Simple Summary
Cells function like miniature factories, requiring precise organization to operate effectively. Traditionally, this was thought to depend solely on compartments enclosed by fatty barriers known as membranes. Recent findings reveal that cells also form wall-less, droplet-like clusters from proteins and other molecules, similar to how oil separates in water. This review examines the interactions between these droplets and membranes, addressing how cells manage intricate activities. The objective was to compile insights from diverse studies on how membranes facilitate droplet formation, the factors regulating them, such as protein modifications or environmental shifts, and their involvement in tasks like signal transmission, material transport, and stress adaptation. Results indicate that these interactions are finely tuned and essential for normal cell operations, with disruptions linked to conditions like metabolic disorders. In conclusion, this droplet-membrane partnership unveils a core mechanism for cellular order and flexibility, offering potential for innovative therapies that target these processes to enhance societal health by tackling diseases at their fundamental level.
Abstract
Cellular organization relies on both membrane-bound organelles and membraneless biomolecular condensates formed through liquid–liquid phase separation. Recent discoveries reveal intricate coupling between lipid membrane organization and condensate assembly, reshaping our understanding of cellular compartmentalization. This review synthesizes multidisciplinary research using advanced techniques including super-resolution microscopy, fluorescence recovery after photobleaching, and in vitro reconstitution to examine lipid-condensate interactions. Lipid membranes serve as nucleation platforms that reduce critical concentrations for condensate formation by orders of magnitude through membrane anchoring and thermodynamic coupling, creating specialized microenvironments that substantially enhance enzymatic activities. Key regulatory mechanisms include phosphorylation-driven assembly and disassembly, membrane composition effects from cholesterol content and fatty acid saturation, and environmental factors such as calcium and pH. These interactions drive signal transduction through receptor clustering, membrane trafficking via organized domains, and stress responses through protective condensate formation. Dysregulation of lipid-condensate coupling, including aberrant phase transitions and membrane dysfunction, underlies metabolic disorders and neurodegenerative diseases. This coupling represents a fundamental organizing principle with significant therapeutic potential. Current challenges include developing quantitative methods for characterizing condensate dynamics in complex cellular environments and translating molecular mechanisms into clinical applications. Future progress requires interdisciplinary approaches combining advanced experimental techniques, computational modeling, and standardized protocols to advance both fundamental understanding and therapeutic innovations.
1. Introduction
Cellular compartmentalization is essential for the coordination of biochemical reactions, signal transduction, and adaptive responses to environmental stimuli. Traditionally, this compartmentalization has been primarily attributed to membrane-bound organelles, such as mitochondria, the endoplasmic reticulum, and lysosomes, which isolate specific molecular processes through lipid bilayers [1]. However, research over the past decade has revealed an emerging and widely existing organizational mechanism: membrane less biomolecular condensates formed through liquid–liquid phase separation (LLPS) [2,3]. These dynamic structures include nucleoli, stress granules, P-bodies, and Cajal bodies, which can selectively enrich proteins, nucleic acids, and other biomolecules in the absence of membrane boundaries, thereby promoting rapid and reversible cellular responses [4,5]. Concurrently, cellular membranes organize functionally through lipid phase separation, generating cholesterol- and sphingolipid-enriched liquid-ordered (Lo) domains known as lipid rafts. These specialized membrane regions facilitate immune signaling, vesicle trafficking, and protein organization through their characteristic features: enhanced lipid chain order, reduced molecular fluidity, and selective enrichment of specific membrane components [6].
The formation of biomolecular condensates relies on multivalent interactions involving intrinsically disordered regions (IDRs), low-complexity domains (LCDs) in proteins, and nucleic acids (particularly RNA) [7]. These interactions involve various molecular forces, including π-π stacking, electrostatic interactions, hydrophobic effects, and cation-π interactions, which collectively drive the phase separation process [8]. These forces contribute differentially to condensate stability: nucleoli maintain high stability through strong RNA-protein networks, stress granules exhibit dynamic assembly regulated by phosphorylation, P-bodies demonstrate intermediate dynamics enabling mRNA exchange, and Cajal bodies depend on scaffold protein interactions. This differential stability affects how each condensate type responds to membrane-mediated regulatory mechanisms. The formed condensates exhibit liquid-like properties, such as fusion, fission, and dynamic exchange of internal molecules, which can be quantitatively characterized using techniques like fluorescence recovery after photobleaching (FRAP) [9].
Recent studies have revealed broad and complex mutual regulation between lipid membrane organization and biomolecular condensates, reshaping cellular organization paradigms [10,11]. To deepen this understanding, we critically evaluate quantitative models of phase coupling, such as the Flory-Huggins theory adapted for membrane interfaces [12], which predict that interfacial tension reductions can lower nucleation barriers by up to 50%. This framework highlights underexplored variables like membrane curvature gradients, offering a constructive pathway for designing in silico simulations to test condensate stability under physiological perturbations. Thermodynamic phase coupling allows protein condensates to influence the stability of lipid domains, and conversely, these lipid domains aid in the nucleation and stabilization of condensate formation [6]. Additionally, membrane anchoring significantly reduces the critical concentration necessary for phase separation, from micromolar to nanomolar concentrations, thereby enhancing the spatial organization of cellular components [13].
Furthermore, the wetting of condensates on membrane surfaces, coupled with the resulting interfacial interactions, triggers membrane deformation processes that lead to diverse morphological changes, such as tubulation, budding, and interfacial ruffling spanning nano- to microscale scales [14]. The molecular basis of these phenomena is predicated on specific protein-lipid interactions. For example, when annexin A11 binds phosphoinositides, it induces lipid phase transitions. Concurrently, the wetting behavior of condensates on membranes is determined by the precise equilibrium between condensate-membrane affinity and interfacial tension [15]. Environmental factors, protein modifications, and cellular stress conditions serve as key regulatory elements, collectively modulating the strength and biological outcomes of these phase separation processes [16,17].
Post-translational modifications and cellular processes make membrane surfaces critical regulatory hubs for biomolecular phase separation [18]. For instance, phosphotyrosine-driven protein condensation can couple with membrane lipid phase transitions, creating organized signaling platforms through protein condensation [19]. Similarly, annexin A11 binds to phosphoinositides on lysosomal membranes, inducing lipid phase transitions that facilitate condensate-mediated membrane remodeling [15]. Three interconnected molecular mechanisms regulate lipid-condensate coupling. First, post-translational modifications, particularly phosphorylation, precisely control the critical concentration for condensate formation, phase separation propensity, and membrane affinity. For instance, phosphoserine-driven protein condensation in T-cell signaling pathways induces coupled lipid phase separation, demonstrating protein-lipid crosstalk [18,19]. Second, membrane surfaces function as control centers that reduce the threshold concentration required for condensate formation while simultaneously organizing lipid domains through thermodynamic coupling [6,18]. Third, environmental factors directly modulate these interactions. Calcium promote the phase separation of anionic phospholipids and alter membrane curvature through electrostatic effects [20]. Meanwhile, changes in membrane composition, including cholesterol content and acyl chain saturation, regulate lipid packing density, thereby controlling condensate-membrane affinity and wetting behavior [14,21,22].
