Diversity and Ecology of Late Glacial Diatoms of the Eastern Baltic Region
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographical Setting
2.2. Geochronology and Lithology
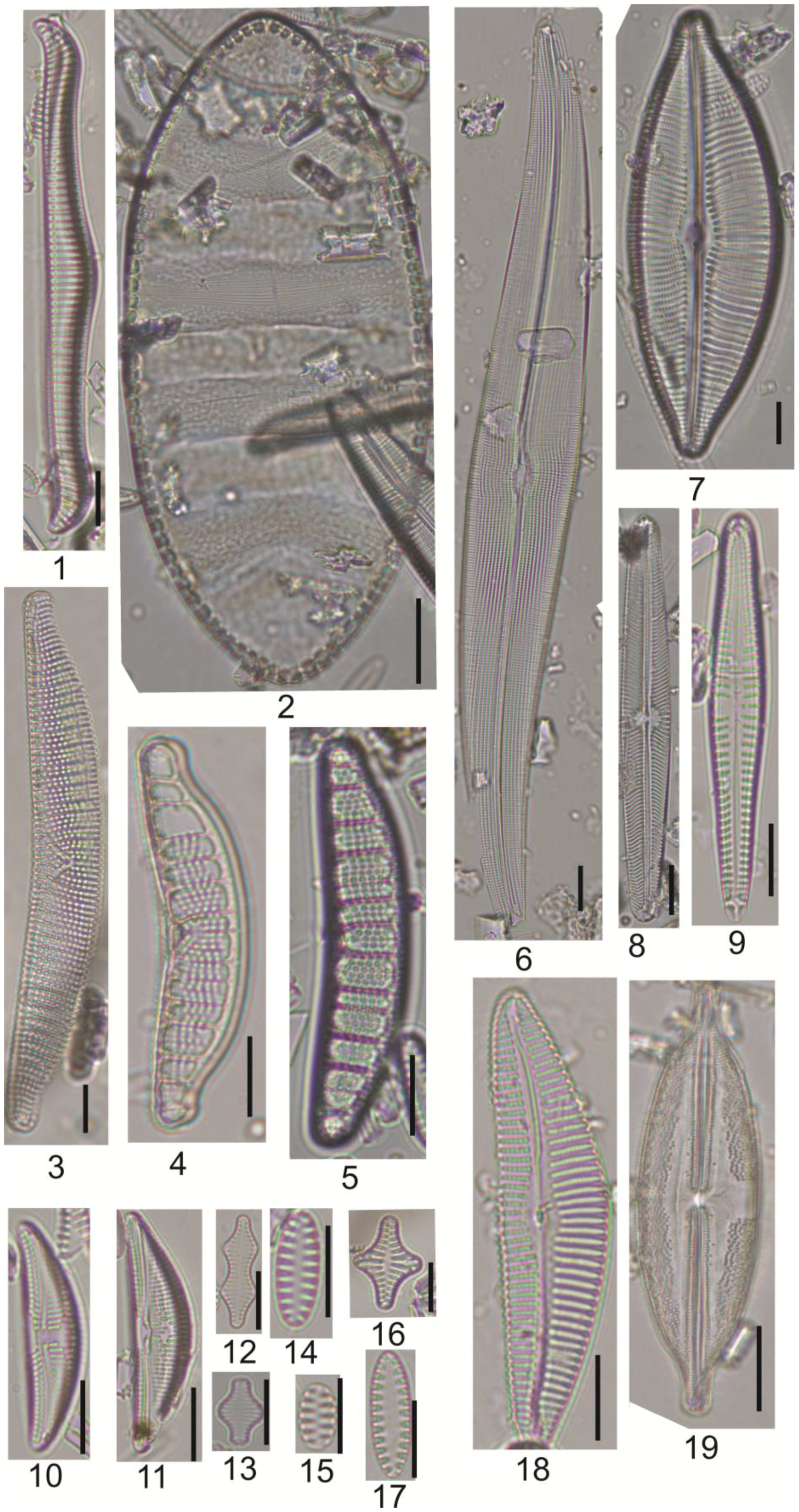
2.3. Diatom Analysis
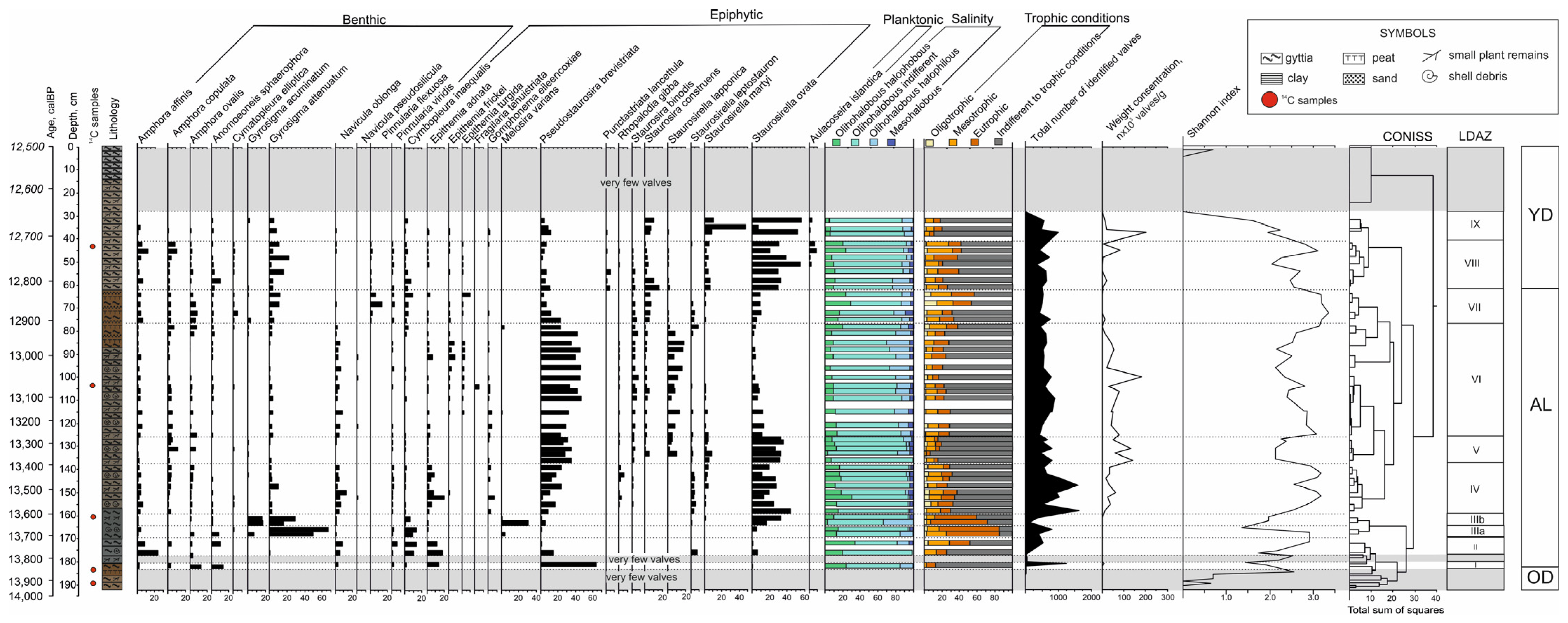
3. Results
3.1. Geochronology and Lithology
3.2. Diatom Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIL | Baltic Ice Lake |
| LDAZ | Local Diatoms Assemblages Zone |
Appendix A
| № Taxon | Ecological and Geographical Characteristics of Indicator Organisms | |||||||
|---|---|---|---|---|---|---|---|---|
| Hb | Hl | T | pH | Temp | F | Geo | ||
| Phylum Bacillariophyta Class Bacillariophyceae Subclass Bacillariophycidae Order Achnanthales Family Achnanthidiaceae | ||||||||
| 1. | Achnanthidium eutrophilum (Lange-Bertalot) Lange-Bertalot 1999 | B | ohi | I | alk | E | ind | k |
| 2. | Crenotia thermalis (Rabenhorst) Wojtal 2013 | B | ohb | M | ind | W | ind | k |
| 3. | Karayevia clevei (Grunow) Bukhtiyarova 1999 | B | mh | E | alk | – | ind | k |
| 4. | Karayevia rostrata (Hustedt) Kulikovskiy & Genkal 2013 | B | ohi | E | alk | – | ind | Ha |
| 5. | Planoplatessa joursacensis (Héribaud) Kulikovskiy, Glushchenko & Kociolek 2022 | B | ohi | M | alk | – | rh | Ha |
| 6. | Planothidium biporomum (M.H.Hohn & Hellerman) Lange-Bertalot 1999 | B | ohi | I | alk | – | rh | k |
| 7. | Planothidium frequentissimum (Lange-Bertalot) Lange-Bertalot 1999 | B | ohi | M | alk | – | ind | k |
| 8. | Planothidium werumianum Lange-Bertalot & Bąk 2015 | B | ohi | E | alk | – | lph | Ha |
| 9. | Platessa conspicua (Ant.Mayer) Lange-Bertalot 2004 | B | ohi | E | alk | – | lph | k |
| 10. | Skabitschewskia peragalloi (Brun & Héribaud) Kuliskovskiy & Lange-Bertalot 2015 | B | ohb | I | neu | – | ind | – |
| Family Bacillariaceae | ||||||||
| 11. | Hantzschia abundans Lange-Bertalot 1993 | B | ohb | M | alk | T | – | k |
| 12. | Hantzschia amphioxys (Ehrenberg) Grunow 1880 | B | ohi | I | alk | T | ind | k |
| 13. | Hantzschia calcifuga E.Reichardt & Lange-Bertalot 2004 | B | ohi | I | acd | – | – | Ha |
| 14. | Nitzschia dissipata (Kützing) Rabenhorst 1860 | B | ohb | E | alk | – | ind | k |
| 15. | Nitzschia heufleriana Grunow 1862 | B | ohi | E | alk | – | rh | k |
| 16. | Nitzschia lanceolata W.Smith 1853 | B | mh | E | alk | – | – | – |
| 17. | Nitzschia linearis W.Smith 1853 | B | ohi | I | alk | T | – | k |
| 18. | Nitzschia macilenta W.Gregory ex Greville 1859 | B | ohl | I | – | – | – | – |
| 19. | Nitzschia semirobusta Lange-Bertalot 1993 | B | ohi | I | alk | – | – | k |
| 20. | Tryblionella angustata W.Smith 1853 | B | ohl | M | alk | – | lph | k |
| 21. | Tryblionella brunoi (Lange-Bertalot) Cantonati & Lange-Bertalot 2017 | B | mh | I | alk | – | – | Ha |
| 22. | Tryblionella hungarica (Grunow) Frenguelli 1942 | B | ohi | I | alk | – | ind | k |
| 23. | Tryblionella scalaris (Ehrenberg) Siver & P.B.Hamilton 2005 | B | ohi | M | neu | – | – | – |
| Family Cocconeidaceae | ||||||||
| 24. | Cocconeis lineata Ehrenberg 1849 | E | mh | E | alk | – | ind | k |
| 25. | Cocconeis placentula Ehrenberg 1838 | E | ohi | I | alk | T | ind | k |
| Order Cymbellales Family Anomoeoneidaceae | ||||||||
| 26. | Anomoeoneis inconcinna Metzeltin, Lange-Bertalot & Soninkhishig 2009 | B | mh | I | – | – | – | – |
| 27. | Anomoeoneis sphaerophora Pfitzer 1871 | B | ohl | I | acd | W | ind | k |
| Family Cymbellaceae | ||||||||
| 28. | Cymbella aspera (Ehrenberg) 1894 | E | ohi | E | alk | – | ind | k |
| 29. | Cymbella helvetica Kützing 1844 | E | ohi | M | alk | – | lph | a, k |
| 30. | Cymbella lanceolata C.Agardh | E | ohi | M | alk | – | lph | k |
| 31. | Cymbella neocistula Krammer 2002 | E | ohb | M | alk | – | – | k |
| 32. | Cymbella neocistula var. islandica Krammer 2002 | E | ohb | O | neu | – | – | – |
| 33. | Cymbella neolanceolata W.Silva 2013 | E | ohi | E | alk | – | – | – |
| 34. | Cymbella parva (W.Smith) Kirchner 1878 | E | ohi | M | ind | – | – | – |
