Changes in the Microbiota of the Scale Insect (Diaspis echinocacti, Bouché, 1833) in Opuntia stricta Cladodes: Taxonomic and Metagenomic Analysis as a Function of Infestation Levels
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
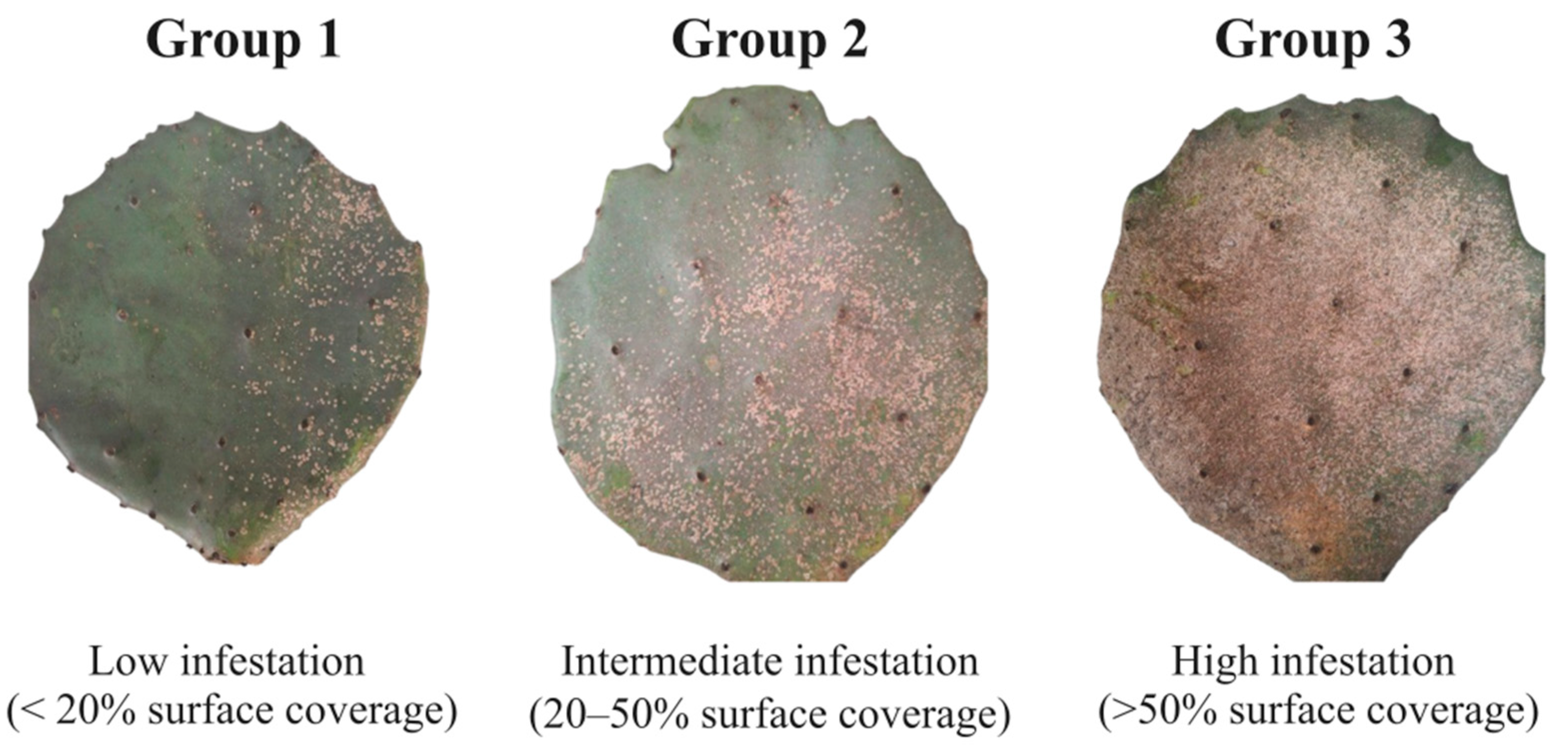
2.1. Sample Collection
2.2. Procedures for 16S Sequencing on the iSeq 100
2.2.1. DNA Extraction and 16S Amplification
2.2.2. Purification and PCR Binding INDEX
2.2.3. Bioinformatics Analysis
3. Results
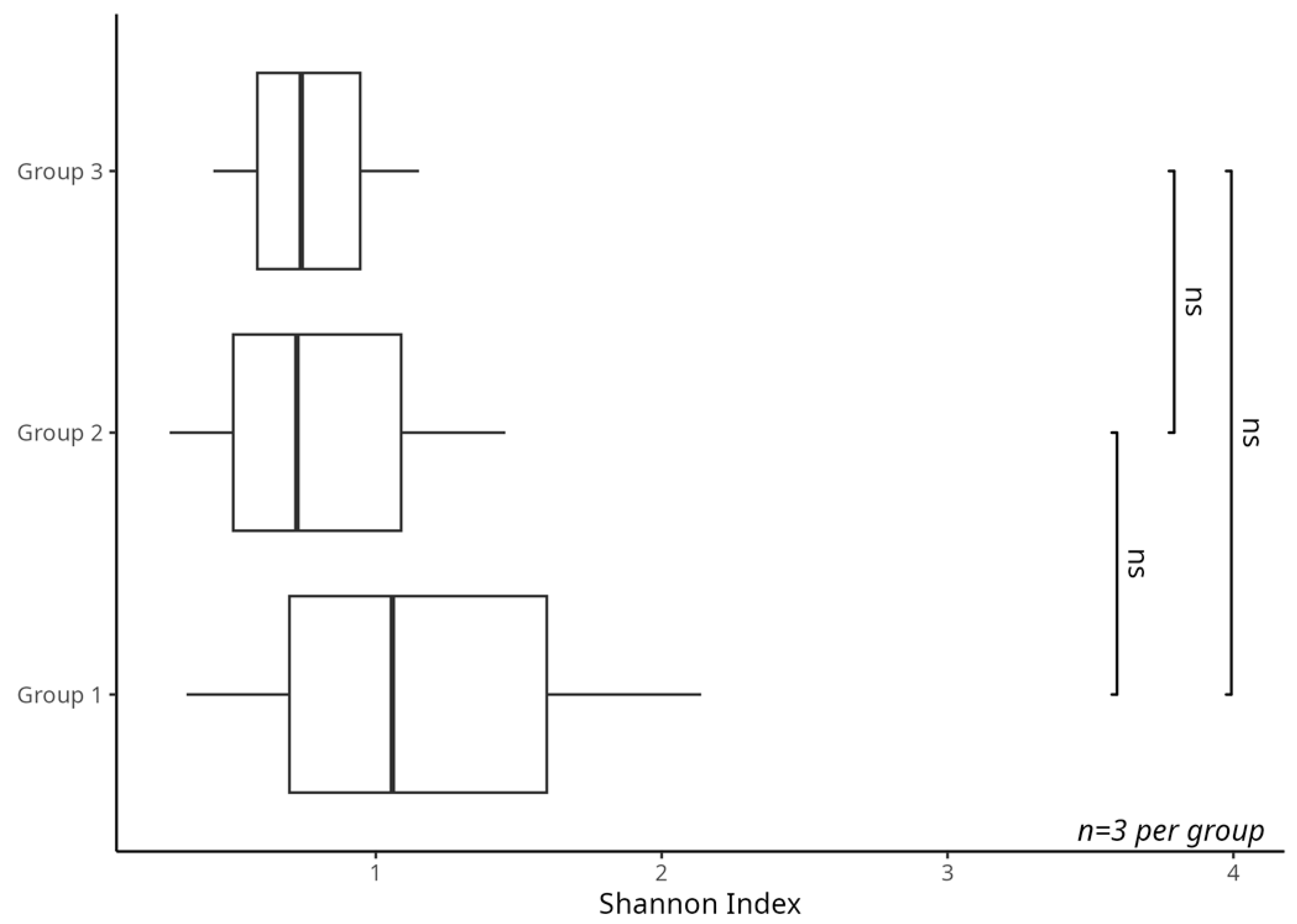
3.1. Analysis of Biological Diversity
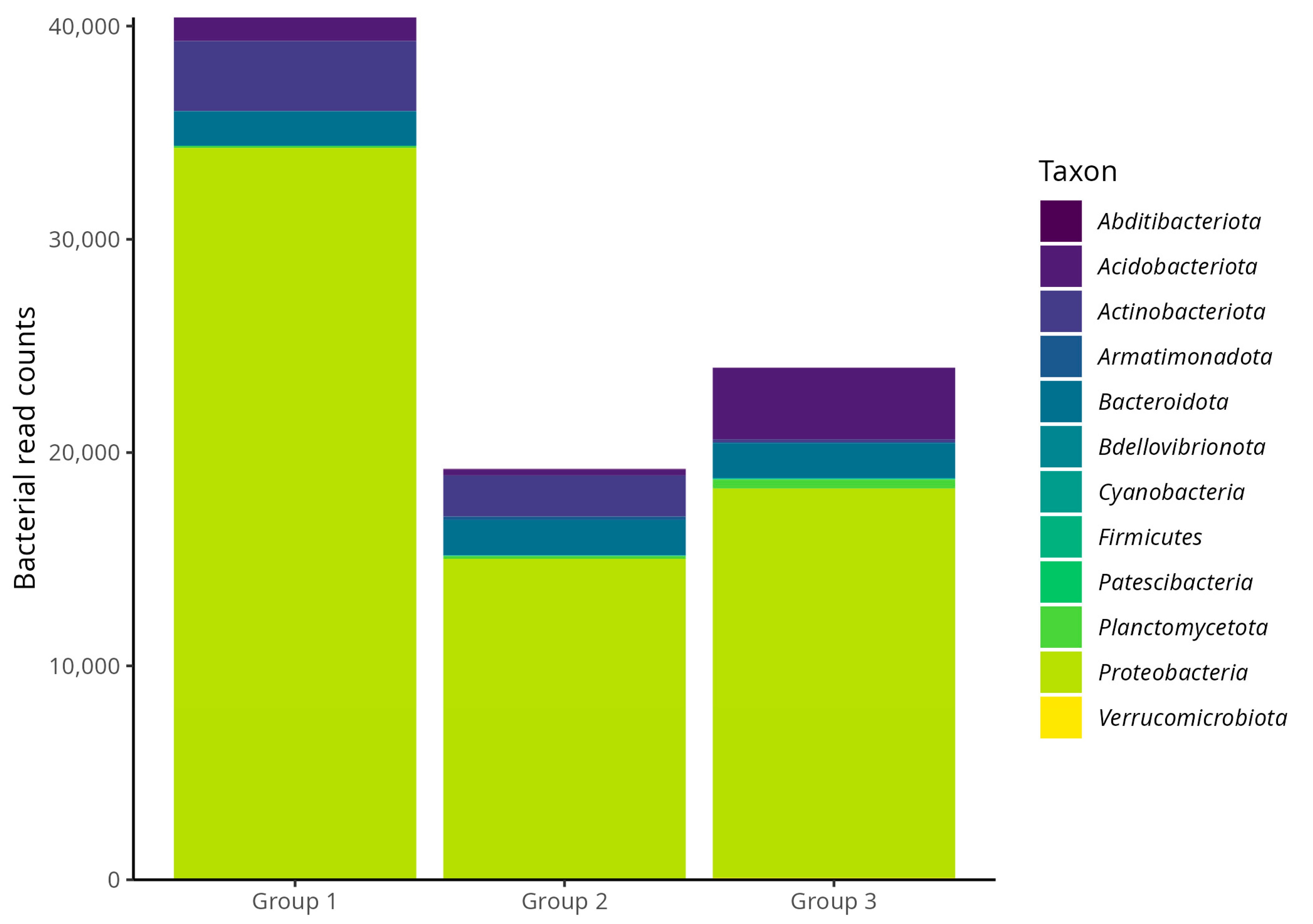
3.2. Taxonomic Composition of the Microbiota Associated with Scale Insects
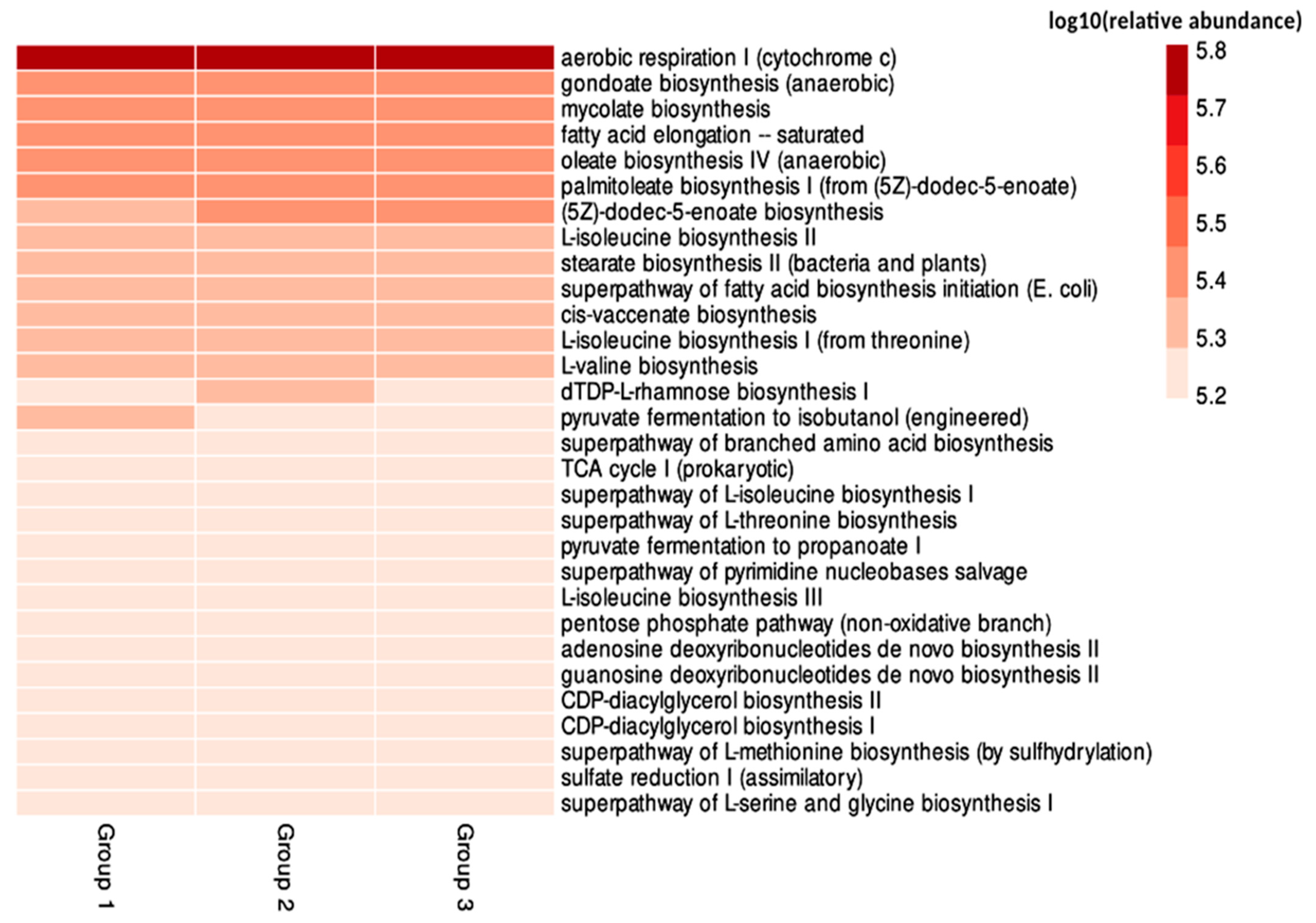
3.3. Functional Profile of the Microbiota Associated with Scale Insects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albuquerque Junior, P.S.; Silva, C.A.D.; Ramos, R.S.; Zanuncio, J.C.; Castellani, M.A. Diaspis echinocacti (Hemiptera: Diaspididae) on Cactus Pear Cladodes: Biological Aspects at Different Temperatures. Braz. J. Biol. 2023, 83, e274016. [Google Scholar] [CrossRef] [PubMed]
- Dubeux, J.C.B.; Santos, M.V.F.; Souza, R.T.A.; Siebert, A. Cactus: The New Green Revolution in Drylands. Acta Hortic. 2022, 233–240. [Google Scholar] [CrossRef]
- Marengo, J.A.; Galdos, M.V.; Challinor, A.; Cunha, A.P.; Marin, F.R.; Vianna, M.d.S.; Alvala, R.C.S.; Alves, L.M.; Moraes, O.L.; Bender, F. Drought in Northeast Brazil: A Review of Agricultural and Policy Adaptation Options for Food Security. Clim. Resil. Sustain. 2022, 1, e17. [Google Scholar] [CrossRef]
- Rodrigues, P.G.; Garcez, D.S.M.; Silva, C.M.; Santana, C.C.S.; Santana, J.C.S.; Lopes, C.D.C.; Muniz, E.N.; Oliveira Júnior, G.M.; de Moura, R.S.; Souza, J.C. Use of Palm Bran (Nopalea cochenillifera (L.) Salm-Dyck) in Partial Replacement of Concentrate in Maintenance Equine Diets—A Pilot Study. Arch. Anim. Breed. 2021, 64, 273–282. [Google Scholar] [CrossRef]
