Spatiotemporal Distribution Shifts of Zelkova schneideriana Under Climate Change: A Biomod2-Driven Modeling Framework
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Processing
2.2. Selection and Processing of Environmental Variables
2.3. Model Construction
2.4. Distribution Area Classification and Change Analysis
3. Results
3.1. Model Accuracy and Performance Evaluation
3.2. Dominant Environmental Factors
3.3. Spatiotemporal Dynamics of Potentially Suitable Areas
4. Discussion
4.1. Model Evaluation
4.2. Environmental Factors
4.3. Spatiotemporal Dynamics of Distribution
4.4. Species Conservation and Restoration Strategies
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IUCN | International Union for Conservation of Nature |
| VU | Vulnerable |
| SDMs | Species distribution models |
| GBIF | Global Biodiversity Information Facility |
| CVH | Chinese Virtual Herbarium |
| NSII | National Specimen Information Infrastructure |
| PPBC | Plant Photo Bank of China |
| LIG | Last Interglacial |
| LGM | Last Glacial Maximum |
| MH | Middle Holocene |
| CMIP6 | Coupled Model Intercomparison Project Phase 6 |
| SSPs | Shared Socioeconomic Pathways |
| DEM | Digital Elevation Model |
| NFGIS | National Fundamental Geographic Information System |
| ANN | Artificial Neural Networks |
| CTA | Classification Tree Analysis |
| GBM | Generalized Boosting Model |
| GLM | Generalized Linear Model |
| MARS | Multivariate Adaptive Regression Splines |
| MAXENT | Maximum Entropy |
| MAXNET | Maximum Network |
| RF | Random Forest |
| SRE | Surface Range Envelope |
| XGBOOST | eXtreme Gradient Boosting |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the ROC Curve |
| TSS | True Skill Statistic |
References
- Fu, L.; Xin, Y.; Alan, W. Ulmaceae. In Flora of China; Missouri Botanical Garden Press: St. Louis, MO, USA; Science Press: Beijing, China, 2003; Volume 5, pp. 1–19. [Google Scholar]
- Song, Y.; Bétrisey, S.; Kozlowski, G. IUCN Zelkova schneideriana. The IUCN Red List of Threatened Species 2018: E.T131155456A131155458 2018. Available online: https://www.iucnredlist.org/species/131155456/131155458 (accessed on 10 May 2024).
- Sun, J.; Qiu, H.; Guo, J.; Xu, X.; Wu, D.; Zhong, L.; Jiang, B.; Jiao, J.; Yuan, W.; Huang, Y.; et al. Modeling the Potential Distribution of Zelkova schneideriana under Different Human Activity Intensities and Climate Change Patterns in China. Glob. Ecol. Conserv. 2020, 21, e00840. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, X.; Zhang, G. Potentially Differential Impacts on Niche Overlap between Chinese Endangered Zelkova schneideriana and Its Associated Tree Species under Climate Change. Front. Ecol. Evol. 2023, 11, 1218149. [Google Scholar] [CrossRef]
- He, J.; Jin, X.; Wu, X.; Zhang, W.; Huang, C.; Zhang, Z.; Chen, Y.; Yu, Q.; Yan, W.; Wang, J.; et al. Environmental Drivers and Conservation Implications of Endangered Ancient Zelkova schneideriana Trees in Hunan, China. Biodivers. Conserv. 2024, 33, 3663–3682. [Google Scholar] [CrossRef]
- Araujo, M.; New, M. Ensemble Forecasting of Species Distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Huo, T.; Xu, H.; Meng, F.; Xu, N.; Peng, C. Predicting High-Quality Ecologically Suitable Areas of Astragalus mongholicus Bunge Based on Secondary Metabolites Content Using Biomod2 Model. Sci. Rep. 2025, 15, 1373. [Google Scholar] [CrossRef]
- Wang, R.; Guo, X.; Song, Y.; Cai, Y.; Wu, Y.; Wang, M. Effects of Ultraviolet Radiation as a Climate Variable on the Geographic Distribution of Oryza sativa under Climate Change Based on Biomod2. Front. Plant Sci. 2025, 16, 1552770. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guan, B.; Chen, R.; Yi, R.; Jiang, X.; Xie, K. Investigating the Distribution Dynamics of the Camellia Subgenus Camellia in China and Providing Insights into Camellia Resources Management under Future Climate Change. Plants 2025, 14, 1137. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Wang, Q.; Huang, R.; Nie, W.; Yang, S.; Cheng, X.; Li, M. Occurrence Data Sources Matter for Species Distribution Modeling: A Case Study of Quercus variabilis Based on Biomod2. Ecol. Evol. 2025, 15, e71390. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, C.; Zhang, H.; Ran, Q.; Tao, S.; Wang, Z.; Xu, X.; Cai, Q.; Fang, J. Possible Refugia for Fagaceae Species in China under Climate Change. J. Plant Ecol. 2025, 18, rtae111. [Google Scholar] [CrossRef]
- Hu, C.; Wu, H.; Zhang, G. Evaluating Habitat Suitability for the Endangered Sinojackia xylocarpa (Styracaceae) in China under Climate Change Based on Ensemble Modeling and Gap Analysis. Biology 2025, 14, 304. [Google Scholar] [CrossRef]
- Koç, D.E.; Ustaoğlu, B.; Biltekin, D. Effect of Climate Change on the Habitat Suitability of the Relict Species Zelkova carpinifolia Spach Using Ensembled Species Distribution Modelling. Sci. Rep. 2024, 14, 27967. [Google Scholar] [CrossRef]
- Hijmans, R.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling 2023. Available online: https://rspatial.org/raster/sdm/ (accessed on 22 February 2025).
