Choline-Mediated Regulation of Follicular Growth: Interplay Between Steroid Synthesis, Epigenetics, and Oocyte Development
Simple Summary
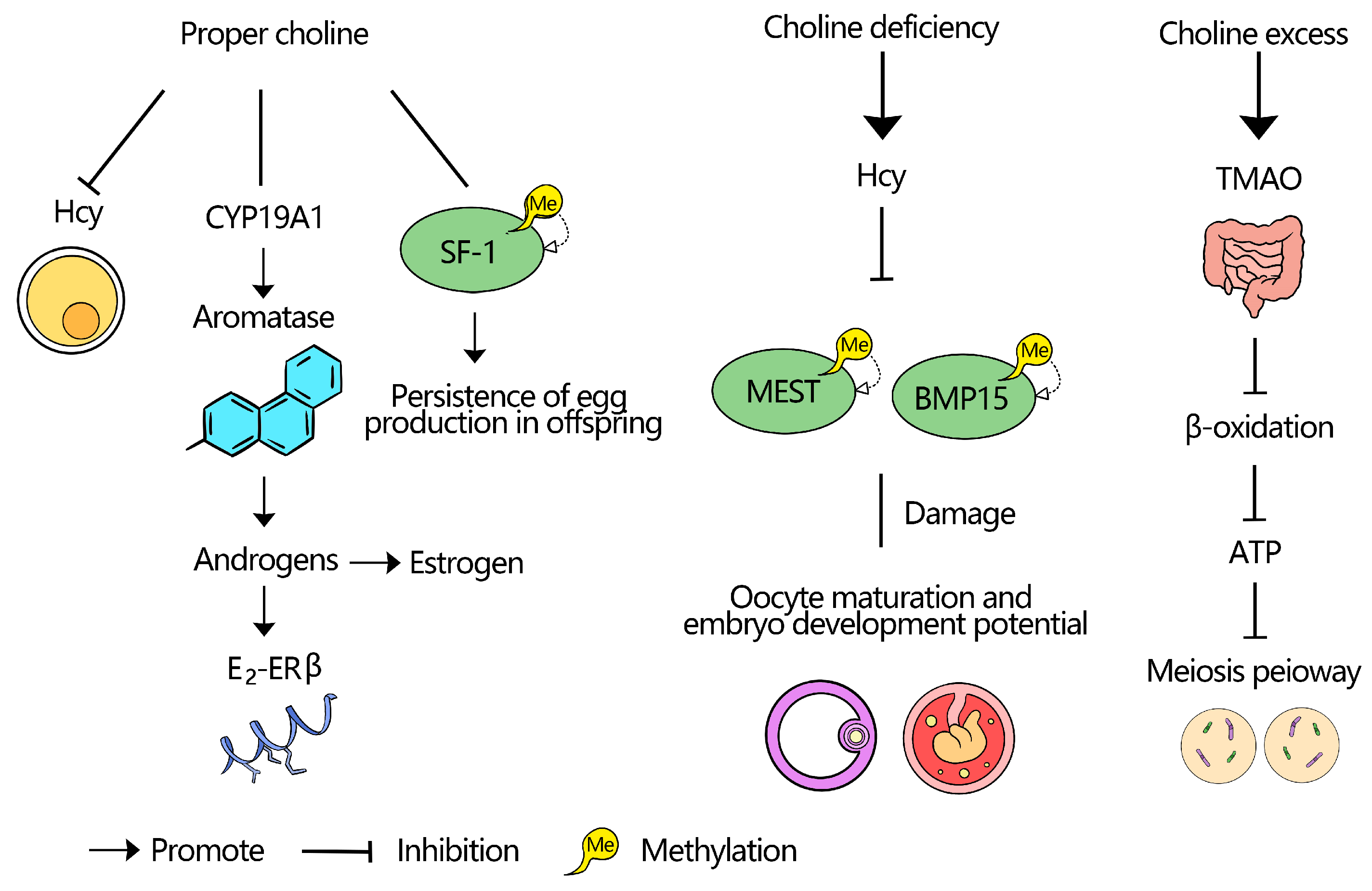
Abstract
1. Introduction
2. Literature Review Methodology
2.1. Literature Search Timeframe and Databases
2.2. Search Keywords
2.3. Literature Selection and Evaluation Criteria
2.4. Literature Assessment
3. Choline Structure, Absorption, and Metabolism
4. Impact of Choline on Follicular Development
4.1. Deficiency
4.2. Toxicity
4.3. Optimal Choline Levels for Follicular Health
5. Mechanism of Choline in Follicular Development
5.1. Choline and DNA Methylation
5.2. DNA Methylation and Folliculogenesis
5.3. Choline Regulation of Steroid Hormone Synthesis and Follicular Development
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crowe, M.A.; Hostens, M.; Opsomer, G. Reproductive Management in Dairy Cows-the Future. Ir. Vet. J. 2018, 71, 1. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.A.; Marques, D.B.D.; De Campos, C.F.; Saraiva, A.; Guimarães, J.D.; Guimarães, S.E.F. Nutrition Influence on Sow Reproductive Performance and Conceptuses Development and Survival: A Review about l-Arginine Supplementation. Livest. Sci. 2019, 228, 97–103. [Google Scholar] [CrossRef]
- Leroy, J.L.M.R.; Rizos, D.; Sturmey, R.; Bossaert, P.; Gutierrez-Adan, A.; Hoeck, V.V.; Valckx, S.; Bols, P.E.J. Intrafollicular Conditions as a Major Link between Maternal Metabolism and Oocyte Quality: A Focus on Dairy Cow Fertility. Reprod. Fertil. Dev. 2011, 24, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Morita, T.; Sugiyama, K. Effects of Betaine Supplementation and Choline Deficiency on Folate Deficiency-Induced Hyperhomocysteinemia in Rats. Pubmed 2012, 58, 69–77. [Google Scholar] [CrossRef][Green Version]
- Berker, B.; Kaya, C.; Aytac, R.; Satiroglu, H. Homocysteine Concentrations in Follicular Fluid Are Associated with Poor Oocyte and Embryo Qualities in Polycystic Ovary Syndrome Patients Undergoing Assisted Reproduction. Hum. Reprod. 2009, 24, 2293–2302. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, J.; An, C.; Li, X.; Xu, S.; He, Y.; Zhang, X.; Liu, L.; Hu, K.; Liang, M. Mechanism of LncRNA Gm2044 in Germ Cell Development. Front. Cell Dev. Biol. 2024, 12, 1410914. [Google Scholar]
- Krzysztof Blusztajn, J.; Mellott, J.T. Choline Nutrition Programs Brain Development via DNA and Histone Methylation. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 82–94. [Google Scholar] [CrossRef]
- Jiang, X.; Greenwald, E.; Jack-Roberts, C. Effects of Choline on DNA Methylation and Macronutrient Metabolic Gene Expression in in Vitro Models of Hyperglycemia. Nutr. Metab. Insights 2016, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.-C.; Zhou, L.-W.; Li, Y.-Y.; Yu, Z.-M.; Li, L.-D.; Wang, Y.-Z.; Xu, M.-C.; Wang, Q.-Y.; Li, C.-Q. SEanalysis 2.0: A Comprehensive Super-Enhancer Regulatory Network Analysis Tool for Human and Mouse. Nucleic Acids Res. 2023, 51, W520–W527. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Wang, K.; He, C.; Hu, K.; Liang, M. The Effects and Molecular Mechanism of Heat Stress on Spermatogenesis and the Mitigation Measures. Syst. Biol. Reprod. Med. 2022, 68, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, D.; Xia, W.; Pan, X.; Huo, W.; Xu, S.; Li, Y. Epigenetic Disruption and Glucose Homeostasis Changes Following Low-Dose Maternal Bisphenol a Exposure. Toxicol. Res. 2016, 5, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, H.; Wang, C.; Yuan, Y.; Tickner, J.; Xu, J.; Zou, J. Molecular Structure and Differential Function of Choline Kinases CHKα and CHKβ in Musculoskeletal System and Cancer. Cytokine Growth Factor. Rev. 2017, 33, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Cortés, E.; Ortiz, W.G.; Chebel, R.C.; Jannaman, E.A.; Moss, J.I.; De Castro, F.C.; Zolini, A.M.; Staples, C.R.; Hansen, P.J. Embryo and Cow Factors Affecting Pregnancy per Embryo Transfer for Multiple-Service, Lactating Holstein Recipients. Transl. Anim. Sci. 2019, 3, 60–65. [Google Scholar] [CrossRef]
- McDowell, L.R. Vitamins in Animal and Human Nutrition, 1st ed.; Wiley: Ames, IA, USA, 2000; ISBN 978-0-8138-2630-1. [Google Scholar]
- Richmond, G.S.; Smith, T.K. Phospholipases A1. Int. J. Mol. Sci. 2011, 12, 588–612. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Blusztajn, J.K. Choline and Human Nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline Deficiency. J. Nutr. Biochem. 1990, 1, 332–349. [Google Scholar] [CrossRef]
- Murota, K. Digestion and Absorption of Dietary Glycerophospholipids in the Small Intestine: Their Significance as Carrier Molecules of Choline and n-3 Polyunsaturated Fatty Acids. Biocatal. Agric. Biotechnol. 2020, 26, 101633. [Google Scholar] [CrossRef]
- Kamath, A.V.; Darling, I.M.; Morris, M.E. Choline Uptake in Human Intestinal Caco-2 Cells Is Carrier-Mediated. J. Nutr. 2003, 133, 2607–2611. [Google Scholar] [CrossRef]
- Hegazy, E.; Schwenk, M. Choline Uptake by Isolated Enterocytes of Guinea Pig. J. Nutr. 1984, 114, 2217–2220. [Google Scholar] [CrossRef]
- Sanford, P.A.; Smyth, D.H. Intestinal Transfer of Choline in Rat and Hamster. J. Physiol. 1971, 215, 769–788. [Google Scholar] [CrossRef]
- Arias, N.; Arboleya, S.; Allison, J.; Kaliszewska, A.; Higarza, S.G.; Gueimonde, M.; Arias, J.L. The Relationship between Choline Bioavailability from Diet, Intestinal Microbiota Composition, and Its Modulation of Human Diseases. Nutrients 2020, 12, 2340. [Google Scholar] [CrossRef]
- Luo, T.; Guo, Z.; Liu, D.; Guo, Z.; Wu, Q.; Li, Q.; Lin, R.; Chen, P.; Ou, C.; Chen, M. Deficiency of PSRC1 Accelerates Atherosclerosis by Increasing TMAO Production via Manipulating Gut Microbiota and Flavin Monooxygenase 3. Gut Microbes 2022, 14, 2077602. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Menze, M.A.; Elmoazzen, H.; Hand, S.C.; Toner, M. Choline Chloride Improves the Desiccation Tolerance of Chinese Hamster Ovary Cells. In Proceedings of the ASME 2010 Summer Bioengineering Conference, Parts A and B, Naples, FL, USA, 16–19 June 2010; American Society of Mechanical Engineers: Naples, FL, USA, 2010; pp. 993–994. [Google Scholar]