| 35. | Cymbella perfossilis Krammer 2002 | E | ohb | O | neu | – | – | – |
| 36. | Cymbella proxima Reimer 1975 | E | ohi | M | – | – | – | k |
| 37. | Cymbella stigmaphora Østrup 1910 | E | ohb | M | acd | – | – | Ha |
| 38. | Cymbella subarctica A.Cleve 1934 | E | ohb | O | – | – | – | a-a |
| 39. | Cymbopleura amphicephala (Nägeli ex Kützing) Krammer 2003 | E | ohb | M | neu | – | ind | b |
| 40. | Cymbopleura apiculata Krammer 2003 | E | ohi | I | neu | – | lph | Ha |
| 41. | Cymbopleura designata (Krammer) Bahls 2019 | E | ohb | O | neu | – | – | – |
| 42. | Cymbopleura inaequalis (Ehrenberg) Krammer 2003 | E | ohb | M | alk | – | – | Ha |
| 43. | Cymbopleura fluminea (R.M.Patrick & Freese) Lange-Bertalot & Krammer ex Lange-Bertalot & Genkal 1999 | E | ohb | O | neu | – | – | Ha |
| 44. | Cymbopleura kuelbsii Krammer 2003 | E | ohi | I | – | – | – | – |
| 45. | Cymbopleura naviculiformis (Auerswald ex Heiberg) Krammer 2003 | E | ohb | O | ind | – | – | b |
| 46. | Cymbopleura subaequalis (Grunow) Krammer 2003 | E | ohb | I | alk | – | ind | – |
| 47. | Cymbopleura subcuspidata (Krammer) Krammer 2003 | E | ohi | I | acd | – | – | a-a |
| 48. | Placoneis amphibola (Cleve) E.J.Cox 2003 | B | ohb | I | ind | C | ind | a-a |
| 49. | Placoneis clementis (Grunow) E.J.Cox 1988 | B | ohi | E | alk | – | – | b |
| 50. | Placoneis explanata (Hustedt) S.Mamaya 2000 | B | ohi | M | neu | – | – | k |
| 51. | Placoneis gastrum (Ehrenberg) Mereschkowsky 1903 | B | ohi | M | ind | – | lph | k |
| 52. | Placoneis ignorata (Schimanski) Lange-Bertalot 2000 | B | ohi | O | – | – | – | k |
| 53. | Placoneis paraelginensis Lange-Bertalot 2000 | B | ohi | I | ind | – | – | Ha |
| 54. | Placoneis parvapolonica Lange-Bertalot & Wojtal 2014 | B | ohi | I | – | – | – | Ha |
| Family Cymbellales incertae sedis | ||||||||
| 55. | Gomphonella olivaceolacua (Lange-Bertalot & E.Reichart) R.Jahn & N.Abarca 2019 | E | ohi | I | alk | – | ind | Ha |
| 56. | Gomphonella stauroneiformis (Grunow) R.Jahn & N.Abarca | E | ohi | I | – | – | – | – |
| Family Gomphonemataceae | ||||||||
| 57. | Gomphonema acuminatum Ehrenberg 1832 | E | ohi | I | alk | – | ind | k |
| 58. | Gomphonema brebissonii Kützing 1846 | E | ohi | E | alk | – | lph | Ha |
| 59. | Gomphonema duplipunctatum Lange-Bertalot & E.Reichardt 1996 | E | ohi | M | acd | – | lph | Ha |
| 60. | Gomphonema eileencoxiae (Bahls) Bahls, C.E.Wetzel & Ector 2018 | E | ohb | M | neu | – | lph | – |
| 61. | Gomphonema khentiiense Metzeltin, Lange-Bertalot & Soninkhishig 2009 | E | ohb | O | acd | – | – | – |
| 62. | Gomphonema lagerheimii A.Cleve 1895 | E | ohb | O | acd | – | – | a-a |
| 63. | Gomphonema parvulum (Kützing) Kützing 1849 | E | ohi | E | alk | – | – | k |
| 64. | Gomphonema pseudotenellum Lange-Bertalot 1985 | E | ohi | I | – | – | – | – |
| 65. | Gomphonema pusillum (Grunow) Kulikovskiy & Kociolek 2015 | E | ohi | I | – | – | – | – |
| 66. | Gomphonema sarcophagus W.Gregory 1856 | E | ohi | M | neu | – | – | – |
| 67. | Gomphonema subclavatum (Grunow) Grunow 1884 | E | ohi | I | – | – | – | – |
| 68. | Gomphonema truncatum Ehrenberg 1832 | E | ohb | E | alk | – | ind | Ha |
| 69 | Gomphonema truncatum var. turgidum (Ehrenberg) R.M.Patrick 1975 | E | ohb | I | – | – | – | k |
| 70. | Gomphonema utae Lange-Bertalot & E.Reichardt | E | ohb | I | acd | – | – | Ha |
| Family Encyonemataceae | ||||||||
| 71. | Encyonema elginense (Krammer) D.G.Mann 1990 | E | ohi | O | acd | – | lph | Ha |
| 72. | Encyonema lange-bertalotii Krammer 1997 | E | ohb | O | alk | – | – | – |
| 73. | Encyonema minutum (Hilse) D.G.Mann 1990 | E | ohi | I | neu | – | ind | Ha |
| 74. | Encyonema obscurum (Krasske) D.G.Mann 1990 | E | ohb | I | – | – | – | – |
| 75 | Encyonema perelginense Krammer 1997 | E | ohb | O | neu | – | – | Ha |
| 76. | Encyonema silesiacum (Bleisch) D.G.Mann 1990 | E | ohi | I | neu | – | ind | Ha |
| Family Rhoicospheniaceae | ||||||||
| 77. | Gomphonemopsis exigua (Kützing) Medlin 1986 | E | mh | E | – | – | – | – |
| Family Witkowskiaceae | ||||||||