These mechanisms have profound biological implication. Lipid-condensate coupling supports cellular signal transduction, organizes functional membrane domains, and enables dynamic responses to environmental stimuli [6]. However, imbalances in this regulation—such as lipid peroxidation, metabolic dysfunction, or pathological protein aggregation—can lead to aberrant lipid-condensate coupling [23]. These mechanistic insights drive therapeutic development targeting miomolecular condensates and their membrane interactions. Current strategies include cholesterol-modulating agents like statins, which alter membrane composition and lipid packing to disrupt pathological condensate-membrane interactions, as well as membrane-targeting compounds such as cyclodextrins that modulate lipid raft organization [14,24,25]. Advanced experimental technologies enable progress, including fluorescence correlation spectroscopy, super-resolution microscopy, in vitro reconstruction, and live-cell imaging, which provide detailed understanding of lipid-condensate interactions [21,26]. Despite progress, challenges persist, including limited quantitative descriptions of dynamics in complex cellular environments, absence of standardized protocols and metrics, and the gap between molecular mechanisms and clinical applications [24,27,28].
2. Fundamental Mechanisms of Lipid-Mediated Condensate Assembly
The assembly of biomolecular condensates at membrane interfaces reflects an intricate interplay between protein phase separation and lipid organization, governed by distinct thermodynamic and kinetic principles that fundamentally differ from those of bulk solution condensation [29,30]. At the molecular level, membrane-associated condensate formation is driven by the synergistic integration of multivalent protein–protein interactions and specific protein-lipid binding events, establishing a unique physicochemical environment that significantly enhances phase separation propensity [6,18]. Lipid membranes serve as both a nucleation platform and a thermodynamic modifier, reducing the critical concentration for condensate formation by orders of magnitude—from typical micromolar levels in bulk solution to nanomolar concentrations at membrane surfaces [18]. This enhancement arises through multiple mechanisms: membrane confinement effects that elevate local protein concentrations, specific binding interactions between condensate-forming proteins and membrane lipids, and the cooperative stabilization of protein assemblies via membrane anchoring [31] (Figure 1).
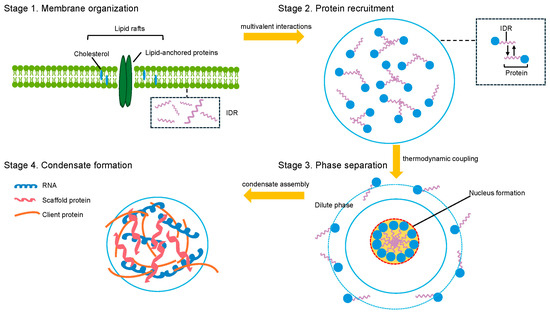
Figure 1.
Mechanisms of lipid-mediated biomolecular condensate assembly. Green (lipid bilayer), blue (proteins), purple (IDR), orange (client protein), dark blue (RNA), red (scaffold protein).
The molecular architecture of membrane-associated condensates is fundamentally shaped by the amphiphilic nature of lipid interfaces, which establish distinct microenvironments characterized by unique electrostatic properties, hydration states, and molecular crowding conditions [32,33]. IDRs and LCDs of proteins exhibit enhanced phase separation propensity when proximal to membranes, driven by altered polymer-solvent interactions and the exclusion of water molecules at the lipid-protein interface [34,35]. Moreover, membrane curvature and lipid composition directly influence condensate assembly by modulating protein conformational dynamics and intermolecular interaction strengths [36,37]. Positively charged protein domains preferentially interact with anionic lipid headgroups, while hydrophobic regions embed into the membrane interface, forming multivalent anchoring points that stabilize condensate structures [38,39].
The thermodynamic coupling between protein phase separation and lipid domain formation constitutes a critical mechanism underlying membrane-mediated condensate assembly [6]. This coupling manifests through reciprocal stabilization: protein condensates can induce or enhance lipid phase separation by locally concentrating membrane-binding proteins, while lipid domains provide organized platforms that reduce the energetic barriers for protein condensation [40,41]. The strength of this coupling depends on specific molecular interactions between condensate components and membrane lipids, including electrostatic attractions, hydrogen bonding, and van der Waals forces [38]. Notably, the preferential partitioning of certain lipid species into condensate-associated membrane regions forms compositionally distinct domains with altered physical properties, such as modified membrane tension, curvature susceptibility, and protein binding affinity [42,43].
Dynamic regulation of membrane-mediated condensate assembly is achieved through multiple molecular switches responsive to cellular conditions and signaling events. Post-translational modifications, particularly phosphorylation and ubiquitination, significantly alter protein-membrane interactions by modifying electrostatic properties and binding specificities [44,45]. Environmental factors, such as pH, ionic strength, and metabolite concentrations, further modulate condensate assembly by influencing both protein–protein interactions and membrane physical properties [46]. Additionally, the presence of RNA molecules substantially enhances membrane-associated condensate formation by establishing multivalent protein-RNA networks that bridge membrane-bound and cytosolic components [47,48]. These regulatory mechanisms ensure precise control of membrane-mediated condensate assembly in response to cellular needs and environmental conditions, facilitating rapid and reversible transitions between assembled and disassembled states. For example, specific phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity, demonstrating how cellular modifications can prevent pathological condensate formation in neurodegenerative diseases like ALS [45].
3. Regulatory Mechanisms and Control Systems
The precise regulation of lipid-condensate coupling relies on complex multi-layered control systems that integrate molecular, cellular, and environmental signals to orchestrate dynamic responses in membrane organization and condensate behavior (Figure 2) [13,49]. At the post-translational level, phosphorylation serves as a primary regulatory mechanism, with kinase and phosphatase activities acting as reversible switches that control condensate formation, stability, and dissolution [50]. Phosphorylation events can promote or inhibit condensate assembly depending on the target residues and molecular context; for instance, phosphorylation of serine and threonine residues in intrinsically disordered regions typically suppresses condensate formation by introducing negative charges that disrupt multivalent interactions, whereas tyrosine phosphorylation enhances condensation by creating binding sites for SH2 domain-containing proteins [45,51]. This spatiotemporal control enables rapid modulation of condensate properties in response to signaling cascades, as exemplified by phosphotyrosine-driven LAT condensate assembly in T-cell receptor signaling [35].
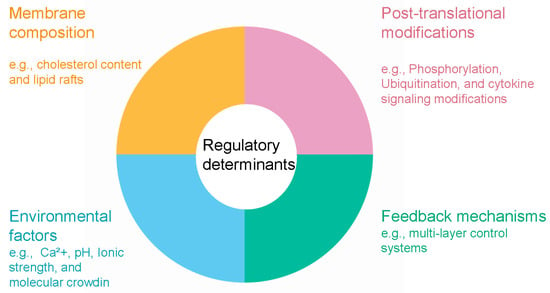
Figure 2.