- Fernando-Warnakulasuriya, G.J.; Eckerson, M.L.; Clark, W.A.; Wells, M.A. Lipoprotein Metabolism in the Suckling Rat: Characterization of Plasma and Lymphatic Lipoproteins. J. Lipid Res. 1983, 24, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Agellon, L.B.; Allen, T.M.; Umeda, M.; Jewell, L.; Mason, A.; Vance, D.E. The Ratio of Phosphatidylcholine to Phosphatidylethanolamine Influences Membrane Integrity and Steatohepatitis. Cell Metab. 2006, 3, 321–331. [Google Scholar] [CrossRef]
- Glunde, K.; Bhujwalla, Z.M.; Ronen, S.M. Choline Metabolism in Malignant Transformation. Nat. Rev. Cancer 2011, 11, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Celemin Sarmiento, A.; Bradford, B.J.; Mamedova, L.K.; Zhou, G.; Estes, K.A.; Swartz, T.H. Effects of Dietary Rumen-Protected Choline Supplementation on Genome-Wide DNA Methylation in Mammary Tissue from Postpartum Dairy Cows. JDS Commun. 2025, 6, 333–338. [Google Scholar] [CrossRef]
- Sato, N.; Mori, N.; Hirashima, T.; Moriyama, T. Diverse Pathways of Phosphatidylcholine Biosynthesis in Algae as Estimated by Labeling Studies and Genomic Sequence Analysis. Plant J. 2016, 87, 281–292. [Google Scholar] [CrossRef]
- DeLong, C.J.; Hicks, A.M.; Cui, Z. Disruption of Choline Methyl Group Donation for Phosphatidylethanolamine Methylation in Hepatocarcinoma Cells. J. Biol. Chem. 2002, 277, 17217–17225. [Google Scholar] [CrossRef]
- Ganz, A.B.; Shields, K.; Fomin, V.G.; Lopez, Y.S.; Mohan, S.; Lovesky, J.; Chuang, J.C.; Ganti, A.; Carrier, B.; Yan, J.; et al. Genetic Impairments in Folate Enzymes Increase Dependence on Dietary Choline for Phosphatidylcholine Production at the Expense of Betaine Synthesis. FASEB J. 2016, 30, 3321–3333. [Google Scholar] [CrossRef]
- Gibellini, F.; Smith, T.K. The Kennedy Pathway-De Novo Synthesis of Phosphatidylethanolamine and Phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Zhong, Z.; Stubelius, A.; Sweeney, S.R.; Booshehri, L.M.; Antonucci, L.; Liu-Bryan, R.; Lodi, A.; Terkeltaub, R.; Lacal, J.C.; et al. Choline Uptake and Metabolism Modulate Macrophage IL-1β and IL-18 Production. Cell Metab. 2019, 29, 1350–1362.e7. [Google Scholar] [CrossRef]
- Okuda, T.; Haga, T. Functional Characterization of the Human High-affinity Choline Transporter. FEBS Lett. 2000, 484, 92–97. [Google Scholar] [CrossRef]
- Koepsell, H.; Schmitt, B.M.; Gorboulev, V. Organic Cation Transporters. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2004; Volume 150, pp. 36–90. ISBN 978-3-540-20214-1. [Google Scholar]
- Taylor, A.; Grapentine, S.; Ichhpuniani, J.; Bakovic, M. Choline Transporter-like Proteins 1 and 2 Are Newly Identified Plasma Membrane and Mitochondrial Ethanolamine Transporters. J. Biol. Chem. 2021, 296, 100604. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Fujiwara, R.; Ishiguro, N.; Oyabu, M.; Nakanishi, T.; Shirasaka, Y.; Maeda, T.; Tamai, I. Involvement of Choline Transporter-like Proteins, CTL1 and CTL2, in Glucocorticoid-Induced Acceleration of Phosphatidylcholine Synthesis via Increased Choline Uptake. Biol. Pharm. Bull. 2010, 33, 691–696. [Google Scholar] [CrossRef]
- Imbard, A.; Smulders, Y.M.; Barto, R.; Smith, D.E.; Kok, R.M.; Jakobs, C.; Blom, H.J. Plasma Choline and Betaine Correlate with Serum Folate, Plasma S-Adenosyl-Methionine and S-Adenosyl-Homocysteine in Healthy Volunteers. Clin. Chem. Lab. Med. 2013, 51, 683–692. [Google Scholar] [CrossRef]