- Dantas, P.C.; de Araújo, R.G.V.; de Abreu, L.A.; Sabino, A.R.; dos Santos Silva, C.; Figueiroa, L.E.; Cunha, J.L.X.L.; Duarte, A.G. Avaliação de Extratos Botânicos No Controle Da Cochonilha de Escama Diaspis Echinocacti (Brouché, 1833) (Hemiptera: Diaspididae). Braz. J. Dev. 2019, 5, 2012–2017. [Google Scholar]
- El Aalaoui, M.; Rammali, S.; Bencharki, B.; Sbaghi, M. Integrated Biological Control of Diaspis Echinocacti (Bouché) on Opuntia Ficus-Indica (L.) Mill (Cactaceae) Using Predatory Ladybirds and Fungal Pathogens. Crop Prot. 2025, 187, 106950. [Google Scholar] [CrossRef]
- Bustamante-Brito, R.; Vera-Ponce de León, A.; Rosenblueth, M.; Martínez-Romero, E. Comparative Genomics of the Carmine Cochineal Symbiont Candidatus Dactylopiibacterium Carminicum Reveals Possible Protection to the Host against Viruses via CRISPR/Cas. Syst. Appl. Microbiol. 2024, 47, 126540. [Google Scholar] [CrossRef]
- Zepeda-Paulo, F.; Romero, V.; Celis-Diez, J.L.; Lavandero, B. A Newly Discovered Bacterial Symbiont in the Aphid Microbiome Identified through 16S RRNA Sequencing. Symbiosis 2024, 93, 223–228. [Google Scholar] [CrossRef]
- Lange, C.; Boyer, S.; Bezemer, T.M.; Lefort, M.-C.; Dhami, M.K.; Biggs, E.; Groenteman, R.; Fowler, S.V.; Paynter, Q.; Verdecia Mogena, A.M.; et al. Impact of Intraspecific Variation in Insect Microbiomes on Host Phenotype and Evolution. ISME J. 2023, 17, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Gruwell, M.E.; Flarhety, M.; Dittmar, K. Distribution of the Primary Endosymbiont (Candidatus Uzinura Diaspidicola) Within Host Insects from the Scale Insect Family Diaspididae. Insects 2012, 3, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Gruwell, M.E.; Morse, G.E.; Normark, B.B. Phylogenetic Congruence of Armored Scale Insects (Hemiptera: Diaspididae) and Their Primary Endosymbionts from the Phylum Bacteroidetes. Mol. Phylogenetics Evol. 2007, 44, 267–280. [Google Scholar] [CrossRef]