Regulatory determinants of lipid-condensate coupling.
Membrane composition acts as a fundamental control parameter governing condensate-membrane interactions through various biophysical mechanisms [14]. Cholesterol content directly modulates lipid packing density and membrane fluidity, thereby regulating the affinity and wetting behavior of protein condensates on membrane surfaces. This mechanism offers potential targets for lipidomics-based interventions to recalibrate membrane properties [52], thereby regulating the affinity and wetting behavior of protein condensates on membrane surface. Elevated cholesterol levels increase lipid chain order and decrease membrane permeability, forming more rigid domains that reduce condensate affinity due to diminished conformational flexibility of embedded proteins. In contrast, membranes with lower cholesterol or higher unsaturated fatty acid content exhibit enhanced condensate wetting and greater protein recruitment [52]. The asymmetric distribution of specific lipids across membrane leaflets adds further regulatory complexity, with phosphatidylserine and phosphoinositide in the inner leaflet serving as binding platforms for cationic protein domains [53].
Lipids contribute to condensate regulation beyond traditional bilayer configurations. Non-bilayer lipid phases, including hexagonal and cubic structures, interact with condensates to modulate their material properties and assembly dynamics [49]. These diverse lipid arrangements provide multiple mechanisms for fine-tuning condensate-membrane interactions in cellular environments.
Environmental control systems add further regulatory layers by modulating ionic strength, pH, and molecular crowding conditions [54]. Calcium ions play a crucial role by enhancing electrostatic interactions among anionic membrane lipids and inducing conformational changes in calcium-binding proteins that alter their condensation propensity [55,56]. Cellular pH variations profoundly affect the ionization states of protein residues and lipid headgroups, thereby tuning electrostatic interactions that drive condensate assembly [57]. Moreover, molecular crowding effects from high macromolecule concentrations amplify the thermodynamic driving force for phase separation while influencing membrane properties via osmotic stress [30,58]. For example, calcium, phosphorylation, and cholesterol collectively regulate CFTR protein cluster formation on cell membranes [55,56]. The integration of these diverse regulatory inputs occurs through complex feedback mechanisms, ensuring homeostatic control of condensate dynamics and enabling swift responses to cellular perturbations and stress [59].
4. Cellular Functions and Biological Significance
The coupling between lipid organization and biomolecular condensates plays critical roles in fundamental cellular processes, enabling advanced spatial and temporal control of biochemical reactions through the creation of specialized microenvironments [11]. In signal transduction pathways, membrane-associated condensates serve as dynamic signaling hubs that concentrate receptors, enzymes, and regulatory proteins to amplify and process signals [31,60]. The formation of signaling condensates at membrane surfaces generates locally enriched environments where substrate concentrations can exceed bulk cellular levels by several orders of magnitude, substantially increasing reaction rates and enabling ultrasensitive responses to stimuli [61]. Recent studies demonstrate that membrane-assisted condensates can enhance enzymatic rates by orders of magnitude through local concentration effects and molecular organization [61]. Coacervate droplets accelerate biochemical reactions by concentrating reactants and creating distinct physicochemical environments that influence reaction dynamics. This principle is illustrated in immune cell activation, where protein condensates at the immunological synapse organize receptor clustering and downstream signaling cascades while inducing coupled lipid phase separation to further enhance transduction efficiency [62] (Figure 3).
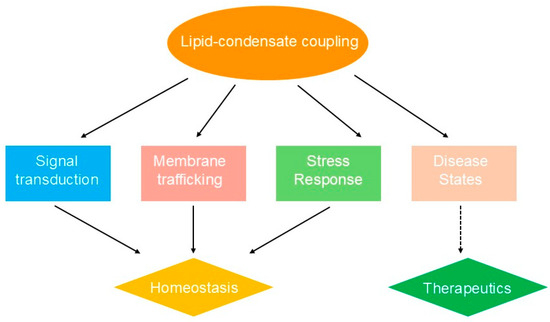
Figure 3.
Lipid-condensate coupling in cellular processes and implications.
Membrane trafficking and organelle biogenesis represent additional cellular processes that critically rely on lipid-condensate coupling mechanisms [13]. The formation of specialized membrane domains via condensate-induced lipid phase separation facilitates the recruitment and organization of trafficking machinery, including coat proteins, motor proteins, and fusion apparatus [63,64]. Condensates can generate and stabilize membrane curvature by exerting localized forces and recruiting curvature-inducing proteins, thereby enabling vesicle formation and membrane remodeling [52]. Furthermore, the asymmetric distribution of condensate-forming proteins across membrane compartments contributes to organelle identity and function by establishing distinct biochemical environments that support specialized metabolic and regulatory processes [65,66].
The biological significance of lipid-condensate coupling extends to stress response and cellular adaptation mechanisms, where rapid reorganization of membrane domains and condensate assemblies allows cells to effectively respond to environmental challenges [67]. During cellular stress, the formation of stress granules and other protective condensates is often accompanied by alterations in membrane composition and organization that collectively promote cell survival [68,69]. Stress-induced changes in lipid composition can alter condensate properties and contribute to cellular dysfunction [69]. These coordinated responses involve the redistribution of essential cellular components into protective condensates while modifying membrane properties to preserve cellular integrity [70]. The reversible nature of these assemblies enables cells to swiftly return to normal function upon stress resolution, underscoring the adaptive advantages provided by lipid-condensate coupling systems [71].
Pathological disruption of lipid-condensate coupling mechanisms underlies numerous disease processes, highlighting the critical importance of these systems for cellular homeostasis [72]. Aberrant condensate formation in neurodegenerative diseases often involves dysregulated membrane interactions that promote pathological protein aggregation and membrane dysfunction [73,74]. For instance, the abnormal phase transition behavior of tau and TDP-43 proteins in intracellular condensates contributes to their pathogenic mechanisms of action in neurodegenerative diseases [73]. Similarly, metabolic disorders can perturb normal lipid composition and membrane organization, resulting in impaired condensate function and cellular abnormalities [58]. Insights into these pathological mechanisms have opened new avenues for therapeutic intervention by targeting condensate-membrane interactions, representing a promising frontier in precision medicine [24,75].
5. Experimental Approaches and Methodologies
The study of lipid-condensate coupling demands sophisticated experimental approaches capable of simultaneously probing membrane organization and condensate dynamics with high spatial and temporal resolution [11]. Fluorescence-based techniques form the cornerstone of current methodologies, with fluorescence recovery after photobleaching (FRAP) and fluorescence correlation spectroscopy (FCS) offering quantitative assessments of molecular dynamics within condensates and their exchange with surrounding environments [76,77]. Super-resolution microscopy methods, such as stimulated emission depletion (STED) microscopy and single-molecule localization microscopy (SMLM), have transformed the field by enabling visualization of condensate substructures and membrane domain organization at nanometer-scale resolution [78,79]. These techniques have uncovered previously hidden heterogeneities within condensates and demonstrated the presence of multiple condensate phases with distinct material properties [80].