- Robinson, J.L.; McBreairty, L.E.; Brunton, J.A.; Bertolo, R.F. The Effects of Folate, Choline and Betaine on Transmethylation in a Methionine Restricted Piglet. FASEB J. 2013, 27, 1077.24. [Google Scholar] [CrossRef]
- Ziganshin, A.U.; Khairullin, A.E.; Hoyle, C.H.V.; Grishin, S.N. Modulatory Roles of ATP and Adenosine in Cholinergic Neuromuscular Transmission. Int. J. Mol. Sci. 2020, 21, 6423. [Google Scholar] [CrossRef] [PubMed]
- Sastry, B.V.R. Human Placental Cholinergic System. Biochem. Pharmacol. 1997, 53, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Ray, K. Cholinergic Activity Is Essential for Maintaining the Anterograde Transport of Choline Acetyltransferase in Drosophila. Sci. Rep. 2018, 8, 8028. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut Liver Brain Axis in Diseases: The Implications for Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 443. [Google Scholar] [CrossRef]
- Satheesh Babu, A.K.; Petersen, C.; Iglesias-Carres, L.; Paz, H.A.; Wankhade, U.D.; Neilson, A.P.; Anandh Babu, P.V. Blueberry Intervention Mitigates Detrimental Microbial Metabolite Trimethylamine N-oxide by Modulating Gut Microbes. BioFactors 2024, 50, 392–404. [Google Scholar] [CrossRef]
- Jaworska, K.; Bielinska, K.; Gawrys-Kopczynska, M.; Ufnal, M. TMA (Trimethylamine), but Not Its Oxide TMAO (Trimethylamine-Oxide), Exerts Haemodynamic Effects: Implications for Interpretation of Cardiovascular Actions of Gut Microbiome. Cardiovasc. Res. 2019, 115, 1948–1949. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.C.; Aitchison, A.J.; Gehrig, K.; Ridgway, N.D. A Mechanism for Suppression of the CDP-Choline Pathway during Apoptosis. J. Lipid Res. 2013, 54, 3373–3384. [Google Scholar] [CrossRef]
- Lai, F.; Liu, X.; Li, N.; Zhang, R.; Zhao, Y.; Feng, Y.; Nyachoti, C.M.; Shen, W.; Li, L. Phosphatidylcholine Could Protect the Defect of Zearalenone Exposure on Follicular Development and Oocyte Maturation. Aging 2018, 10, 3486–3506. [Google Scholar] [CrossRef]
- Noga, A.A.; Vance, D.E. Insights into the Requirement of Phosphatidylcholine Synthesis for Liver Function in Mice. J. Lipid Res. 2003, 44, 1998–2005. [Google Scholar] [CrossRef]
- West, A.A.; Yan, J.; Jiang, X.; Perry, C.A.; Innis, S.M.; Caudill, M.A. Choline Intake Influences Phosphatidylcholine DHA Enrichment in Nonpregnant Women but Not in Pregnant Women in the Third Trimester123. Am. J. Clin. Nutr. 2013, 97, 718–727. [Google Scholar] [CrossRef]
- Anckaert, E.; Romero, S.; Adriaenssens, T.; Smitz, J. Effects of Low Methyl Donor Levels in Culture Medium during Mouse Follicle Culture on Oocyte Imprinting Establishment. Biol. Reprod. 2010, 83, 377–386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bokor, S.; Vass, R.A.; Funke, S.; Ertl, T.; Molnár, D. Epigenetic Effect of Maternal Methyl-Group Donor Intake on Offspring’s Health and Disease. Life 2022, 12, 609. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Maternal Methyl Donor Supplementation: A Potential Therapy for Metabolic Disorder in Offspring. J. Nutr. Biochem. 2024, 124, 109533. [Google Scholar] [CrossRef] [PubMed]