| 78. | Witkowskia abiskoensis (Hustedt) Kulikovskiy, Glushchenko, Mironov & Kociolek 2024 | B | ohb | O | ind | – | – | a-a |
| Order Lyrellales Family Lyrellaceae | ||||||||
| 79. | Petroneis humerosa (Brébisson ex W.Smith) Stickle & D.G.Mann 1990 | B | ohp | I | – | – | – | k |
| Order Mastogloiales Family Mastogloiaceae | ||||||||
| 80. | Aneumastus apiculatus (Østrup) Lange-Bertalot 1999 | B | ohb | M | ind | – | – | b |
| 81. | Aneumastus tusculus (Ehrenberg) D.G.Mann & Stickle | B | ohb | E | alk | - | lph | k |
| Order Naviculales Suborder Diploneidineae Family Diploneidaceae | ||||||||
| 82. | Diploneis elliptica (Kützing) Cleve 1894 | B | ohb | O | alk | T | lph | k |
| Suborder Neidiineae Family Diadesmidaceae | ||||||||
| 83. | Luticola acidoclinata Lange-Bertalot 1996 | B | ohb | O | acd | – | ind | Ha |
| Suborder Naviculineae Family Naviculaceae | ||||||||
| 84. | Caloneis amphisbaena (Bory) Cleve 1894 | B | ohp | E | alk | – | ind | k |
| 85. | Caloneis holarctica Kulikovskiy, Lange-Bertalot & Witkowski 2010 | B | ohp | M | – | – | – | – |
| 86. | Caloneis hyalina Hustedt 1937 | B | ohi | I | neu | – | + | k |
| 87. | Caloneis molaris (Grunow) Krammer 1985 | B | ohi | I | ind | – | rh | k |
| 88. | Caloneis silicula var. elliptica Mayer 1913 | B | ohb | O | alk | – | lph | k |
| 89. | Caloneis tenuis (W.Gregory) Krammer 1985 | B | ohb | O | neu | – | – | Ha |
| 90. | Caloneis vasileyevae Lange-Bertalot, Genkal & Vekhov 2004 | B | ohb | O | neu | – | – | Ha |
| 91. | Cavinula variostriata (Krasske) D.G.Mann & Stickle 1990 | B | ohi | I | ind | – | ind | k |
| 92. | Cavinula vincentii Antoniades & P.B.Hamilton 2014 | B | ohb | O | acd | – | lph | Ha |
| 93. | Gyrosigma acuminatum (Kützing) Rabenhorst 1853 | B | ohi | E | alk | C | ind | k |
| 94. | Gyrosigma attenuatum (Kützing) Rabenhorst 1853 | B | ohi | E | alk | – | ind | k |
| 95. | Hippodonta capitata (Ehrenberg) Lange-Bertalot, Metzeltin & Witkowski 1996 | B | ohi | E | alk | – | ind | – |
| 96. | Navicula angusta Grunow 1860 | B | ohb | O | acd | – | ind | k |
| 97. | Navicula associata Lange-Bertalot 2001 | B | ohp | E | alk | – | – | – |
| 98. | Navicula catalanogermanica Lange-Bertalot & G.Hofmann 1993 | B | ohi | I | – | – | – | – |
| 99. | Navicula libonensis Schoeman 1970 | B | ohi | E | alk | – | – | – |
| 100. | Navicula oblonga (Kützing) Kützing | B | ohi | E | alk | + | ind | k |
| 101. | Navicula pseudosilicula Hustedt 1942 | B | ohi | I | – | – | – | – |
| 102. | Navicula radiosa Kützing 1844 | B | ohi | E | neu | T | ind | k |
| 103. | Navicula reinhardtii (Grunow) Grunow 1880 | B | ohi | I | alk | – | ind | k |
| 104. | Navicula schweigeri Bahls 2012 | B | ohb | I | neu | – | – | – |
| 105. | Navicula subalpina E.Reichardt 1988 | B | ohi | M | alk | – | – | – |
| 106. | Navicula wildii Lange-Bertalot 1993 | B | ohp | O | alk | – | – | Ha |
| Family Stauroneidaceae | ||||||||
| 107. | Stauroneis acidoclinata Lange-Bertalot & Werum 2004 | B | ohb | O | neu | – | – | – |
| 108. | Stauroneis acidoclinatopsis Van de Vijver & Lange-Bertalot 2004 | B | mh | I | ind | – | lph | – |
| 109. | Stauroneis acuta W.Smith 1853 | B | mh | O | alk | – | ind | k |
| 110. | Stauroneis amphicephala Kützing 1844 | B | ohb | O | ind | – | lph | Ha |
| 111. | Stauroneis anceps Ehrenberg 1843 | B | ohb | M | neu | – | ind | – |
| 112. | Stauroneis gracilis Ehrenberg 1843 | B | ohb | M | neu | – | – | Ha |
| 113. | Stauroneis neohyalina Lange-Bertalot & Krammer 1996 | B | ohb | I | neu | – | lph | – |
| 114. | Stauroneis phoenicenteron (Nitzsch) Ehrenberg 1843 | B | mh | I | neu | T | ind | k |
| 115. | Stauroneis producta Grunow 1880 | B | ohi | I | alk | – | lph | Ha |
| 116. | Stauroneis schulzii Jousé 1936 | B | ohi | – | – | – | – | b |
| 117. | Stauroneis separanda Lange-Bertalot & Werum 2004 | B | ohi | I | alk | – | ind | – |
| 118. | Stauroneis sonyae Kulikovskiy, Lange-Bertalot, Witkowski & Dorofeyuk 2010 | B | ohb | I | neu | – | ind | – |