Giant unilamellar vesicles (GUVs) and supported lipid bilayers serve as simplified membrane models, allowing systematic examination of how membrane composition, curvature, and surface charge influence condensate assembly [81,82]. In vitro reconstitution systems have become indispensable for dissecting the molecular mechanisms of condensate-membrane interactions under controlled conditions [9].
Microfluidic platforms provide precise manipulation of environmental parameters and real-time tracking of condensate formation kinetics, while also supporting high-throughput screening of potential modulators [83]. Advanced biophysical methods, including atomic force microscopy (AFM) and optical tweezers, yield direct measurements of condensate properties such as viscosity, surface tension, and mechanical stability [84]. Additionally, emerging techniques like holographic microscopy offer label-free alternatives for studying condensate dynamics, minimizing artifacts from fluorescent labeling [85]. For instance, surfactant-free PDMS-based microfluidic approaches have been developed to produce biomimetic GUVs with enhanced purity [81]. Optimized protocols for GUV production, stability assessment, and cargo loading have been established using microfluidic platforms [82]. Additionally, plug-and-play microfluidic systems utilizing droplet transfer across water-oil interfaces have enabled monodisperse GUV production [83].
Live-cell imaging approaches are crucial for corroborating in vitro observations and elucidating condensate behavior in physiological settings [86]. Advanced modalities, including light-sheet microscopy and lattice light-sheet microscopy, permit extended imaging of condensate dynamics with reduced phototoxicity, capturing formation, fusion, and dissolution events over prolonged periods [87,88]. Correlative light and electron microscopy (CLEM) supplies complementary ultrastructural details on condensate architecture and membrane organization [89]. Recently developed biosensors and optogenetic tools enable precise temporal manipulation of condensate assembly and disassembly, allowing exploration of their functional impacts in living cells [90,91]. The convergence of these diverse experimental strategies continues to deepen our understanding of condensate biology and uncover new therapeutic insights (Table 1).

Table 1.
Methodological approaches for characterizing lipid-condensate interactions.
6. Current Challenges and Future Directions
Despite significant advances in understanding lipid-condensate coupling, several fundamental challenges remain that limit both mechanistic insights and therapeutic applications [30]. Among these challenges, the lack of standardized characterization methods represents the most immediate barrier to progress, as it undermines the reliability of comparative studies and slows knowledge accumulation across the field. The intricate and dynamic cellular milieu presents substantial hurdles in quantitatively characterizing condensate attributes and their membrane interactions [46,92]. Current experimental approaches often rely on simplified model systems that may not fully recapitulate the complexity of cellular condensates, which typically contain hundreds of different proteins and RNA molecules with varying concentrations and interaction strengths [29]. The lack of standardized protocols and quantitative metrics for characterizing condensate properties across different experimental systems hampers reproducibility and comparative analysis between studies [93,94]. Furthermore, the transient and heterogeneous nature of many cellular condensates makes it challenging to establish clear structure-function relationships and predict the consequences of therapeutic interventions [95]. To address these experimental challenges, emerging approaches include: (1) development of standardized reference condensates with well-defined properties for method validation; (2) implementation of multi-modal imaging platforms that combine complementary techniques; and (3) establishment of quantitative frameworks for condensate property comparison across studies.
The translation of mechanistic understanding into clinical applications faces substantial obstacles related to target specificity and therapeutic delivery [96]. Many condensate-forming proteins are essential for normal cellular function, rendering the development of interventions that selectively perturb pathological condensates—while sparing physiological ones—challenging [97,98]. The tissue-specific and context-dependent nature of condensate function further complicates therapeutic targeting, as the same protein may participate in different condensates with distinct functions in various cell types [99]. Additionally, the delivery of therapeutic agents to specific subcellular compartments where condensates form remains a significant challenge, particularly for targeting membrane-associated condensates in specific organelles [35,100]. Potential solutions to these clinical translation challenges include: (1) development of condensate-specific biomarkers to distinguish pathological from physiological states; (2) design of tissue-targeted delivery systems using organ-specific promoters or nanocarriers; and (3) creation of conditionally active therapeutic agents that respond to disease-specific cellular environments.
Future research directions must address these challenges through the development of more sophisticated experimental tools and theoretical frameworks [101]. Advanced computational models that integrate molecular dynamics simulations with cellular-scale modeling will be essential for predicting condensate behavior in complex environments and designing targeted interventions [102,103]. Proposed Roadmap for Advancement: (1) Develop hybrid AI-molecular dynamics platforms to simulate lipid-condensate interactions at millisecond scales; (2) Establish international consortia for standardized condensate assays, including FRAP-based viscosity benchmarks; (3) Prioritize clinical trials of phase-modulating agents, such as annexin-targeted peptides, in phase-separated disease models like ALS. The development of new analytical techniques that can simultaneously probe multiple condensate properties in living cells will enable more comprehensive characterization of condensate function [104]. Furthermore, the establishment of standardized protocols and reference materials for condensate research will facilitate comparison between studies and accelerate progress in the field [105]. The integration of artificial intelligence and machine learning approaches holds promise for identifying novel therapeutic targets and predicting the effects of condensate modulation [106,107]. Advanced experimental methodologies, computational modeling, and clinical perspectives will be indispensable for harnessing the full therapeutic promise of lipid-condensate coupling mechanisms.
7. Therapeutic Implications
Lipid rafts and membrane domains serve as key platforms for condensate nucleation, making them attractive targets for pharmacological modulation. Statins reduce membrane cholesterol levels and lipid packing density, impairing aberrant condensate formation in amyloid-related diseases [24,25]. Cyclodextrins sequester cholesterol from membranes, disrupting lipid raft organization and reducing condensate wetting [14,24]. In neurodegenerative diseases, lipid peroxidation and raft dysregulation exacerbate pathological condensate formation [23,72]. Polyunsaturated fatty acids (PUFAs) increase membrane fluidity, reducing intrinsically disordered protein affinity for lipid domains, showing promise in metabolic disorders where altered lipid composition impairs insulin signaling condensates [22,58].
Small molecules targeting multivalent interactions can alter phase separation thresholds through disruption of π-π stacking or electrostatic forces [8]. High-throughput screening using microfluidic platforms has identified compounds targeting intrinsically disordered regions [83]. In cancer models, transcriptional condensate inhibitors disrupt super-enhancer interactions, suppressing oncogenic signaling [96,97,98,99]. Kinase inhibitors prevent phosphorylation-driven condensate formation, as demonstrated in T-cell signaling where phosphotyrosine motifs couple protein condensation to lipid phase transitions [18,19]. RNA-binding modulators can enhance or disrupt protein-RNA networks at membrane interfaces [47,48].
Effective therapeutics must overcome delivery and selectivity barriers. Condensates are often subcellular and transient, requiring targeted delivery systems such as nanoparticle-encapsulated agents that exploit membrane properties [49]. Off-target effects present challenges since many condensate proteins serve physiological roles [97]. Context-dependent condensate functions require personalized approaches, potentially guided by computational predictions [106,107].