- Velker, B.A.M.; Denomme, M.M.; Krafty, R.T.; Mann, M.R.W. Maintenance of Mest Imprinted Methylation in Blastocyst-Stage Mouse Embryos Is Less Stable than Other Imprinted Loci Following Superovulation or Embryo Culture. Environ. Epigenet. 2017, 3, dvx015. [Google Scholar] [CrossRef]
- Fang, S.; Chang, K.; Lefebvre, L. Roles of Endogenous Retroviral Elements in the Establishment and Maintenance of Imprinted Gene Expression. Front. Cell Dev. Biol. 2024, 12, 1369751. [Google Scholar] [CrossRef]
- Shojaei Saadi, H.A.; Gagné, D.; Fournier, É.; Baldoceda Baldeon, L.M.; Sirard, M.-A.; Robert, C. Responses of Bovine Early Embryos to S-Adenosyl Methionine Supplementation in Culture. Epigenomics 2016, 8, 1039–1060. [Google Scholar] [CrossRef]
- Patel, J.; Chaudhary, H.; Chudasama, A.; Panchal, J.; Trivedi, A.; Panchal, S.; Joshi, T.; Joshi, R. Comparing the metabolomic landscape of polycystic ovary syndrome within urban and rural environments. Commun. Med. 2025, 5, 253. [Google Scholar] [CrossRef]
- Sun, L.; Hu, W.; Liu, Q.; Hao, Q.; Sun, B.; Zhang, Q.; Mao, S.; Qiao, J.; Yan, X. Metabonomics Reveals Plasma Metabolic Changes and Inflammatory Marker in Polycystic Ovary Syndrome Patients. J. Proteome Res. 2012, 11, 2937–2946. [Google Scholar] [CrossRef]
- Ersahin, A.A.; Çalışkan, E. Clomiphene Citrate Changes Metabolite Content of Follicular Fluid of PCOS Women. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4359–4362. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Fletcher, L.; Dingle, S.; Baracuhy, E.; Wang, B.; Huber, L.-A.; Li, J. Choline Supplementation Influences Ovarian Follicular Development. Front. Biosci. Landmark 2021, 26, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Choi, S.; Chang, J.; Choi, D.; Son, J.S.; Kim, K.; Kim, S.M.; Jeong, S.; Park, S.M. Association of L-α Glycerylphosphorylcholine with Subsequent Stroke Risk after 10 Years. JAMA Netw. Open 2021, 4, e2136008. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Downs, S.M.; Mosey, J.L.; Klinger, J. Fatty Acid Oxidation and Meiotic Resumption in Mouse Oocytes. Mol. Reprod. Dev. 2009, 76, 844–853. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wei, Y.; Zhu, P.; Yin, T.; Wan, Q. Metabonomic Analysis of Follicular Fluid in Patients with Diminished Ovarian Reserve. Front. Endocrinol. 2023, 14, 1132621. [Google Scholar] [CrossRef]
- Nagy, R.A.; Homminga, I.; Jia, C.; Liu, F.; Anderson, J.L.C.; Hoek, A.; Tietge, U.J.F. Trimethylamine-N-Oxide Is Present in Human Follicular Fluid and Is a Negative Predictor of Embryo Quality. Hum. Reprod. 2020, 35, 81–88. [Google Scholar] [CrossRef]
- Wallace, M.; Cottell, E.; Gibney, M.J.; McAuliffe, F.M.; Wingfield, M.; Brennan, L. An Investigation into the Relationship between the Metabolic Profile of Follicular Fluid, Oocyte Developmental Potential, and Implantation Outcome. Fertil. Steril. 2012, 97, 1078–1084.e8. [Google Scholar] [CrossRef] [PubMed]
- Júnior, D.N.; Pereira, A.A.; Júnior, G.M.O.; de Almeida, V.V.; da Silva, E.F.; da Silva, E.F.; da Silva, W.A.; de Alcântara, R.S.; da Silva, W.A.; Júnior, G.S.C.; et al. Choline and Digestible Methionine + Cystine Supplementation for Quail in the Laying Phase. Semin. Ciênc. Agrár. 2020, 41, 305–312. [Google Scholar] [CrossRef]