- Sheppard, C.S.; Burns, B.R.; Stanley, M.C. Predicting Plant Invasions under Climate Change: Are Species Distribution Models Validated by Field Trials? Glob. Change Biol. 2014, 20, 2800–2814. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) Experimental Design and Organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Hijmans, R. Terra: Spatial Data Analysis 2020. Available online: https://rspatial.github.io/terra/ (accessed on 22 February 2025).
- Wei, T.; Simko, V. Corrplot: Visualization of a Correlation Matrix 2010. Available online: https://github.com/taiyun/corrplot (accessed on 22 February 2025).
- Thuiller, W.; Georges, D.; Gueguen, M.; Engler, R.; Breiner, F.; Lafourcade, B.; Patin, R.; Blancheteau, H. Biomod2: Ensemble Platform for Species Distribution Modeling. 2012. Available online: https://biomodhub.github.io/biomod2/ (accessed on 28 February 2025).
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Wang, R.; Guo, L.; Ji, Y.; Chen, Y.; Hao, L.; Lin, K. Impacts of Human Activity and Climate Change on the Suitable Habitats for Xanthium spinosum in China. Plants 2025, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next Generation Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Tong, J.; Bai, Y.; Alatalo, J.M.; Liu, G.; Fang, Z.; Zhang, F. Impacts of Environment and Human Activity on Grid-Scale Land Cropping Suitability and Optimization of Planting Structure, Measured Based on the MaxEnt Model. Sci. Total Environ. 2022, 836, 155356. [Google Scholar] [CrossRef]
- Yao, Y.-F.; Song, X.-Y.; Wortley, A.H.; Wang, Y.-F.; Blackmore, S.; Li, C.-S. Pollen-Based Reconstruction of Vegetational and Climatic Change over the Past ~30 Ka at Shudu Lake in the Hengduan Mountains of Yunnan, Southwestern China. PLoS ONE 2017, 12, e0171967. [Google Scholar] [CrossRef]