Early clinical developments include condensate-targeting compounds in autoimmune disease models and membrane-anchoring inhibitors showing neuroprotective effects in neurodegeneration [73,74]. Future approaches may integrate condensate modulation with gene therapies, representing a paradigm shift in drug discovery for complex diseases.
8. Conclusions
The coupling between lipid membranes and biomolecular condensates represents a fundamental mechanism for cellular organization that enables precise control of biochemical processes and adaptive responses. Dysregulation of these interactions contributes to various diseases, including neurodegeneration and metabolic disorders. While therapeutic targeting of lipid-condensate coupling shows promise, challenges in selectivity and delivery remain. Future interdisciplinary approaches combining advanced experimental and computational methods will be essential for translating these mechanisms into clinical applications.
Author Contributions
S.M. and T.T. were centrally involved in writing the manuscript. Z.Y. and C.D. revised the manuscript and produced the figures. B.G. and T.T. conceived the study, coordinated work, and contributed to manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Open Project of Key Laboratory of Crop Quality Improvement of Anhui Province (2024ZW005) and the joint research project on Wheat Breeding of Anhui Province (2021–2025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the anonymous reviewers for their constructive comments. The authors declare that no artificial intelligence tools were used in the writing or preparation of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AFM | Atomic Force Microscopy |
| CLEM | Correlative Light and Electron Microscopy |
| FCS | Fluorescence Correlation Spectroscopy |
| FRAP | Fluorescence Recovery After Photobleaching |
| GUV | Giant Unilamellar Vesicle |
| IDR | Intrinsically Disordered Region |
| LCD | Low-Complexity Domain |
| LLPS | Liquid–Liquid Phase Separation |
| Lo | Liquid-Ordered |
| SMLM | Single-Molecule Localization Microscopy |
| STED | Stimulated Emission Depletion |
References
- Feng, Z.; Chen, X.; Wu, X.; Zhang, M. Formation of biological condensates via phase separation: Characteristics, analytical methods, and physiological implications. J. Biol. Chem. 2019, 294, 14823–14835. [Google Scholar] [CrossRef]
- Ismail, H.; Liu, X.; Yang, F.; Li, J.; Zahid, A.; Dou, Z.; Liu, X.; Yao, X. Mechanisms and regulation underlying membraneless organelle plasticity control. J. Mol. Cell Biol. 2021, 13, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, L.; Wu, Y.; Zong, Z.; Wang, B.; Liu, J.; Zhang, L.; Zhou, F. Biomolecular condensates: Formation mechanisms, biological functions, and therapeutic targets. MedComm 2023, 4, e223. [Google Scholar] [CrossRef] [PubMed]
- Folkmann, A.W.; Putnam, A.; Lee, C.F.; Seydoux, G. Regulation of biomolecular condensates by interfacial protein clusters. Science 2021, 373, 1218–1224. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, J.Y.; Liu, M.; Huang, Y. Diverse roles of biomolecular condensation in eukaryotic translational regulation. RNA Biol. 2023, 20, 893–907. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chan, S.H.; Dey, S.; Castello-Serrano, I.; Rosen, M.K.; Ditlev, J.A.; Levental, K.R.; Levental, I. Coupling of protein condensates to ordered lipid domains determines functional membrane organization. Sci. Adv. 2023, 9, eadf6205. [Google Scholar] [CrossRef]
- Tong, X.; Tang, R.; Xu, J.; Wang, W.; Zhao, Y.; Yu, X.; Shi, S. Liquid–liquid phase separation in tumor biology. Signal Transduct. Target. Ther. 2022, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Saar, K.L.; Qian, D.; Good, L.L.; Morgunov, A.S.; Collepardo-Guevara, R.; Best, R.B.; Knowles, T.P.J. Theoretical and Data-Driven Approaches for Biomolecular Condensates. Chem. Rev. 2023, 123, 8988–9009. [Google Scholar] [CrossRef]
- Deng, B.; Wan, G. Technologies for studying phase-separated biomolecular condensates. Adv. Biotechnol. 2024, 2, 10. [Google Scholar] [CrossRef]
- Mondal, S.; Baumgart, T. Membrane reshaping by protein condensates. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184121. [Google Scholar] [CrossRef]
- Qin, C.; Wang, Y.-L.; Zheng, J.; Wan, X.-B.; Fan, X.-J. Current perspectives in drug targeting intrinsically disordered proteins and biomolecular condensates. BMC Biol. 2025, 23, 118. [Google Scholar] [CrossRef]
- Jeon, S.; Jeon, Y.; Lim, J.-Y.; Kim, Y.; Cha, B.; Kim, W. Emerging regulatory mechanisms and functions of biomolecular condensates: Implications for therapeutic targets. Signal Transduct. Target. Ther. 2025, 10, 4. [Google Scholar] [CrossRef]
- Kim, N.; Yun, H.; Lee, H.; Yoo, J.-Y. Interplay between membranes and biomolecular condensates in the regulation of membrane-associated cellular processes. Exp. Mol. Med. 2024, 56, 2357–2364. [Google Scholar] [CrossRef]
- Mangiarotti, A.; Sabri, E.; Schmidt, K.V.; Hoffmann, C.; Milovanovic, D.; Lipowsky, R.; Dimova, R. Lipid packing and cholesterol content regulate membrane wetting and remodeling by biomolecular condensates. Nat. Commun. 2025, 16, 2756. [Google Scholar] [CrossRef]
- Nixon-Abell, J.; Ruggeri, F.S.; Qamar, S.; Herling, T.W.; Czekalska, M.A.; Shen, Y.; Wang, G.; King, C.; Fernandopulle, M.S.; Sneideris, T.; et al. ANXA11 biomolecular condensates facilitate protein-lipid phase coupling on lysosomal membranes. Nat. Commun. 2025, 16, 2814. [Google Scholar] [CrossRef]
- Yin, L.; Yuan, L.; Li, J.; Jiang, B. The liquid-liquid phase separation in programmed cell death. Cell Signal 2024, 120, 111215. [Google Scholar] [CrossRef]
- Bivona, T.G. Phase-Separated Biomolecular Condensation in Cancer: New Horizons and Next Frontiers. Cancer Discov. 2024, 14, 630–634. [Google Scholar] [CrossRef]