| 119. | Stauroneis subborealis Bahls 2010 | B | ohb | O | ind | – | lph | – |
| 120. | Stauroneis supergracilis Van de Vijver & Lange-Bertalot 2004 | B | ohi | E | ind | – | lph | – |
| Suborder Neidiineae Family Neidiaceae | ||||||||
| 121. | Neidium ampliatum (Ehrenberg) Krammer 1985 | B | ohi | I | neu | – | lph | k |
| 122. | Neidium bobmarshallense Bahls 2013 | B | ohp | M | neu | – | – | – |
| 123. | Neidium dubium (Ehrenberg) Cleve 1894 | B | ohb | M | alk | – | – | k |
| Suborder Sellaphorineae Family Pinnulariaceae | ||||||||
| 124. | Pinnularia divergens W.Smith 1853 | B | ohb | O | neu | – | lph | a-a |
| 125. | Pinnularia flexuosa Cleve 1895 | B | ohb | O | – | – | – | Ha |
| 126. | Pinnularia legumen Ehrenberg 1843 | B | ohb | O | neu | – | – | k |
| 127. | Pinnularia major (Kützing) Rabenhorst 1853 | B | ohi | O | neu | T | ind | k |
| 128. | Pinnularia marchica I.Schönfelder 2000 | B | ohb | I | – | – | – | – |
| 129. | Pinnularia neohalophila Kulikovskiy, Genkal & Mikheeva 2010 | B | ohi | E | alk | – | – | Ha |
| 130. | Pinnularia saprophila Lange-Bertalot, Kobayasi & Krammer 2000 | B | ohi | I | – | – | ind | – |
| 131. | Pinnularia spitsbergensis Cleve 1895 | B | ohi | O | ind | – | – | a-a |
| 132. | Pinnularia suchlandtii Hustedt 1943 | B | ohb | O | acd | C | lph | a-a |
| 133. | Pinnularia subanglica Krammer 2000 | B | ohb | I | acd | – | lph | – |
| 134. | Pinnularia transversa (Cleve) Ant.Mayer 1939 | B | ohb | O | – | – | – | – |
| 135. | Pinnularia turfosiphila E.E.Gaiser & J.R.Johansen 2000 | B | ohb | I | acd | – | lph | – |
| 136. | Pinnularia viridis (Nitzsch) Ehrenberg 1843 | B | ohb | M | neu | T | ind | k |
| Family Sellaphoraceae | ||||||||
| 137. | Fallacia helensis (P.Schulz) D.G.Mann 1990 | B | ohi | I | alk | – | – | b |
| 138. | Sellaphora alastos (M.H.Hohn & Hellerman) Lange-Bertalot & Metzeltin 1996 | B | ohi | O | neu | – | – | – |
| 139. | Sellaphora bacillum (Ehrenberg) D.G.Mann 2018 | B | ohi | M | alk | – | ind | k |
| 140. | Sellaphora laevissima (Kützing) D.G.Mann 1989 | B | ohi | E | alk | – | ind | – |
| 141. | Sellaphora pseudopupula (Krasske) Lange-Bertalot 1996 | B | ohi | E | alk | – | – | Ha |
| 142. | Sellaphora stroemii (Hustedt) H.Kobayasi 2002 | B | ohi | I | alk | E | – | k |
| Order Rhopalodiales Family Rhopalodiaceae | ||||||||
| 143. | Epithema adnata (Kützing) Brébisson 1838 | E | ohi | I | alk | T | ind | k |
| 144. | Epithemia argus var. alpestris (W.Smith) Grunow 1860 | E | ohi | M | alk | – | ind | b |
| 145. | Epithemia frickei Krammer 1987 | E | ohp | E | alk | – | lph | k |
| 146. | Epithemia hyndmanii W.Smith 1850 | E | ohi | E | alk | – | lph | b |
| 147. | Epithemia sorex Kützing 1844 | E | ohp | E | alk | T | ind | k |
| 148. | Epithemia sorex var. gracilis Hustedt 1922 | E | ohp | E | alk | – | ind | b |
| 149. | Epithemia turgida (Ehrenberg) Kützing 1844 | E | ohi | E | alk | T | ind | k |
| 150. | Rhopalodia acuminata Krammer 1987 | E | mh | E | alk | – | – | – |
| 151. | Rhopalodia gibba (Ehrenberg) O.Müller 1895 | E | ohi | I | alk | T | ind | k |
| Family Stauroneidaceae | ||||||||
| 152. | Craticula ambigua (Ehrenberg) 1990 | B | ohp | E | alk | W | ind | k |
| 153. | Craticula cuspidata (Kützing) 1990 | B | ohp | M | alk | T | ind | k |
| Order Surirellales Family Surirellaceae | ||||||||
| 154. | Cymatopleura apiculata W.Smith | B | mh | E | – | – | + | – |
| 155. | Iconella bifrons (Ehrenberg) Ruck & Nakov 2016 | B | ohi | I | alk | – | lph | k |
| 156. | Iconella biseriata (Brébisson) Ruck & Nakov 2016 | B | ohp | I | alk | – | lph | k |
| 157. | Iconella linearis (W.Smith) Ruck & Nakov 2016 | B | ohi | I | ind | – | – | Ha |
| 158. | Iconella turgida (W.Smith) E.Reichardt 2020 | B | ohb | I | ind | – | – | b |
| 159. | Surirella clementis Grunow 1882 | B | mh | I | – | – | – | – |
| 160. | Surirella hibernica (W.Smith) Kapustin & Kryvosheia 2019 | B | ohi | E | alk | – | – | Ha |
| 161. | Surirella librile (Ehrenberg) Ehrenberg 1845 | B | mh | I | alk | – | ind | k |