- Sabree, Z.L.; Huang, C.Y.; Okusu, A.; Moran, N.A.; Normark, B.B. The Nutrient Supplying Capabilities of Uzinura, an Endosymbiont of Armoured Scale Insects. Environ. Microbiol. 2013, 15, 1988–1999. [Google Scholar] [CrossRef]
- Szklarzewicz, T.; Michalik, A.; Michalik, K. The Diversity of Symbiotic Systems in Scale Insects. In Symbiosis: Cellular, Molecular, Medical and Evolutionary Aspects; Kloc, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 469–495. ISBN 978-3-030-51849-3. [Google Scholar]
- van den Bosch, T.J.M.; Welte, C.U. Detoxifying Symbionts in Agriculturally Important Pest Insects. Microb. Biotechnol. 2017, 10, 531–540. [Google Scholar] [CrossRef]
- Silva, D.P.; Epstein, H.E.; Vega Thurber, R.L. Best Practices for Generating and Analyzing 16S RRNA Amplicon Data to Track Coral Microbiome Dynamics. Front. Microbiol. 2023, 13, 1007877. [Google Scholar] [CrossRef]
- Straub, D.; Blackwell, N.; Langarica-Fuentes, A.; Peltzer, A.; Nahnsen, S.; Kleindienst, S. Interpretations of Environmental Microbial Community Studies Are Biased by the Selected 16S RRNA (Gene) Amplicon Sequencing Pipeline. Front. Microbiol. 2020, 11, 550420. [Google Scholar] [CrossRef]
- Agredo-Gomez, A.D.; Molano-Molano, J.A.; Portela-Patiño, M.C.; Rodríguez-Páez, J.E. Use of ZnO Nanoparticles as a Pesticide: In Vitro Evaluation of Their Effect on the Phytophagous Puto Barberi (Mealybug). Nano-Struct. Nano-Objects 2024, 37, 101095. [Google Scholar] [CrossRef]