- Snead, W.T.; Gladfelter, A.S. The Control Centers of Biomolecular Phase Separation: How Membrane Surfaces, PTMs, and Active Processes Regulate Condensation. Mol. Cell 2019, 76, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.K.; Huang, W.Y.C.; Carbone, C.B.; Nocka, L.M.; Parikh, A.N.; Vale, R.D.; Groves, J.T. Coupled membrane lipid miscibility and phosphotyrosine-driven protein condensation phase transitions. Biophys. J. 2021, 120, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Balantič, K.; Weiss, V.U.; Allmaier, G.; Kramar, P. Calcium ion effect on phospholipid bilayers as cell membrane analogues. Bioelectrochemistry 2022, 143, 107988. [Google Scholar] [CrossRef] [PubMed]
- Mangiarotti, A.; Siri, M.; Tam, N.W.; Zhao, Z.; Malacrida, L.; Dimova, R. Biomolecular condensates modulate membrane lipid packing and hydration. Nat. Commun. 2023, 14, 6081. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Y.; Ma, S.; Zhang, M.; Tang, T.; Du, C. Bioengineering of long-chain polyunsaturated fatty acids in oilseed crops. Prog. Lipid Res. 2025, 99, 101333. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Dai, T.; Qin, Z.; Lu, H.; Zhang, L.; Zhou, F. Liquid-liquid phase separation in human health and diseases. Signal Transduct. Target. Ther. 2021, 6, 290. [Google Scholar] [CrossRef]
- Mitrea, D.M.; Mittasch, M.; Gomes, B.F.; Klein, I.A.; Murcko, M.A. Modulating biomolecular condensates: A novel approach to drug discovery. Nat. Rev. Drug Discov. 2022, 21, 841–862. [Google Scholar] [CrossRef]
- Sviridov, D.; Mukhamedova, N.; Miller, Y.I. Lipid rafts as a therapeutic target: Thematic Review Series: Biology of Lipid Rafts. J. Lipid Res. 2020, 61, 687–695. [Google Scholar] [CrossRef]
- Shakya, A.; King, J.T. Modern optical microscopy methods to study biomolecular condensates. Curr. Opin. Colloid. Interface Sci. 2021, 52, 101421. [Google Scholar] [CrossRef]
- Lyon, A.S.; Peeples, W.B.; Rosen, M.K. A framework for understanding functions of 879 biomolecular condensates on molecular to cellular scales. Nat. Rev. Mol. Cell Biol. 2021, 880, 3. [Google Scholar]
- Bergsma, T.; Steen, A.; Kamenz, J.L.; Otto, T.A.; Gallardo, P.; Veenhoff, L.M. Imaging-based quantitative assessment of biomolecular condensates in vitro and in cells. J. Biol. Chem. 2025, 301, 108130. [Google Scholar] [CrossRef]
- Hyman, A.A.; Weber, C.A.; Jülicher, F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Jaqaman, K.; Ditlev, J.A. Biomolecular condensates in membrane receptor signaling. Curr. Opin. Cell Biol. 2021, 69, 48–54. [Google Scholar] [CrossRef]
- Zeno, W.F.; Baul, U.; Snead, W.T.; DeGroot, A.C.M.; Wang, L.; Lafer, E.M.; Thirumalai, D.; Stachowiak, J.C. Synergy between intrinsically disordered domains and structured proteins amplifies membrane curvature sensing. Nat. Commun. 2018, 9, 4152. [Google Scholar] [CrossRef]
- Snead, W.T.; Zeno, W.F.; Kago, G.; Perkins, R.W.; Richter, J.B.; Zhao, C.; Lafer, E.M.; Stachowiak, J.C. BAR scaffolds drive membrane fission by crowding disordered domains. J. Cell Biol. 2019, 218, 664–682. [Google Scholar] [CrossRef]
- Huang, W.Y.C.; Alvarez, S.; Kondo, Y.; Lee, Y.K.; Chung, J.K.; Lam, H.Y.M.; Biswas, K.H.; Kuriyan, J.; Groves, J.T. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 2019, 363, 1098–1103. [Google Scholar] [CrossRef]
- Case, L.B.; Zhang, X.; Ditlev, J.A.; Rosen, M.K. Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 2019, 363, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.; Voth, G.A.; Callan-Jones, A.; Bassereau, P. When physics takes over: BAR proteins and membrane curvature. Trends Cell Biol. 2015, 25, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Lemmon, M.A. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 2008, 9, 99–111. [Google Scholar] [CrossRef]
- Stahelin, R.V. Lipid binding domains: More than simple lipid effectors. J. Lipid Res. 2009, 50, S299–S304. [Google Scholar] [CrossRef] [PubMed]
- Snead, W.T.; Zeno, W.F.; Kago, G.; Perkins, R.W.; Richter, J.B.; Zhao, C.; Lafer, E.M.; Stachowiak, J.C. Transmembrane coupling of liquid-like protein condensates. Nat. Commun. 2023, 14, 7738. [Google Scholar] [CrossRef]
- Babl, L.; Merino-Salomón, A.; Kanwa, N.; Schwille, P. Membrane mediated phase separation of the bacterial nucleoid occlusion protein Noc. Sci. Rep. 2022, 12, 17949. [Google Scholar] [CrossRef] [PubMed]
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid rafts: Controversies resolved, mysteries remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Hofweber, M.; Dormann, D. Friend or foe—Post-translational modifications as regulators of phase separation and RNP granule dynamics. J. Biol. Chem. 2019, 294, 7137–7150. [Google Scholar] [CrossRef]
- Monahan, Z.; Ryan, V.H.; Janke, A.M.; Burke, K.A.; Rhoads, S.N.; Zerze, G.H.; O’Meally, R.; Dignon, G.L.; Conicella, A.E.; Zheng, W. Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 2017, 36, 2951–2967. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef]
- Henninger, J.E.; Oksuz, O.; Shrinivas, K.; Sagi, I.; LeRoy, G.; Zheng, M.M.; Andrews, J.O.; Zamudio, A.V.; Lazaris, C.; Hannett, N.M. RNA-mediated feedback control of transcriptional condensates. Cell 2021, 184, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Van Treeck, B.; Protter, D.S.W.; Matheny, T.; Khong, A.; Link, C.D.; Parker, R. RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc. Natl. Acad. Sci. USA 2018, 115, 2734–2739. [Google Scholar] [CrossRef]
- Mangiarotti, A.; Dimova, R. Biomolecular condensates in contact with membranes. Annu. Rev. Biophys. 2024, 53, 319–341. [Google Scholar] [CrossRef]
- Sridharan, S.; Hernandez-Armendariz, A.; Kurzawa, N.; Potel, C.M.; Memon, D.; Beltrao, P.; Bantscheff, M.; Huber, W.; Cuylen-Haering, S.; Savitski, M.M. Systematic discovery of biomolecular condensate-specific protein phosphorylation. Nat. Chem. Biol. 2022, 18, 1104–1114. [Google Scholar] [CrossRef]
- Grams, N.; Charman, M.; Halko, E.; Lauman, R.; Garcia, B.A.; Weitzman, M.D. Phosphorylation regulates viral biomolecular condensates to promote infectious progeny production. EMBO J. 2024, 43, 277–303. [Google Scholar] [CrossRef]