| 162. | Surirella microlibrile Van de Vijver, Pottiez & Jüttner 2024 | B | mh | I | alk | – | ind | – |
| 163. | Surirella peisonis Pantocsek 1902 | B | ohi | I | – | – | – | Ha, Pt |
| 164. | Surirella undulata (Ehrenberg) Ehrenberg 1845 | B | ohi | I | alk | – | ind | k |
| 165. | Surirella visurgis Hustedt 1957 | B | ohi | M | alk | – | – | – |
| Order Thalassiophysales Family Catenulaceae | ||||||||
| 166. | Amphora affinis Kützing 1844 | B | ohi | I | alk | T | ind | k |
| 167. | Amphora copulata (Kützing) Schoeman & R.E.M.Archibald 1986 | B | ohb | I | alk | T | ind | k |
| 168. | Amphora ovalis (Kützing) Kützing 1844 | B | ohi | I | alk | T | ind | k |
| 169. | Amphora pediculus (Kützing) Grunow 1875 | B | ohi | I | alk | T | ind | k |
| Subclass Eunotiophycidae Order Eunotiales Family Eunotiaceae Subclass Eunotiophycidae Order Eunotiales Family Eunotiaceae | ||||||||
| 170 | Eunotia minor (Kützing) Grunow 1881 | E | ohi | M | neu | – | ind | Ha |
| 171. | Eunotia parapraerupta Lange-Bertalot & Metzeltin 2009 | E | ohi | O | ind | – | lph | – |
| 172. | Eunotia pararepens Kulikovskiy, Lange-Bertalot, Witkowski & N.I.Dorofeyuk 2010 | E | ohb | O | acd | – | lph | – |
| 173. | Eunotia septentrionalis Østrup 1897 | B | ohb | O | acd | – | lph | a-a |
| 174. | Eunotia superbidens Lange-Bertalot 2011 | B | ohb | O | acd | – | lph | – |
| Subclass Fragilariophycidae Order Fragilariales Family Fragilariaceae | ||||||||
| 175. | Fragilaria aequalis Heiberg 1863 | E | ohi | I | alk | – | – | k |
| 176. | Fragilaria capucina Desmazières 1830 | E | ohi | I | alk | – | ind | k |
| 177. | Fragilaria crotonensis Kitton 1869 | E | ohi | M | alk | – | lph | k |
| 178. | Fragilariforma mesolepta (Rabenhorst) Kharitonov 2005 | E | ohi | M | alk | – | – | k |
| 178. | Fragilariforma virescens (Ralfs) D.M.Williams & Round 1988 | E | ohi | O | neu | – | – | k |
| 179. | Punctastriata lancettula (Schumann) P.B.Hamilton & Siver 2008 | E | ind | E | alk | – | – | Ha |
| Order Licmophorales Family Ulnariaceae | ||||||||
| 180. | Ulnaria capitata (Ehrenberg) Compère 2001 | P | ohb | I | alk | – | lph | Ha |
| 181. | Ulnaria delicatissima (W.Smith) Aboal & P.C.Silva 2004 | P | ohi | I | – | – | ind | k |
| 182. | Ulnaria ulna (Nitzsch) Compère | P | ohb | I | alk | – | – | k |
| Family Staurosiraceae | ||||||||
| 183. | Nanofrustulum trainorii (E.Morales) E.Morales 2019 | E | ohi | I | alk | – | – | – |
| 184. | Pseudostaurosira brevistriata (Grunow) D.M.Williams & Round 1988 | E | ohi | I | alk | – | lph | k |
| 185. | Pseudostaurosira elliptica (Schumann) Edlund, Morales & Spaulding 2006 | E | ohi | I | alk | – | ind | – |
| 186. | Pseudostaurosira parasitica (W.Smith) E.Morales 2003 | E | ohi | I | alk | – | – | k |
| 187. | Staurosira binodis (Ehrenberg) Lange-Bertalot 2011 | E | ohi | I | alk | – | – | Ha |
| 188. | Staurosira construens Ehrenberg 1843 | E | ohp | I | alk | T | ind | k |
| 189. | Staurosira pseudoconstruens (Marciniak) Lange-Bertalot 2000 | E | ohi | I | alk | – | – | – |
| 190. | Staurosira venter (Ehrenberg) Cleve & J.D.Möller 1879 | E | ohp | E | neu | – | – | k |
| 191. | Staurosirella lapponica (Grunow) D.M.Williams & Round 1987 | E | ohp | I | alk | – | lph | – |
| 192. | Staurosirella leptostauron (Ehrenberg) D.M.Williams & Round 1988 | E | ohi | I | alk | – | lph | b |
| 193. | Staurosirella martyi (Héribaud) Morales & Manoylov 2006 | E | ohi | I | alk | – | ind | k |
| 194. | Staurosirella ovata E.A.Morales 2006 | E | ohi | I | alk | – | – | Ha |
| 195. | Staurosirella pinnata (Ehrenberg) D.M.Williams & Round 1988 | E | ohi | I | alk | T | lph | k |
| Family Staurosiraceae | ||||||||
| 196. | Tabellaria fenestrata (Lyngbye) Kützing 1844 | E | ohb | M | acd | – | ind | k |
| 197. | Tabellaria flocculosa (Roth) Kützing 1844 | E | ohi | I | acd | E | ind | k |
| Phylum Heterokontophyta Subphylum Bacillariophytina Class Mediophyceae Subclass Thalassiosirophycidae Order Stephanodiscales Family Stephanodiscaceae | ||||||||