- Ullah, M.S.; Sharif, M.M.H.; Dey, A.; Haque, M.M.; Rashed, M.T.N.N. Capacity of Detergent to Increase the Efficacy of Bio-Pesticides and Chemical Pesticides against Giant Mealybug, Drosicha Mangiferae (Homoptera: Pseudococcidae). Int. J. Trop. Insect Sci. 2024, 44, 2889–2896. [Google Scholar] [CrossRef]
- El Aalaoui, M.; Sbaghi, M. Population Fluctuations, Diversity and Effectiveness of Natural Enemies Associated with the Cactus Scale Diaspis Echinocacti (Bouché) (Hemiptera: Diaspididae) in Morocco. Phytoparasitica 2023, 51, 1059–1072. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal Insects: Diversity and Function of Resident Microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Chen, B.; Teh, B.-S.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y. Biodiversity and Activity of the Gut Microbiota across the Life History of the Insect Herbivore Spodoptera Littoralis. Sci. Rep. 2016, 6, 29505. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Roh, S.W.; Whon, T.W.; Jung, M.-J.; Kim, M.-S.; Park, D.-S.; Yoon, C.; Nam, Y.-D.; Kim, Y.-J.; Choi, J.-H.; et al. Insect Gut Bacterial Diversity Determined by Environmental Habitat, Diet, Developmental Stage, and Phylogeny of Host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Midford, P.E.; Ong, Q.; Ong, W.K.; et al. The MetaCyc Database of Metabolic Pathways and Enzymes. Nucleic Acids Res. 2018, 46, D633–D639. [Google Scholar] [CrossRef]
- Nicol, M.; Alexandre, S.; Luizet, J.-B.; Skogman, M.; Jouenne, T.; Salcedo, S.P.; Dé, E. Unsaturated Fatty Acids Affect Quorum Sensing Communication System and Inhibit Motility and Biofilm Formation of Acinetobacter Baumannii. Int. J. Mol. Sci. 2018, 19, 214. [Google Scholar] [CrossRef]
- Heath, R.J.; Rock, C.O. Roles of the FabA and FabZ β-Hydroxyacyl-Acyl Carrier Protein Dehydratases in Escherichia Coli Fatty Acid Biosynthesis. J. Biol. Chem. 1996, 271, 27795–27801. [Google Scholar] [CrossRef]