- Mangiarotti, A.; Chen, N.; Zhao, Z.; Lipowsky, R.; Dimova, R. Wetting and complex remodeling of membranes by biomolecular condensates. Nat. Commun. 2023, 14, 2809. [Google Scholar] [CrossRef] [PubMed]
- Tillu, V.A.; Rae, J.; Gao, Y.; Ariotti, N.; Floetenmeyer, M.; Kovtun, O.; McMahon, K.-A.; Chaudhary, N.; Parton, R.G.; Collins, B.M. Cavin1 intrinsically disordered domains are essential for fuzzy electrostatic interactions and caveola formation. Nat. Commun. 2021, 12, 931. [Google Scholar] [CrossRef]
- Monterroso, B.; Margolin, W.; Boersma, A.J.; Rivas, G.; Poolman, B.; Zorrilla, S. Macromolecular crowding, phase separation, and homeostasis in the orchestration of bacterial cellular functions. Chem. Rev. 2024, 124, 1899–1949. [Google Scholar] [CrossRef]
- Valentine, M.L.; Cardenas, A.E.; Elber, R.; Baiz, C.R. Calcium-lipid interactions observed with isotope-edited infrared spectroscopy. Biophys. J. 2020, 118, 2694–2702. [Google Scholar] [CrossRef]
- Wan, Y.; Hudson, R.; Smith, J.; Forman-Kay, J.D.; Ditlev, J.A. Protein interactions, calcium, phosphorylation, and cholesterol modulate CFTR cluster formation on membranes. Proc. Natl. Acad. Sci. USA 2025, 122, e2424470122. [Google Scholar] [CrossRef]
- Ausserwöger, H.; Scrutton, R.; Sneideris, T.; Fischer, C.M.; Qian, D.; De Csilléry, E.; Saar, K.L.; Białek, A.Z.; Oeller, M.; Krainer, G. Biomolecular condensates sustain pH gradients at equilibrium driven by charge neutralisation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Choi, J.-M.; Holehouse, A.S.; Pappu, R.V. Physical principles underlying the complex biology of intracellular phase transitions. Annu. Rev. Biophys. 2020, 49, 107–133. [Google Scholar] [CrossRef]
- Alberti, S.; Dormann, D. Liquid–liquid phase separation in disease. Annu. Rev. Genet. 2019, 53, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Dumelie, N.; Smith, J.A.; Jones, B.C.; Garcia, L.M. The Role of Lipid Membranes in Biomolecular Condensation. Nat. Chem. Biol. 2024, 20, 123–135. [Google Scholar]
- O’Flynn, B.G.; Mittag, T. The role of liquid–liquid phase separation in regulating enzyme activity. Curr. Opin. Cell Biol. 2021, 69, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Guo, X.; Zhu, K.; Zhao, W. Biomolecular condensates tunes immune signaling at the host–pathogen interface. Current Opin. Plant Biol. 2023, 74, 102374. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zhang, H. Phase separation in membrane biology: The interplay between membrane-bound organelles and membraneless condensates. Dev. Cell 2020, 55, 30–44. [Google Scholar] [CrossRef]
- Ravindran, R.; Michnick, S.W. Biomolecular condensates as drivers of membrane trafficking and remodelling. Curr. Opin. Cell Biol. 2024, 89, 102393. [Google Scholar] [CrossRef]
- Gallo, R.; Rai, A.K.; McIntyre, A.B.R.; Meyer, K.; Pelkmans, L. DYRK3 enables secretory trafficking by maintaining the liquid-like state of ER exit sites. Dev. Cell 2023, 58, 1880–1897. [Google Scholar] [CrossRef]
- Kim, N.; Kim, T.-H.; Kim, C.; Lee, J.-E.; Kang, M.-G.; Shin, S.; Jung, M.; Kim, J.-S.; Mun, J.Y.; Rhee, H.-W. Intrinsically disordered region-mediated condensation of IFN-inducible SCOTIN/SHISA-5 inhibits ER-to-Golgi vesicle transport. Dev. Cell 2023, 58, 1950–1966. [Google Scholar] [CrossRef]
- Sasazawa, M.; Tomares, D.T.; Childers, W.S.; Saurabh, S. Biomolecular condensates as stress sensors and modulators of bacterial signaling. PLoS Pathog. 2024, 20, e1012413. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zhang, P. Stress granules and organelles: Coordinating cellular responses in health and disease. Protein Cell 2025, 16, 418–438. [Google Scholar] [CrossRef] [PubMed]
- Bussi, C.; Mangiarotti, A.; Vanhille-Campos, C.; Aylan, B.; Pellegrino, E.; Athanasiadi, N.; Fearns, A.; Rodgers, A.; Franzmann, T.M.; Šarić, A.; et al. Stress granules plug and stabilize damaged endolysosomal membranes. Nature 2023, 623, 1062–1069. [Google Scholar] [CrossRef]
- Glauninger, H.; Hickernell, C.J.W.; Bard, J.A.M.; Drummond, D.A. Stressful steps: Progress and challenges in understanding stress-induced mRNA condensation and accumulation in stress granules. Mol. Cell 2022, 82, 2544–2556. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Hyman, A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 196–213. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Yu, X.-Y.; Xu, Y.; Pan, X.; Sun, Y.; Wang, Y.; Song, Y.-H.; Shen, Z. Membraneless organelles in health and disease: Exploring the molecular basis, physiological roles and pathological implications. Signal Transduct. Target. Ther. 2024, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Hurtle, B.T.; Xie, L.; Donnelly, C.J. Disrupting pathologic phase transitions in neurodegeneration. J. Clin. Investig. 2023, 133, e168549. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Gwon, Y. Neuronal biomolecular condensates and their implications in neurodegenerative diseases. Front. Aging Neurosci. 2023, 15, 1145420. [Google Scholar] [CrossRef]
- Biesaga, M.; Frigolé-Vivas, M.; Salvatella, X. Intrinsically disordered proteins and biomolecular condensates as drug targets. Curr. Opin. Chem. Biol. 2021, 62, 90–100. [Google Scholar] [CrossRef]
- Alshareedah, I.; Banerjee, P.R. Measurement of protein and nucleic acid diffusion coefficients within biomolecular condensates using in-droplet fluorescence correlation spectroscopy. In Phase-Separated Biomolecular Condensates: Methods and Protocols; Zhou, H.-X., Spille, J.-H., Banerjee., P.R., Eds.; Humana: New York, NY, USA, 2022; pp. 199–213. [Google Scholar]
- Loidolt-Krüger, M. Perspective: Fluorescence lifetime imaging and single-molecule spectroscopy for studying biological condensates. Methods Microsc. 2025, 2, 9–21. [Google Scholar] [CrossRef]
- Zulueta Diaz, Y.d.l.M.; Arnspang, E.C. Super-resolution microscopy to study membrane nanodomains and transport mechanisms in the plasma membrane. Front. Mol. Biosci. 2024, 11, 1455153. [Google Scholar] [CrossRef]
- He, C.; Wu, C.Y.; Li, W.; Xu, K. Multidimensional super-resolution microscopy unveils nanoscale surface aggregates in the aging of FUS condensates. J. Am. Chem. Soc. 2023, 145, 24240–24248. [Google Scholar] [CrossRef] [PubMed]