| 198. | Lindavia affinis (Grunow) Nakov, Guillory, M.L.Julius, E.C.Theriot & A.J.Alverson 2015 | P | ohb | I | neu | – | lph | – |
| 199. | Lindavia radiosa (Grunow) De Toni & Forti 1900 | P | ohb | M | alk | – | – | k |
| 200. | Pantocsekiella ocellata (Pantocsek) K.T.Kiss & Ács 2016 | P | ohb | M | alk | – | lph | k |
| 201. | Pantocsekiella tripartita (Håkansson) K.T.Kiss & E.Ács 2016 | P | ohi | O | ind | C | – | Ha |
| 202. | Stephanocyclus gamma (Sovereign) Kulikovskiy, Genkal & Kociolek 2022 | P | ohb | M | neu | – | lph | – |
| 203. | Stephanodiscus alpinus Hustedt 1942 | P | ohi | O | neu | C | lph | – |
| 204. | Stephanodiscus neoastraea Håkansson & Hickel 1986 | P | ohi | I | ind | – | lph | k |
References
- Mangerud, J.; Jakobsson, M.; Alexanderson, H.; Astakhov, V.; Clarke, G.K.C.; Henriksen, M.; Hjort, C.; Krinner, G.; Lunkka, J.-P.; Möller, P.; et al. Ice-dammed lakes and rerouting of the drainage of Northern Eurasia during the Last Glaciation. Quat. Sci. Rev. 2004, 23, 1313–1332. [Google Scholar] [CrossRef]
- Seppä, H.; Poska, A. Holocene annual mean temperature changes in Estonia and their relationship to solar insolation and atmospheric circulation patterns. Quat. Res. 2004, 61, 22–31. [Google Scholar] [CrossRef]
- Heikkilä, M.; Seppä, H. Holocene climate dynamics in Latvia, eastern Baltic region: A pollen-based summer temperature reconstruction and regional comparison. Boreas 2010, 39, 705–719. [Google Scholar] [CrossRef]
- Veski, S.; Seppä, H.; Stančikaitė, M.; Zernitskaya, V.; Reitalu, T.; Gryguc, G.; Atko Heinsalu, A.; Normunds Stivrins, N.; Amon, L.; Vassiljev, J.; et al. Quantitative summer and winter temperature reconstructions from pollen and chironomid data between 15 and 8 ka BP in the Baltic-Belarus area. Quat. Int. 2015, 388, 4–11. [Google Scholar] [CrossRef]
- Druzhinina, O.; Subetto, D.; Stančikaitė, M.; Vaikutienė, G.; Kublitsky, J.; Arslanov, K. Sediment record from the Kamyshovoe Lake: History of vegetation during Late Pleistocene and Early Holocene (Kaliningrad District, Russia). Baltica 2015, 28, 121–134. [Google Scholar] [CrossRef]
- Kabailiné, M. Freshwater and marine diatoms in the Holocene in Lithuania. In Iskopayemye Diatomovye Vodorosli SSSR; Vozshennikova, T.F., Glezer, Z.I., Eds.; Nauka: Moscow, Russia, 1968; pp. 102–107. (In Russian) [Google Scholar]
- Kabailiné, M. Lakes of South-Eastern Lithuania and their environment in the Late Glacial and Holocene according to diatom and pollen analysis of sediments. Prikl. Limnol. 2002, 3, 123–132. (In Russian) [Google Scholar]
- Kabailiné, M. Late Glacial and Holocene stratigraphy of Lithuania based on pollen and diatom data. Geologija 2006, 54, 42–48. [Google Scholar]
- Shelekhova, T.S.; Tihkonova, J.S.; Lazareva, O.V. Late Glacial and holocene natural environment dynamics and evolution of lake okunozero, south karelia: Micropalaeontological data. Proc. Karelian Res. Cent. Russ. Acad. Sci. 2021, 4, 134–152. (In Russian) [Google Scholar] [CrossRef]
- Ludikova, A.V.; Sapelko, T.V.; Kuznetsov, D.D.; Shikhirina, K.A. Sediment Record of the Earliest Stage of the Evolution of Lake Kanozero (Sw Kola Peninsula): New Data for Regional Deglaciation Reconstructions and Relative Sea-Level Studies. Geomorfol. i Paleogeogr. 2023, 54, 90–104. (In Russian) [Google Scholar] [CrossRef]
- Gaigalas, A.; Vaikutienė, G.; Vainorius, J.; Kazlauskas, M. Development of Lake Rėkyva and its environment in Late Pleistocene and Holocene. Geologija 2008, 1, 28–36. [Google Scholar] [CrossRef]
- Šeirienė, V.; Kabailienė, M.; Kasperovičienė, J.; Mažeika, J.; Petrošius, R.; Paškauskas, R. Reconstruction of postglacial palaeoenvironmental changes in eastern Lithuania: Evidence from lacustrine sediment data. Quat. Int. 2009, 207, 58–68. [Google Scholar] [CrossRef]
- Stančikaitė, M.; Šeirienė, V.; Kisielienė, D.; Martma, T.; Gražyna Gryguc, G.; Zinkutė, R.; Mažeika, J.; Šinkūnas, P. Lateglacial and early Holocene environmental dynamics in northern Lithuania: A multi-proxy record from Ginkūnai Lake. Quat. Int. 2015, 357, 44–57. [Google Scholar] [CrossRef]
