proNGF in Aging and Alzheimer’s Disease. Cells 2021, 10, 1983. [Google Scholar] [CrossRef]
- Seager, R.; Lee, L.; Henley, J.M.; Wilkinson, K.A. Mechanisms and Roles of Mitochondrial Localisation and Dynamics in Neuronal Function. Neuronal Signal 2020, 4, NS20200008. [Google Scholar] [CrossRef]
- Faria-Pereira, A.; Morais, V.A. Synapses: The Brain’s Energy-Demanding Sites. Int. J. Mol. Sci. 2022, 23, 3627. [Google Scholar] [CrossRef]
- Jurk, D.; Wang, C.; Miwa, S.; Maddick, M.; Korolchuk, V.; Tsolou, A.; Gonos, E.S.; Thrasivoulou, C.; Saffrey, M.J.; Cameron, K.; et al. Postmitotic Neurons Develop a P21-Dependent Senescence-like Phenotype Driven by a DNA Damage Response. Aging Cell 2012, 11, 996–1004. [Google Scholar] [CrossRef]
- Dos Santos, L.M.; Trombetta-Lima, M.; Eggen, B.; Demaria, M. Cellular Senescence in Brain Aging and Neurodegeneration. Ageing Res. Rev. 2024, 93, 102141. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Blas, D.; Gorostieta-Salas, E.; Pommer-Alba, A.; Muciño-Hernández, G.; Gerónimo-Olvera, C.; Maciel-Barón, L.A.; Konigsberg, M.; Massieu, L.; Castro-Obregón, S. Cortical Neurons Develop a Senescence-like Phenotype Promoted by Dysfunctional Autophagy. Aging 2019, 11, 6175–6198. [Google Scholar] [CrossRef]
- Bañuelos, C.; Kittleson, J.R.; LaNasa, K.H.; Galiano, C.S.; Roth, S.M.; Perez, E.J.; Long, J.M.; Roberts, M.T.; Fong, S.; Rapp, P.R. Cognitive Aging and the Primate Basal Forebrain Revisited: Disproportionate GABAergic Vulnerability Revealed. J. Neurosci. 2023, 43, 8425–8441. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Li, J.; Deng, Z.; Xu, Y.; Li, X.; Ren, M.; Li, X. A Simple Three-Dimensional Compartmentalized Co-Culture Model for Basal Forebrain and Hippocampal Neurons. Biology 2025, 14, 1238. https://doi.org/10.3390/biology14091238
Luo X, Li J, Deng Z, Xu Y, Li X, Ren M, Li X. A Simple Three-Dimensional Compartmentalized Co-Culture Model for Basal Forebrain and Hippocampal Neurons. Biology. 2025; 14(9):1238. https://doi.org/10.3390/biology14091238
Chicago/Turabian StyleLuo, Xiaoman, Jing Li, Zhiyu Deng, Yali Xu, Xixi Li, Miao Ren, and Xiangning Li. 2025. "A Simple Three-Dimensional Compartmentalized Co-Culture Model for Basal Forebrain and Hippocampal Neurons" Biology 14, no. 9: 1238. https://doi.org/10.3390/biology14091238
APA StyleLuo, X., Li, J., Deng, Z., Xu, Y., Li, X., Ren, M., & Li, X. (2025). A Simple Three-Dimensional Compartmentalized Co-Culture Model for Basal Forebrain and Hippocampal Neurons. Biology, 14(9), 1238. https://doi.org/10.3390/biology14091238