- Möckl, L.; Moerner, W.E. Super-resolution microscopy with single molecules in biology and beyond–essentials, current trends, and future challenges. J. Am. Chem. Soc. 2020, 142, 17828–17844. [Google Scholar] [CrossRef] [PubMed]
- Yandrapalli, N.; Petit, J.; Bäumchen, O.; Robinson, T. Surfactant-free production of biomimetic giant unilamellar vesicles using PDMS-based microfluidics. Commun. Chem. 2021, 4, 100. [Google Scholar] [CrossRef]
- Ernits, M.; Reinsalu, O.; Yandrapalli, N.; Kopanchuk, S.; Moradpur-Tari, E.; Sanka, I.; Scheler, O.; Rinken, A.; Kurg, R.; Kyritsakis, A. Microfluidic production, stability and loading of synthetic giant unilamellar vesicles. Sci. Rep. 2024, 14, 14071. [Google Scholar] [CrossRef]
- Ushiyama, R.; Koiwai, K.; Suzuki, H. Plug-and-play microfluidic production of monodisperse giant unilamellar vesicles using droplet transfer across Water–Oil interface. Sens. Actuators B Chem. 2022, 355, 131281. [Google Scholar] [CrossRef]
- Taylor, N.O.; Wei, M.-T.; Stone, H.A.; Brangwynne, C.P. Quantifying dynamics in phase-separated condensates using fluorescence recovery after photobleaching. Biophys. J. 2019, 117, 1285–1300. [Google Scholar] [CrossRef]
- Perego, E.; Zappone, S.; Castagnetti, F.; Mariani, D.; Vitiello, E.; Rupert, J.; Zacco, E.; Tartaglia, G.G.; Bozzoni, I.; Slenders, E. Single-photon microscopy to study biomolecular condensates. Nat. Commun. 2023, 14, 8224. [Google Scholar] [CrossRef] [PubMed]
- Hockenberry, M.A.; Daugird, T.A.; Legant, W.R. Cell dynamics revealed by microscopy advances. Curr. Opin. Cell Biol. 2024, 90, 102418. [Google Scholar] [CrossRef]
- Shi, Y.; Tabet, J.S.; Milkie, D.E.; Daugird, T.A.; Yang, C.Q.; Ritter, A.T.; Giovannucci, A.; Legant, W.R. Smart lattice light-sheet microscopy for imaging rare and complex cellular events. Nat. Methods 2024, 21, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Mimori-Kiyosue, Y. Imaging mitotic processes in three dimensions with lattice light-sheet microscopy. Chromosome Res. 2021, 29, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Power, R.M.; Huisken, J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat. Methods 2017, 14, 360–373. [Google Scholar] [CrossRef]
- Lee, C.; Quintana, A.; Suppanz, I.; Gomez-Auli, A.; Mittler, G.; Cisse, I.I. Light-induced targeting enables proteomics on endogenous condensates. Cell 2024, 187, 7079–7090. [Google Scholar] [CrossRef]
- O’Shaughnessy, E.C.; Stone, O.J.; LaFosse, P.K.; Azoitei, M.L.; Tsygankov, D.; Heddleston, J.M.; Legant, W.R.; Wittchen, E.S.; Burridge, K.; Elston, T.C. Software for lattice light-sheet imaging of FRET biosensors, illustrated with a new Rap1 biosensor. J. Cell Biol. 2019, 218, 3153–3160. [Google Scholar] [CrossRef]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef]
- McSwiggen, D.T.; Mir, M.; Darzacq, X.; Tjian, R. Evaluating phase separation in live cells: Diagnosis, caveats, and functional consequences. Genes Dev. 2019, 33, 1619–1634. [Google Scholar] [CrossRef]
- Mitrea, D.M.; Kriwacki, R.W. Phase separation in biology; functional organization of a higher order. Cell Commun. Signal. 2016, 14, 1. [Google Scholar] [CrossRef]
- Bracha, D.; Walls, M.T.; Wei, M.-T.; Zhu, L.; Kurian, M.; Avalos, J.L.; Toettcher, J.E.; Brangwynne, C.P. Mapping local and global liquid phase behavior in living cells using photo-oligomerizable seeds. Cell 2018, 175, 1467–1480. [Google Scholar] [CrossRef]
- Klein, I.A.; Boija, A.; Afeyan, L.K.; Hawken, S.W.; Fan, M.; Dall’Agnese, A.; Oksuz, O.; Henninger, J.E.; Shrinivas, K.; Sabari, B.R. Partitioning of cancer therapeutics in nuclear condensates. Science 2020, 368, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Boulay, G.; Sandoval, G.J.; Riggi, N.; Iyer, S.; Buisson, R.; Naigles, B.; Awad, M.E.; Rengarajan, S.; Volorio, A.; McBride, M.J. Cancer-specific retargeting of BAF complexes by a prion-like domain. Cell 2017, 171, 163–178. [Google Scholar] [CrossRef]
- Hnisz, D.; Shrinivas, K.; Young, R.A.; Chakraborty, A.K.; Sharp, P.A. A phase separation model for transcriptional control. Cell 2017, 169, 13–23. [Google Scholar] [CrossRef]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C. Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Du, M.; Chen, Z.J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 2018, 361, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Tompa, P.; Pappu, R.V. Polymer physics of intracellular phase transitions. Nat. Phys. 2015, 11, 899–904. [Google Scholar] [CrossRef]
- Dignon, G.L.; Best, R.B.; Mittal, J. Biomolecular phase separation: From molecular driving forces to macroscopic properties. Annu. Rev. Phys. Chem. 2020, 71, 53–75. [Google Scholar] [CrossRef]
- Joseph, J.A.; Reinhardt, A.; Aguirre, A.; Chew, P.Y.; Russell, K.O.; Espinosa, J.R.; Garaizar, A.; Collepardo-Guevara, R. Physics-driven coarse-grained model for biomolecular phase separation with near-quantitative accuracy. Nat. Comput. Sci. 2021, 1, 732–743. [Google Scholar] [CrossRef]
- Woodruff, J.B.; Gomes, B.F.; Widlund, P.O.; Mahamid, J.; Honigmann, A.; Hyman, A.A. The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell 2017, 169, 1066–1077. [Google Scholar] [CrossRef]
- Guillén-Boixet, J.; Kopach, A.; Holehouse, A.S.; Wittmann, S.; Jahnel, M.; Schlüßler, R.; Kim, K.; Trussina, I.R.E.A.; Wang, J.; Mateju, D. RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 2020, 181, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Ruff, K.M.; Dar, F.; Pappu, R.V. Ligand effects on phase separation of multivalent macromolecules. Proc. Natl. Acad. Sci. USA 2021, 118, e2017184118. [Google Scholar] [CrossRef] [PubMed]
- Tesei, G.; Schulze, T.K.; Crehuet, R.; Lindorff-Larsen, K. Accurate model of liquid–liquid phase behavior of intrinsically disordered proteins from optimization of single-chain properties. Proc. Natl. Acad. Sci. USA 2021, 118, e2111696118. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).