- Olgun, O.; Gül, E.T.; Kılınç, G.; Yıldız, A.; Çolak, A.; Sarmiento-García, A. Performance, Egg Quality, and Yolk Antioxidant Capacity of the Laying Quail in Response to Dietary Choline Levels. Animals 2022, 12, 3361. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Wang, J.; Ji, R.; Wang, Z.; Yang, H. Effects of Choline on Reproductive Performance, Egg Quality, and Ovarian Morphological Development in Laying Geese. Anim. Feed. Sci. Technol. 2025, 324, 116288. [Google Scholar] [CrossRef]
- Zhong, W.; Hu, L.; Zhao, Y.; Li, Z.; Zhuo, Y.; Jiang, X.; Li, J.; Zhao, X.; Che, L.; Feng, B.; et al. Effects of Dietary Choline Levels During Pregnancy on Reproductive Performance, Plasma Metabolome and Gut Microbiota of Sows. Front. Vet. Sci. 2022, 8, 771228. [Google Scholar] [CrossRef]
- Zhan, X. The Effect of Choline Supplement and miR-29b-3p in Porcine Ovarian Follicular Development. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2023. [Google Scholar]
- Acosta, D.A.V.; Rivelli, M.I.; Skenandore, C.; Zhou, Z.; Keisler, D.H.; Luchini, D.; Corrêa, M.N.; Cardoso, F.C. Effects of Rumen-Protected Methionine and Choline Supplementation on Steroidogenic Potential of the First Postpartum Dominant Follicle and Expression of Immune Mediators in Holstein Cows. Theriogenology 2017, 96, 1–9. [Google Scholar] [CrossRef]
- Kotsampasi, B.; Tsiplakou, E.; Karatzia, M.-A.; Oikonomou, S.; Mitsiopoulou, C.; Kalogiannis, D.; Dovolou, E.; Lymperopoulos, A.; Sotirakoglou, K.; Anastasiadou, M.; et al. Effects of Rumen-Protected Methionine, Choline, and Betaine Supplementation on Ewes’ Pregnancy and Reproductive Outcomes. Vet. Sci. 2025, 12, 723. [Google Scholar] [CrossRef]
- Davenport, C.; Yan, J.; Taesuwan, S.; Shields, K.; West, A.A.; Jiang, X.; Perry, C.A.; Malysheva, O.V.; Stabler, S.P.; Allen, R.H.; et al. Choline Intakes Exceeding Recommendations during Human Lactation Improve Breast Milk Choline Content by Increasing PEMT Pathway Metabolites. J. Nutr. Biochem. 2015, 26, 903–911. [Google Scholar] [CrossRef]
- Taesuwan, S.; McDougall, M.Q.; Malysheva, O.V.; Bender, E.; Nevins, J.E.H.; Devapatla, S.; Vidavalur, R.; Caudill, M.A.; Klatt, K.C. Choline Metabolome Response to Prenatal Choline Supplementation across Pregnancy: A Randomized Controlled Trial. FASEB J. 2021, 35, e22063. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, Y.; Su, Y.; Zhou, Y.; Yang, T.; Li, Y.; Wang, Y.; Sun, Y.; Chen, L.; Zhang, F.; et al. Oroxylin a Regulates cGAS DNA Hypermethylation Induced by Methionine Metabolism to Promote HSC Senescence. Pharmacol. Res. 2023, 187, 106590. [Google Scholar] [CrossRef]
- Ferré, V.M.; Brousseau, J.; Charpentier, C.; Péré, H. Nouveaux Marqueurs Développés Pour Le Dépistage Des Cancers Liés Aux HPV. Rev. Francoph. Lab. 2024, 2024, 46–58. [Google Scholar] [CrossRef]
- Yang, G.; Li, S.; Cai, S.; Zhou, J.; Ye, Q.; Zhang, S.; Chen, F.; Wang, F.; Zeng, X. Dietary Methionine Supplementation during the Estrous Cycle Improves Follicular Development and Estrogen Synthesis in Rats. Food Funct. 2024, 15, 704–715. [Google Scholar] [CrossRef]