- Wang, L.; Jackson, D.A. Effects of Sample Size, Data Quality, and Species Response in Environmental Space on Modeling Species Distributions. Landsc. Ecol. 2023, 38, 4009–4031. [Google Scholar] [CrossRef]
- Williams, J.W.; Jackson, S.T.; Kutzbach, J.E. Projected Distributions of Novel and Disappearing Climates by 2100 AD. Proc. Natl. Acad. Sci. USA 2007, 104, 5738–5742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Xiao, H.; Li, H.; Liao, D.; Xue, Y.; Huang, X.; Su, Q.; Xiao, Y. Changes in the Distribution Range of the Genus Cardiocrinum in China under Climate Change and Human Activities. Biology 2025, 14, 581. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Wang, Y.; Landis, J.B.; Wang, X.; He, Y.; Wang, H.; Huang, X.; Liu, Q.; Yang, J.; Shang, F.; et al. Genome Sequencing and Population Genomics Provide Insights into the Demographic History, Genetic Load, and Local Adaptation of an Endangered Tertiary Relict. Plant J. 2025, 123, e70425. [Google Scholar] [CrossRef] [PubMed]
- Yihui, D.; Chan, J.C.L. The East Asian Summer Monsoon: An Overview. Meteorol. Atmos. Phys. 2005, 89, 117–142. [Google Scholar] [CrossRef]
- Walther, G.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Hewitt, G. The Genetic Legacy of the Quaternary Ice Ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef]
- Ehlers, J.; Gibbard, P.L. The Extent and Chronology of Cenozoic Global Glaciation. Quat. Int. 2007, 164–165, 6–20. [Google Scholar] [CrossRef]
- Tian, S.; Lei, S.; Hu, W.; Deng, L.; Li, B.; Meng, Q.-L.; Soltis, D.E.; Soltis, P.S.; Fan, D.-M.; Zhang, Z.-Y. Repeated Range Expansions and Inter-/Postglacial Recolonization Routes of Sargentodoxa cuneata (Oliv.) Rehd. et Wils. (Lardizabalaceae) in Subtropical China Revealed by Chloroplast Phylogeography. Mol. Phylogenet. Evol. 2015, 85, 238–246. [Google Scholar] [CrossRef]