- Huerta-García, A.; Álvarez-Cervantes, J. The Gut Microbiota of Insects: A Potential Source of Bacteria and Metabolites. Int. J. Trop. Insect Sci. 2024, 44, 13–30. [Google Scholar] [CrossRef]
- Beltrame, W.S.; Daquila, B.V.; Caleffe, R.R.T.; dos Santos Wolff, V.R.; da Silva Santos, É.; da Silva Machado, M.D.F.P.; Conte, H.; Ruvolo-Takasusuki, M.C.C. Gall Formation in Cereus Sp. Infected with Diaspis Echinocacti Bouché, 1833 (Hemiptera: Diaspididae). Flora 2022, 290, 152042. [Google Scholar] [CrossRef]
- El Fakhouri, K.; Ramdani, C.; Aasfar, A.; Boulamtat, R.; Sijilmassi, B.; El Bouhssini, M.; Kadmiri, I.M. Isolation, Identification and Pathogenicity of Local Entomopathogenic Bacteria as Biological Control Agents against the Wild Cochineal Dactylopius Opuntiae (Cockerell) on Cactus Pear in Morocco. Sci. Rep. 2023, 13, 21647. [Google Scholar] [CrossRef] [PubMed]
- Callejas-Chavero, A.; Martínez-Hernández, D.; Flores-Martínez, A.; Moncada-Orellana, A.; Diaz-Quiñones, Y.; Vargas-Mendoza, C.F. Herbivory in Cacti: Fitness Effects of Two Herbivores, One Tending Ant on Myrtillocactus Geometrizans (Cactaceae). In Evolutionary Ecology of Plant-Herbivore Interaction; Springer International Publishing: Cham, Switzerland, 2020; pp. 109–134. [Google Scholar]
- Husnik, F.; McCutcheon, J.P. Repeated Replacement of an Intrabacterial Symbiont in the Tripartite Nested Mealybug Symbiosis. Proc. Natl. Acad. Sci. USA 2016, 113, E5416–E5424. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, F.; Yuan, Y.; Pan, H. Structural Composition and Diversity of Bacterial Communities in High- and Low-Yielding Moso Bamboo Forests. Front. Biosci.-Landmark 2023, 28, 290. [Google Scholar] [CrossRef]