- Usman, M.; Li, A.; Wu, D.; Qinyan, Y.; Yi, L.X.; He, G.; Lu, H. The Functional Role of lncRNAs as ceRNAs in Both Ovarian Processes and Associated Diseases. Non-Coding RNA Res. 2024, 9, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Annisa, N.G.; Febri, R.R.; Darmawi; Kinasih, T.; Muharam, R. Asmarinah Analysis of the Methylation Profiles of the Steroidogenic Factor-1 (SF-1) Gene in Peritoneal and Ovarian Endometriosis. J. Phys. Conf. Ser. 2018, 1073, 32080. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, C.; Liu, Y.; Li, Y.; Wang, L.; Zhang, W. Promoter Methylation of CYP19A1 Gene in Chinese Polycystic Ovary Syndrome Patients. Gynecol. Obstet. Investig. 2013, 76, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.; Shahhoseini, M.; Afsharian, P.; Karimian, L.; Ashrafi, M.; Mehraein, F.; Afatoonian, R. Role of Epigenetic Modifications in the Aberrant CYP19A1 Gene Expression in Polycystic Ovary Syndrome. Arch. Med. Sci. 2019, 15, 887–895. [Google Scholar] [CrossRef]
- Bulun, S.E.; Takayama, K.; Suzuki, T.; Sasano, H.; Yilmaz, B.; Sebastian, S. Organization of the Human Aromatase P450 (CYP19) Gene. Semin. Reprod. Med. 2004, 22, 5–9. [Google Scholar] [CrossRef]
- Ding, Y.; Long, C.; Liu, X.; Chen, X.; Guo, L.; Xia, Y.; He, J.; Wang, Y. 5-Aza-2’-Deoxycytidine Leads to Reduced Embryo Implantation and Reduced Expression of DNA Methyltransferases and Essential Endometrial Genes. PLoS ONE 2012, 7, e45364. [Google Scholar] [CrossRef]
- Geng, Y.; Gao, R.; Chen, X.; Liu, X.; Liao, X.; Li, Y.; Liu, S.; Ding, Y.; Wang, Y.; He, J. Folate Deficiency Impairs Decidualization and Alters Methylation Patterns of the Genome in Mice. Mol. Hum. Reprod. 2015, 21, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Li, W.; Chen, M.; Ding, Y.; Chen, X.; Tong, C.; Li, N.; Liu, X.; He, J.; Peng, C.; et al. Uterine Deficiency of Dnmt3b Impairs Decidualization and Causes Consequent Embryo Implantation Defects. Cell Biol. Toxicol. 2023, 39, 1077–1098. [Google Scholar] [CrossRef]
- Kumar, P.; Sait, S.F. Luteinizing Hormone and Its Dilemma in Ovulation Induction. J. Hum. Reprod. Sci. 2011, 4, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Zhang, X.; Deng, Z.; Xiang, Z.; Wang, H.; Yi, L.; Gao, Q.; Wang, Y. Epigenetic Pattern Changes in Prenatal Female Sprague-Dawley Rats Following Exposure to Androgen. Reprod. Fertil. Dev. 2015, 28, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Mao, Y.; Zhou, W.; Jiang, Y. Dynamic Changes in the Follicular Transcriptome and Promoter DNA Methylation Pattern of Steroidogenic Genes in Chicken Follicles throughout the Ovulation Cycle. PLoS ONE 2015, 10, e0146028. [Google Scholar] [CrossRef]
- Kovacheva, V.P.; Mellott, T.J.; Davison, J.M.; Wagner, N.; Lopez-Coviella, I.; Schnitzler, A.C.; Blusztajn, J.K. Gestational Choline Deficiency Causes Global and Igf2 Gene DNA Hypermethylation by Up-Regulation of Dnmt1 Expression. J. Biol. Chem. 2007, 282, 31777–31788. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Y.; Zhi, L.; Han, X.; Shen, J.; Liu, Y.; Yao, J.; Yang, X. DNA Methylation Variation Trends during the Embryonic Development of Chicken. PLoS ONE 2016, 11, e0159230. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Xue, Q.; Li, G.; Yin, J.; Zhang, H.; Zhu, Y.; Xing, W.; Cao, Y.; Su, Y.; Wang, K.; et al. Genome-Wide Analysis of the Role of DNA Methylation in Inbreeding Depression of Reproduction in Langshan Chicken. Genomics 2020, 112, 2677–2687. [Google Scholar] [CrossRef]