- Liu, X.; Huang, H.; Meng, X.; Li, M.; Qin, Z. The Past, Present, and Future Distribution of Sargentodoxa: Perspectives from Fossil Record and Species Distribution Models. Ecol. Evol. 2025, 15, e71831. [Google Scholar] [CrossRef]
- Cheng, Y.; Hwang, S.; Lin, T. Potential Refugia in Taiwan Revealed by the Phylogeographical Study of Castanopsis carlesii Hayata (Fagaceae). Mol. Ecol. 2005, 14, 2075–2085. [Google Scholar] [CrossRef]
- Huang, S.S.F.; Hwang, S.; Lin, T. Spatial Pattern of Chloroplast DNA Variation of Cyclobalanopsis glauca in Taiwan and East Asia. Mol. Ecol. 2002, 11, 2349–2358. [Google Scholar] [CrossRef]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.-F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T.; et al. Identifying Long-Term Stable Refugia for Relict Plant Species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.I.; Abbott, R.J. The Origin and Evolution of Tertiary Relict Floras. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2002; Volume 38, pp. 281–314. ISBN 978-0-12-005938-6. [Google Scholar]
- Sun, H.; Zhang, J.; Deng, T.; Boufford, D.E. Origins and Evolution of Plant Diversity in the Hengduan Mountains, China. Plant Divers. 2017, 39, 161–166. [Google Scholar] [CrossRef]
- Ye, X.; Ma, P.; Yang, G.; Guo, C.; Zhang, Y.; Chen, Y.; Guo, Z.; Li, D. Rapid Diversification of Alpine Bamboos Associated with the Uplift of the Hengduan Mountains. J. Biogeogr. 2019, 46, 2678–2689. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, J.; Li, Z.; Li, W.; Ma, X.; Sun, W. Phylogeography of Pleurospermum foetens (Apiaceae) from the Sky Islands of Southwest China. Ecol. Evol. 2024, 14, e70542. [Google Scholar] [CrossRef]