- Kondo, T.; Watson, G.W. Encyclopedia of Scale Insect Pests; CAB International: Wallingford, UK, 2022; ISBN 978-1-80062-065-0. [Google Scholar]
- Rosenblueth, M.; Martínez-Romero, J.; Ramírez-Puebla, S.T.; Vera-Ponce de León, A.; Rosas-Pérez, T.; Bustamante-Brito, R.; Rincón-Rosales, R.; Martínez-Romero, E. Microorganismos Endosimbiontes de Insectos Escama. TIP Rev. Espec. Cienc. Quím.-Biol. 2018, 21, 53–69. [Google Scholar]
- El Aalaoui, M.; Sbaghi, M. Ecological Dynamics of Phenacoccus Solenopsis Tinsley (Hemiptera: Pseudococcidae) and Its Parasitoids in Varied Host Environments. Phytoparasitica 2024, 52, 44. [Google Scholar] [CrossRef]
- Yasika, Y.; Shivakumar, M.S. A Comprehensive Account of Functional Role of Insect Gut Microbiome in Insect Orders. J. Nat. Pestic. Res. 2025, 11, 100110. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An Insight into Diversity and Functionalities of Gut Microbiota in Insects. Curr. Microbiol. 2020, 77, 1976–1986. [Google Scholar] [CrossRef]
- Lu, Q.-C.; Yu, J.-M.; Liu, H.-L.; Wu, X.-L.; Wei, S.-J.; Lei, M.; Cai, P.; He, H.-G.; Pu, D.-Q. Stable Composition of Gut Microbiome in the Asian Ladybeetle Coccinella Septempunctata Reared on Natural and Artificial Diets. Sci. Rep. 2024, 14, 71. [Google Scholar] [CrossRef]
- Kumar, D.; Sun, Z.; Cao, G.; Xue, R.; Hu, X.; Gong, C. Bombyx Mori Bidensovirus Infection Alters the Intestinal Microflora of Fifth Instar Silkworm (Bombyx Mori) Larvae. J. Invertebr. Pathol. 2019, 163, 48–63. [Google Scholar] [CrossRef]
- Ranjith, M.T.; ManiChellappan; Harish, E.R.; Girija, D.; Nazeem, P.A. Bacterial Communities Associated with the Gut of Tomato Fruit Borer, Helicoverpa Armigera (Hübner) (Lepidoptera: Noctuidae) Based on Illumina Next-Generation Sequencing. J. Asia Pac. Entomol. 2016, 19, 333–340. [Google Scholar] [CrossRef]