- Stančikaitė, M.; Kisielienė, D.; Moeb, D.; Vaikutienė, G. Lateglacial and early Holocene environmental changes in northeastern Lithuania. Quat. Int. 2009, 207, 80–92. [Google Scholar] [CrossRef]
- Stančikaitė, M.; Šinkūnas, P.; Šeirienė, V.; Kisielienė, D. Patterns and chronology of the Lateglacial environmental development at Pamerkiai and Kašučiai, Lithuania. Quat. Sci. Rev. 2008, 27, 127–147. [Google Scholar] [CrossRef]
- Witkowski, A.; Cedro, B.; Kierzek, A.; Baranowski, D. Diatoms as a proxy in reconstructing the Holocene environmental changes in the south-western Baltic Sea: The lower Rega River Valley sedimentary record. Hydrobiologia 2009, 631, 155–172. [Google Scholar] [CrossRef]
- Gałka, M.; Tobolski, K.; Bubak, I. Late Glacial and Early Holocene lake level fluctuations in NE Poland tracked by macro-fossil, pollen and diatom records. Quat. Int. 2015, 388, 23–38. [Google Scholar] [CrossRef]
- Słowinski, M.; Zawiska, I.; Ott, F.; Noryśkiewicz, A.; Plessen, B.; Apolinarska, K.; Rzodkiewicz, M.; Michczyńska, D.; Wulf, S.; Skubała, P.; et al. Differential proxy responses to late Allerød and early Younger Dryas climatic change recorded in varved sediments of the Trzechowskie palaeolake in Northern Poland. Quat. Sci. Rev. 2017, 158, 94–106. [Google Scholar] [CrossRef]
- Zaretskaya, N.; Ludikova, A.; Kuzhetsov, D.; Lugovoy, N.; Uspenskaya, O.; Frolov, P. Late Glacial palaeoenvironment and development of proglacial lakes on the northern coast of the Sambian (Kaliningrad) Peninsula. Geomorfol. i Paleogeogr. 2023, 54, 7–25. (In Russian) [Google Scholar] [CrossRef]
- Druzhinina, O.; Rudinskaya, A.; Filippova, K.; Lazukova, L.; Lavrova, N.; Zharov, A.; Skhodnov, I.; Burko, A.; van denBerghe, K. The Bølling–AllerødTransition in the Eastern Baltic: Environmental Responses to Climate Change. Biology 2023, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Úscinowicz, S. An Outline of the History of the Baltic Sea. In Geochemistry of Baltic Sea Surface Sediments; Úscinowicz, S., Ed.; Polish Geological Institute-National Research Institute: Warsaw, Poland, 2001; pp. 70–73. [Google Scholar]
- Geographical Atlas of the Kaliningrad Region. CNIT: Kaliningrad, Russia, 2002; p. 302. (In Russian)
- Blaauw, M.; Christen, J.A. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 2011, 6, 457474. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.J.; Austin, W.E.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Druzhinina, O.; Skhodnov, I.; van den Berghe, K.; Filippova, K. Allerød–Younger Dryas Boundary (12.9–12.8 ka) as a “New” Geochronological Marker in Late Glacial Sediments of the Eastern Baltic Region. Quaternary 2025, 8, 28. [Google Scholar] [CrossRef]
- Battarbee, R.W.; Jones, V.J.; Flower, R.J. Diatoms. In Tracking Environmental Change Using Lake Sediments. Terrestrial, Algaland Siliceous Indicators; Smol, J.P., Birks, H.J.-B., Last, W.M., Eds.; Springer Netherlands: Berlin/Heidelberg, Germany, 2001; pp. 155–202. [Google Scholar]
- Kulikovskiy, M.S.; Gluschenko, A.M.; Genkal, S.I.; Kuznetchova, I.V. Identification Book for Diatoms of Russia; Filigran: Yaroslavl’, Russia, 2015; p. 804. (In Russian) [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1.Teil: Naviculaceae. In Süßwasserflora von Mitteleuropa; Springer: Berlin/Heidelberg, Germany, 2001; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2.Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süßwasserflora Vonmitteleuropa; Springer: Berlin/Heidelberg, Germany, 2001; p. 596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3.Teil: Centrales, Fragilariaceae, Eunoticeae. In Süßwasserflora von Mitteleuropa; Springer: Berlin/Heidelberg, Germany, 2001; p. 640. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4.Teil: Achnantaceae. In Süßwasserflora von Mitteleuropa; Springer: Berlin/Heidelberg, Germany, 2001; p. 468. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2020; Available online: https://www.algaebase.org (accessed on 30 July 2025).