- Zhai, T.; Wang, J.; Zhan, G.; Hu, J.; Yu, L. Population Genomic Landscapes and Insights for Conservation of the Critically Endangered Island-Endemic Chinese Pangolin in Taiwan. Sci. China Life Sci. 2025, 68, 2768–2783. [Google Scholar] [CrossRef]
- López-Pujol, J.; Zhang, F.; Sun, H.; Ying, T.; Ge, S. Centres of Plant Endemism in China: Places for Survival or for Speciation? J. Biogeogr. 2011, 38, 1267–1280. [Google Scholar] [CrossRef]
- Meng, H.; Su, T.; Gao, X.; Li, J.; Jiang, X.; Sun, H.; Zhou, Z. Warm–Cold Colonization: Response of Oaks to Uplift of the Himalaya–Hengduan Mountains. Mol. Ecol. 2017, 26, 3276–3294. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Brachi, B.; Yun, Q.; Zhang, W.; Lu, W.; Li, H.; Li, W.; Sun, X.; Wang, G.; et al. Genome-Wide Analysis of Cushion Willow Provides Insights into Alpine Plant Divergence in a Biodiversity Hotspot. Nat. Commun. 2019, 10, 5230. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yang, J.; Dao, Z.; Sun, W. Conservation Genetics and Modeling Potential Geographic Distribution of Corybas taliensis, a Small ‘Sky Island’ Orchid Species in China. BMC Plant Biol. 2023, 24, 11. [Google Scholar] [CrossRef]
- Cao, Y.; Comes, H.P.; Sakaguchi, S.; Chen, L.; Qiu, Y. Evolution of East Asia’s Arcto-Tertiary Relict Euptelea (Eupteleaceae) Shaped by Late Neogene Vicariance and Quaternary Climate Change. BMC Evol. Biol. 2016, 16, 66. [Google Scholar] [CrossRef]
- Hewitt, G. Some Genetic Consequences of Ice Ages, and Their Role in Divergence and Speciation. Biol. J. Linn. Soc. 1996, 58, 247–276. [Google Scholar] [CrossRef]