- Ali, I.; Zhang, S.; Luo, J.-Y.; Wang, C.-Y.; Lv, L.-M.; Cui, J.-J. Artificial Diet Development and Its Effect on the Reproductive Performances of Propylea Japonica and Harmonia Axyridis. J. Asia Pac. Entomol. 2016, 19, 289–293. [Google Scholar] [CrossRef]
- Pongen, Y.L.; Thirumurugan, D.; Ramasubburayan, R.; Prakash, S. Harnessing Actinobacteria Potential for Cancer Prevention and Treatment. Microb. Pathog. 2023, 183, 106324. [Google Scholar] [CrossRef]
- Salgado, J.F.M.; Hervé, V.; Vera, M.A.G.; Tokuda, G.; Brune, A. Unveiling Lignocellulolytic Potential: A Genomic Exploration of Bacterial Lineages within the Termite Gut. Microbiome 2024, 12, 201. [Google Scholar] [CrossRef]
- Chen, L.; He, Z.; Zhang, D.; Zhao, F.; Zhang, Y.; Ding, R. The Role of Gut Microbiota at Different Developmental Stages in the Adaptation of the Etiella Zinckenella to a Plant Host. Sci. Rep. 2025, 15, 4971. [Google Scholar] [CrossRef]
- Moran, N.A.; McCutcheon, J.P.; Nakabachi, A. Genomics and Evolution of Heritable Bacterial Symbionts. Annu. Rev. Genet. 2008, 42, 165–190. [Google Scholar] [CrossRef]
- Wikström, B.; Huemer, P.; Mutanen, M.; Tyllinen, J.; Kaila, L. Pyralis Cardinalis, a Charismatic New Species Related to P. Regalis [Denis & Schiffermüller], 1775, First Recognized in Finland (Lepidoptera, Pyralidae). Nota Lepidopterol. 2020, 43, 337–364. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Rock, C.O. Membrane Lipid Homeostasis in Bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Kinjo, Y.; Bourguignon, T.; Hongoh, Y.; Lo, N.; Tokuda, G.; Ohkuma, M. Coevolution of Metabolic Pathways in Blattodea and Their Blattabacterium Endosymbionts, and Comparisons with Other Insect-Bacteria Symbioses. Microbiol. Spectr. 2022, 10, e02779-22. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.B.; Medeiros, A.B.; dos Anjos, A.I.M.; Ferreira Cavalcante, J.V.; Santiago, B.C.F.; Monteiro, S.S.; Vital, A.C., Júnior; Dalmolin, R.J.S.; Lisboa, H.M.; Pasquali, M.A.d.B. Changes in the Microbiota of the Scale Insect (Diaspis echinocacti, Bouché, 1833) in Opuntia stricta Cladodes: Taxonomic and Metagenomic Analysis as a Function of Infestation Levels. Biology 2025, 14, 1233. https://doi.org/10.3390/biology14091233
da Silva MB, Medeiros AB, dos Anjos AIM, Ferreira Cavalcante JV, Santiago BCF, Monteiro SS, Vital AC Júnior, Dalmolin RJS, Lisboa HM, Pasquali MAdB. Changes in the Microbiota of the Scale Insect (Diaspis echinocacti, Bouché, 1833) in Opuntia stricta Cladodes: Taxonomic and Metagenomic Analysis as a Function of Infestation Levels. Biology. 2025; 14(9):1233. https://doi.org/10.3390/biology14091233
Chicago/Turabian Styleda Silva, Mikaelly Batista, Ana Beatriz Medeiros, Antonia Isabelly Monteiro dos Anjos, João Vitor Ferreira Cavalcante, Bianca Cristiane Ferreira Santiago, Shênia Santos Monteiro, Antonio Carlos Vital, Júnior, Rodrigo Juliani Siqueira Dalmolin, Hugo M. Lisboa, and Matheus Augusto de Bittencourt Pasquali. 2025. "Changes in the Microbiota of the Scale Insect (Diaspis echinocacti, Bouché, 1833) in Opuntia stricta Cladodes: Taxonomic and Metagenomic Analysis as a Function of Infestation Levels" Biology 14, no. 9: 1233. https://doi.org/10.3390/biology14091233
APA Styleda Silva, M. B., Medeiros, A. B., dos Anjos, A. I. M., Ferreira Cavalcante, J. V., Santiago, B. C. F., Monteiro, S. S., Vital, A. C., Júnior, Dalmolin, R. J. S., Lisboa, H. M., & Pasquali, M. A. d. B. (2025). Changes in the Microbiota of the Scale Insect (Diaspis echinocacti, Bouché, 1833) in Opuntia stricta Cladodes: Taxonomic and Metagenomic Analysis as a Function of Infestation Levels. Biology, 14(9), 1233. https://doi.org/10.3390/biology14091233