- Grimm, E.C. CONISS: A FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incrementalsum of squares. Comput. Geosci. 1987, 13, 13–35. [Google Scholar] [CrossRef]
- Denys, L. A check-list of the Diatoms in the Holocene deposits of the Western Belgian Coastal Plane with a Survey of Their Apparent Ecological Requirements. In I.Intriduction, Ecological Code and Complete List; Ministere des Affairs Economiques, Service Geologique de Belgique, Berchem, Kingdom of Belgium: Berchem, Belgium, 1991; p. 41. [Google Scholar]
- Kolbe, R. Grundlinien einer allgemeinen Ökologie der Diatomeen. In Ergebnisse der Biologie; Frisch, K.V., Goldschmidt, R.R., Ruhland, W., Winterstein, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1932; Volume 8, pp. 221–348. [Google Scholar]
- Hustedt, F. Die Systematik der Diatomeen in ihren Beziehungen zur Geologie und Okologie nebst einer Revision des Halobien-systems. Sv. Bot. Tidskr. 1953, 47, 509–519. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E, Ltd.; Plymouth Marine Laboratory: Plymouth, UK, 2001; 176p. [Google Scholar]
- Subbeto, D.A. Lake Sediments: Paleolimnological Reconstructions; Herzen University Publ: Saint-Petersburg, Russia, 2009; p. 348. (In Russian) [Google Scholar]
- Rasmussen, S.O.; Bigler, M.; Blockley, S.P.; Blunier, T.; Buchardt, S.L.; Clausen, H.B.; Cvijanovic, I.; Dahl-Jensen, D.; Johnsen, S.J.; Fischer, H.; et al. A stratigraphic framework for abrupt climatic changes during the Last Glacial period based on three synchronized Greenland ice-core records: Refining and extending the INTIMATE event stratigraphy. Quat. Sci. Rev. 2014, 106, 14–28. [Google Scholar] [CrossRef]
- Lange-Bertalot, H.; Hoffman, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Book: Oberreifenberg, Germany, 2017; p. 908. [Google Scholar]

| Sample | Depth (cm) | Material | Age, 14C | Age, calBP |
|---|---|---|---|---|
| LuS-18436 | 45 | macroremains (wood) | 10,940 ± 60 | 12,773 ± 240 |
| LuS-18462 | 106 | macroremains (wood) | 11,060 ± 60 | 13,102 ± 160 |
| LuS-18461 | 163 | macroremains (wood) | 11,790 ± 60 | 13,693 ± 130 |
| LuS-18460 | 186 | macroremains (wood) | 11,980 ± 80 | 13,957 ± 140 |
| LuS-17811 | 192 | gyttja | 12,200 ± 60 | 14,038 ± 160 |
| Depth (cm) | Layer Description |
|---|---|
| 0–14 | Gray dense clay. |
| 14–63 | Clay gyttja, from gray to light brown, with rare organic matter. The upper contact is clear and sharp. |
| 63–88 | Dark brown peaty gyttja with a thin sandy layer. The upper contact is gradual, clear. |
| 88–158 | Clay gyttja, from dark gray to brown, with organic matter and shell. The upper contact is gradual, clear. |
| 158–171 | Dark gray clay gyttja with organic matter and shell. The upper contact is gradual. |
| 171–181 | Clay dark gray gyttja with rare organic matter and shell. The upper contact is gradual. |
| 181–186 | Dark brown peaty gyttja. The upper contact is gradual. |
| 186–192 | Clay brown gyttja with rare organic matter. The upper contact is gradual. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudinskaya, A.; Druzhinina, O. Diversity and Ecology of Late Glacial Diatoms of the Eastern Baltic Region. Biology 2025, 14, 1226. https://doi.org/10.3390/biology14091226
Rudinskaya A, Druzhinina O. Diversity and Ecology of Late Glacial Diatoms of the Eastern Baltic Region. Biology. 2025; 14(9):1226. https://doi.org/10.3390/biology14091226
Chicago/Turabian StyleRudinskaya, Anna, and Olga Druzhinina. 2025. "Diversity and Ecology of Late Glacial Diatoms of the Eastern Baltic Region" Biology 14, no. 9: 1226. https://doi.org/10.3390/biology14091226
APA StyleRudinskaya, A., & Druzhinina, O. (2025). Diversity and Ecology of Late Glacial Diatoms of the Eastern Baltic Region. Biology, 14(9), 1226. https://doi.org/10.3390/biology14091226