- Qiu, Y.; Fu, C.; Comes, H.P. Plant Molecular Phylogeography in China and Adjacent Regions: Tracing the Genetic Imprints of Quaternary Climate and Environmental Change in the World’s Most Diverse Temperate Flora. Mol. Phylogenet. Evol. 2011, 59, 225–244. [Google Scholar] [CrossRef]
- Song, Y.; Xu, G.-B.; Long, K.-X.; Wang, C.-C.; Chen, R.; Li, H.; Jiang, X.-L.; Deng, M. Ensemble Species Distribution Modeling and Multilocus Phylogeography Provide Insight into the Spatial Genetic Patterns and Distribution Dynamics of a Keystone Forest Species, Quercus Glauca. BMC Plant Biol. 2024, 24, 168. [Google Scholar] [CrossRef]
- Jin, S.; Chi, Y.; Li, X.; Shu, P.; Zhu, M.; Yuan, Z.; Liu, Y.; Chen, W.; Han, Y. Predicting the Response of Three Common Subtropical Tree Species in China to Climate Change. Front. For. Glob. Change 2023, 6, 1299120. [Google Scholar] [CrossRef]
- Pearson, R. Climate Change and the Migration Capacity of Species. Trends Ecol. Evol. 2006, 21, 111–113. [Google Scholar] [CrossRef]
- McLeman, R.; Smit, B. Migration as an Adaptation to Climate Change. Clim. Change 2006, 76, 31–53. [Google Scholar] [CrossRef]
- Thomas, C.D. Climate, Climate Change and Range Boundaries. Divers. Distrib. 2010, 16, 488–495. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, J.; Huang, H. Parallel situ Conservation: A New Plant Conservation Strategy to Integrate In Situ and Ex Situ Conservation of Plants. Biodivers. Sci. 2023, 31, 23184. [Google Scholar] [CrossRef]
- Morelli, T.L.; Maher, S.P.; Lim, M.C.W.; Kastely, C.; Eastman, L.M.; Flint, L.E.; Flint, A.L.; Beissinger, S.R.; Moritz, C. Climate Change Refugia and Habitat Connectivity Promote Species Persistence. Clim. Change Responses 2017, 4, 8. [Google Scholar] [CrossRef]
- Volis, S. Conservation-Oriented Restoration—A Two for One Method to Restore Both Threatened Species and Their Habitats. Plant Divers. 2019, 41, 50–58. [Google Scholar] [CrossRef]
- Zeren Cetin, I.; Ozel, H.B.; Varol, T.; Canturk, U.; Sevik, H. Climate Change Impacts on Taxus baccata Distribution and Conservation. J. For. Res. 2025, 36, 95. [Google Scholar] [CrossRef]
- Yan, F.; Wei, T.; Yang, C.; Yang, Y.; Luo, Z.; Jiang, Y. Combined Analysis of Untargeted Metabolomics and Transcriptomics Revealed Seed Germination and Seedling Establishment in Zelkova schneideriana. Genes 2024, 15, 488. [Google Scholar] [CrossRef]

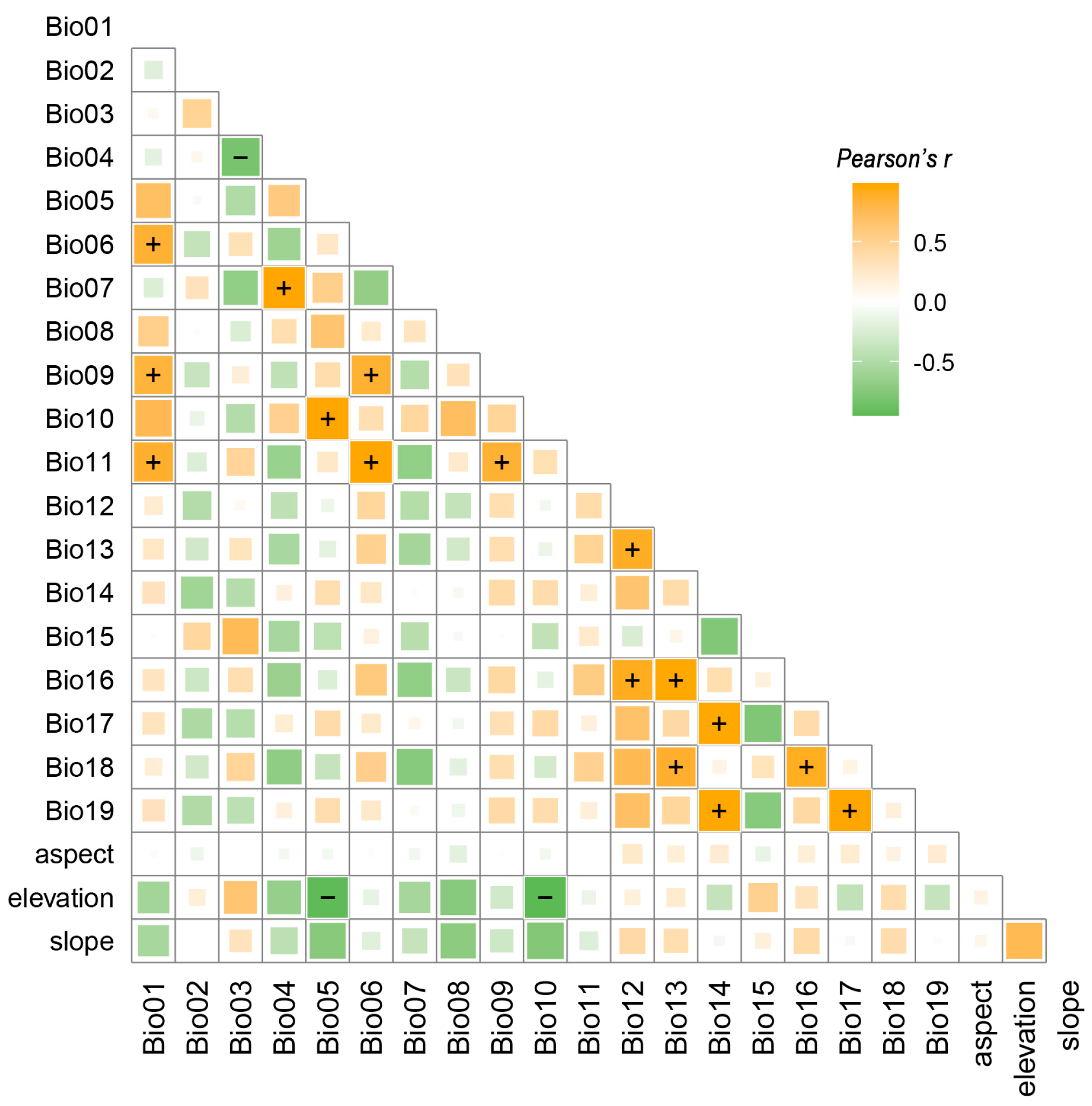
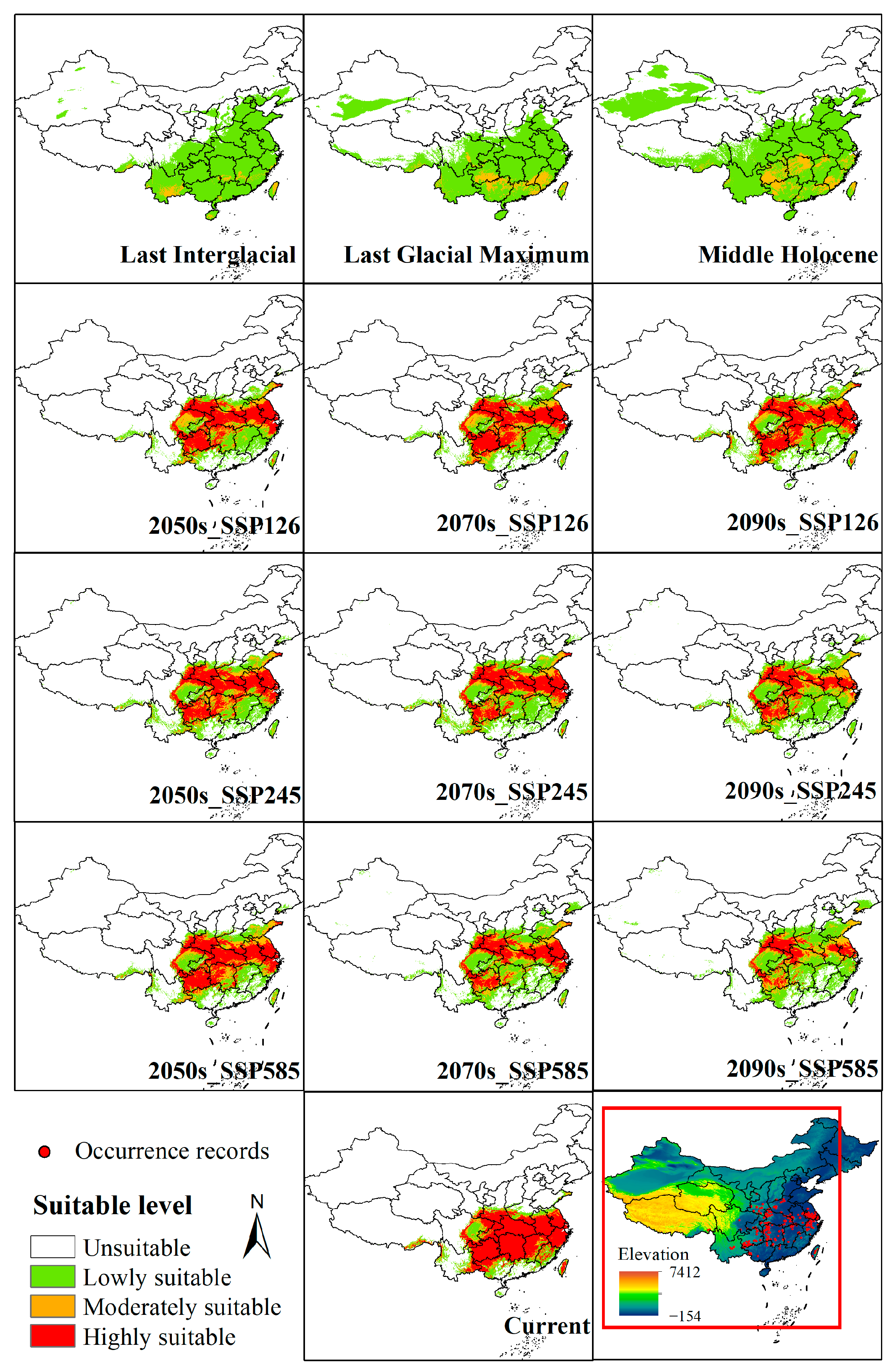
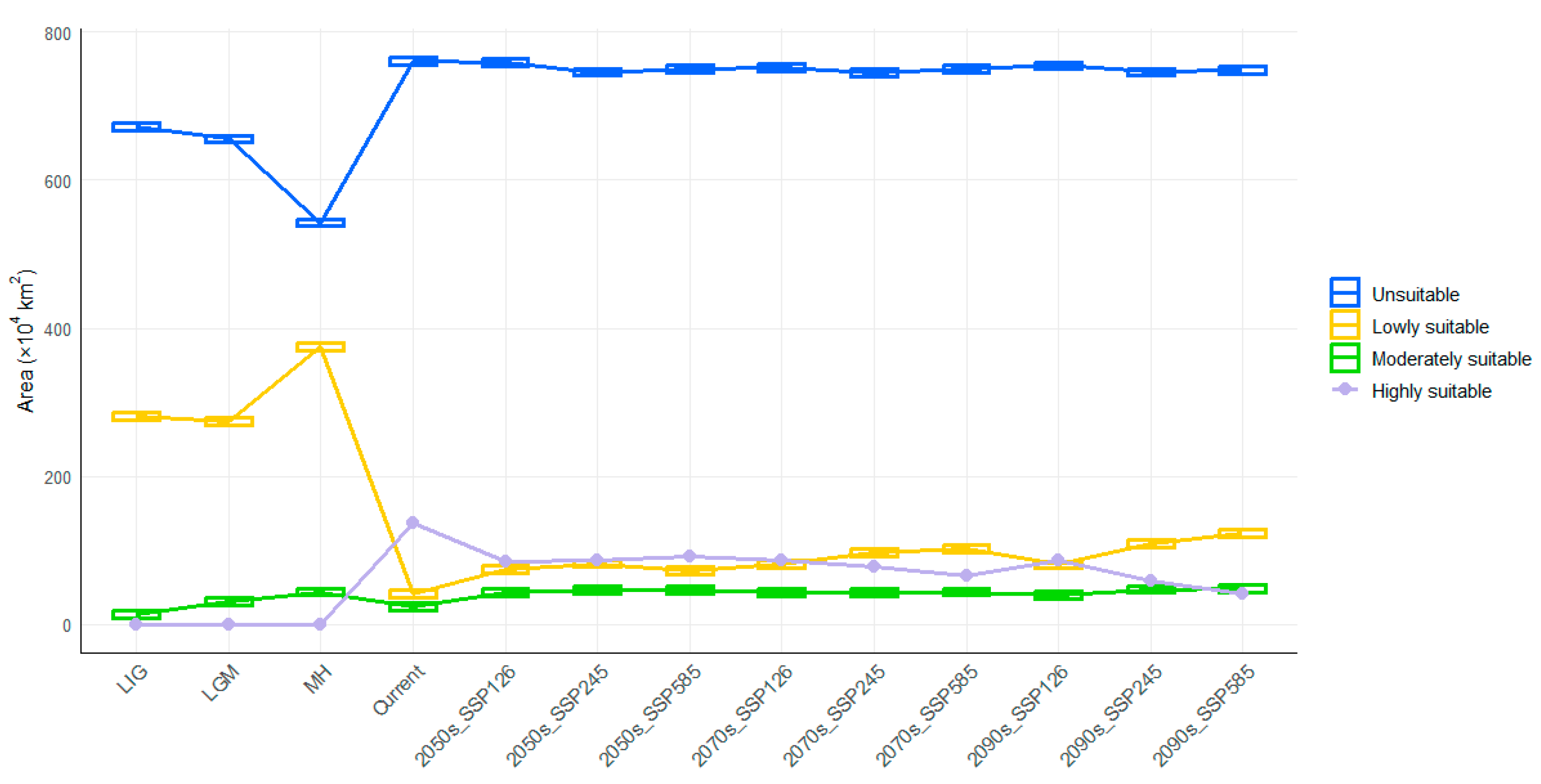
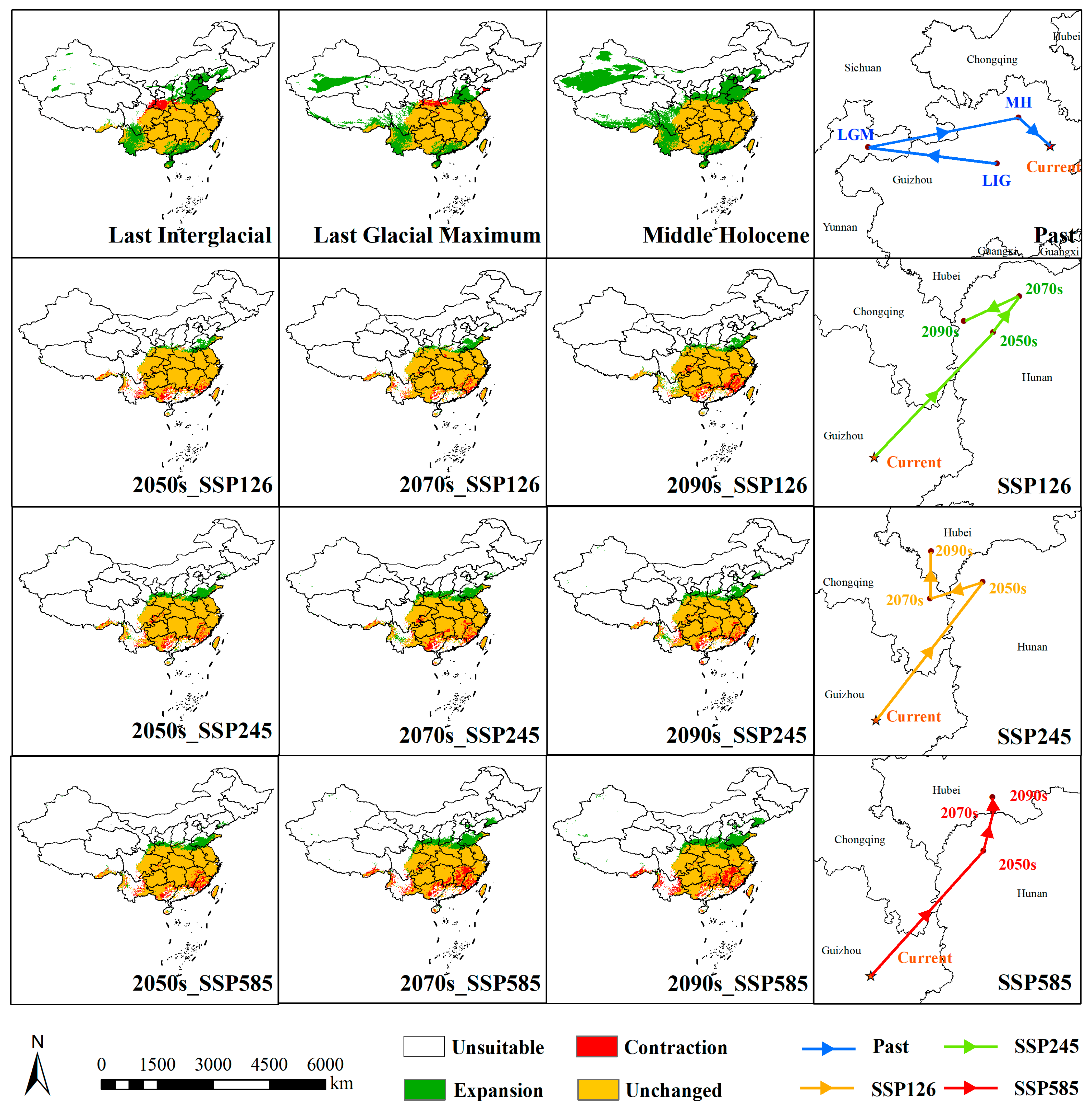
| Code | Description | Percent Contribution (%) |
|---|---|---|
| Bio06 | Min temperature of coldest month | 21.57 |
| Bio02 | Mean diurnal range | 19.81 |
| Bio17 | Precipitation of driest quarter | 13.52 |
| Bio15 | Precipitation seasonality (coefficient of variation) | 8.32 |
| Bio07 | Temperature annual range (Bio05–Bio06) | 8.15 |
| Bio12 | Annual precipitation | 6.58 |
| elevation | Elevation | 6.57 |
| Bio18 | Precipitation of warmest quarter | 6.47 |
| Bio03 | Isothermality (Bio02/Bio07) (×100) | 5.79 |
| slope | Slope | 2.03 |
| Bio08 | Mean temperature of wettest quarter | 1.04 |
| aspect | Aspect | 0.16 |
| Period | Scenario | Lowly Suitable | Moderately Suitable | Highly Suitable | Total | Expansion | Unchanged | Contraction |
|---|---|---|---|---|---|---|---|---|
| LIG | 280.84 | 13.60 | 0.00 | 294.44 | 107.17 | 205.35 | 12.52 | |
| LGM | 274.54 | 30.91 | 0.00 | 305.45 | 120.10 | 207.52 | 10.35 | |
| MH | 374.92 | 43.56 | 0.00 | 418.48 | 219.87 | 217.68 | 0.02 | |
| Current | 41.19 | 23.85 | 136.82 | 201.86 | ||||
| 2050s | SSP126 | 74.00 | 43.34 | 84.58 | 201.92 | 16.87 | 199.58 | 18.29 |
| SSP245 | 81.69 | 46.45 | 87.00 | 215.14 | 29.59 | 200.43 | 17.44 | |
| SSP585 | 72.03 | 46.79 | 92.07 | 210.88 | 28.24 | 197.02 | 20.86 | |
| 2070s | SSP126 | 80.48 | 42.54 | 85.44 | 208.46 | 20.88 | 202.69 | 15.19 |
| SSP245 | 96.09 | 42.78 | 77.34 | 216.21 | 33.89 | 196.95 | 20.92 | |
| SSP585 | 102.06 | 43.44 | 65.15 | 210.66 | 38.91 | 184.80 | 33.07 | |
| 2090s | SSP126 | 80.20 | 39.63 | 86.41 | 206.24 | 24.70 | 196.28 | 21.59 |
| SSP245 | 109.26 | 46.88 | 58.94 | 215.09 | 32.70 | 196.81 | 21.06 | |
| SSP585 | 122.74 | 48.42 | 41.37 | 212.53 | 42.75 | 182.56 | 35.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, L.; Liu, H.; Sun, Y.; Li, N.; Geng, M. Spatiotemporal Distribution Shifts of Zelkova schneideriana Under Climate Change: A Biomod2-Driven Modeling Framework. Biology 2025, 14, 1221. https://doi.org/10.3390/biology14091221
Li M, Wang L, Liu H, Sun Y, Li N, Geng M. Spatiotemporal Distribution Shifts of Zelkova schneideriana Under Climate Change: A Biomod2-Driven Modeling Framework. Biology. 2025; 14(9):1221. https://doi.org/10.3390/biology14091221
Chicago/Turabian StyleLi, Mimi, Lingdan Wang, Hailong Liu, Yueqi Sun, Naiwei Li, and Maolin Geng. 2025. "Spatiotemporal Distribution Shifts of Zelkova schneideriana Under Climate Change: A Biomod2-Driven Modeling Framework" Biology 14, no. 9: 1221. https://doi.org/10.3390/biology14091221
APA StyleLi, M., Wang, L., Liu, H., Sun, Y., Li, N., & Geng, M. (2025). Spatiotemporal Distribution Shifts of Zelkova schneideriana Under Climate Change: A Biomod2-Driven Modeling Framework. Biology, 14(9), 1221. https://doi.org/10.3390/biology14091221