- Haimon, M.L.J.; Estrada-Cortés, E.; Amaral, T.F.; Martin, H.; Jeensuk, S.; Block, J.; Heredia, D.; Venturini, M.; Rojas, C.S.; Gonella-Diaza, A.M.; et al. Provision of Choline Chloride to the Bovine Preimplantation Embryo Alters Postnatal Body Size and DNA Methylation. Biol. Reprod. 2024, 111, 567–579. [Google Scholar] [CrossRef]
- Ju, X.; Wang, Z.; Cai, D.; Bello, S.F.; Nie, Q. DNA Methylation in Poultry: A Review. J. Anim. Sci. Biotechnol. 2023, 14, 138. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, Z.; Li, D.; Kang, L.; Jiang, Y. Long-Read Sequencing Reveals the Effect of Follicle-Stimulating Hormone on the mRNA Profile of Chicken Granulosa Cells from Prehierarchical Follicles. Poult. Sci. 2023, 102, 102600. [Google Scholar] [CrossRef]
- Zhang, T.; He, M.; Zhao, L.; Qin, S.; Zhu, Z.; Du, X.; Zhou, B.; Yang, Y.; Liu, X.; Xia, G.; et al. HDAC6 Regulates Primordial Follicle Activation through mTOR Signaling Pathway. Cell Death Dis. 2021, 12, 559. [Google Scholar] [CrossRef]
- Lin, X.; Liu, X.; Guo, C.; Liu, M.; Mi, Y.; Zhang, C. Promotion of the Prehierarchical Follicle Growth by Postovulatory Follicles Involving PGE2-EP2 Signaling in Chickens. J. Cell. Physiol. 2018, 233, 8984–8995. [Google Scholar] [CrossRef]
- Shen, M.; Wu, P.; Li, T.; Wu, P.; Chen, F.; Chen, L.; Xie, K.; Wang, J.; Zhang, G. Transcriptome Analysis of circRNA and mRNA in Theca Cells during Follicular Development in Chickens. Genes. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, Y.; Cao, Z.F.; Gu, T.; Xu, Q.; Chen, G. Histological Characteristics of Follicles and Reproductive Hormone Secretion during Ovarian Follicle Development in Laying Geese. Poult. Sci. 2019, 98, 6063–6070. [Google Scholar] [CrossRef]
- Sreesujatha, R.M.; Jeyakumar, S.; Kundu, A.; Balasundaram, C. Use of Transcutaneous Ultrasonography to Characterize Ovarian Status, Size Distribution, and Hierarchical Status of Follicles in Japanese Quail (Coturnix Coturnix Japonica). Theriogenology 2016, 86, 1231–1239. [Google Scholar] [CrossRef]
- Li, C.; Guo, S.; Zhang, M.; Guo, Y.; Gao, J. DNA Methylation and Histone Modification Patterns during the Late Embryonic and Early Postnatal Development of Chickens. Poult. Sci. 2015, 94, 706–721. [Google Scholar] [CrossRef]
- Kress, C.; Jouneau, L.; Pain, B. Reinforcement of Repressive Marks in the Chicken Primordial Germ Cell Epigenetic Signature: Divergence from Basal State Resetting in Mammals. Epigenet. Chromatin 2024, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Seo, H.W.; Lee, B.R.; Yoo, M.; Womack, J.E.; Han, J.Y. Gene Expression and DNA Methylation Status of Chicken Primordial Germ Cells. Mol. Biotechnol. 2013, 54, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, D.F.; Cao, W.; Chen, X.; Du, W. Effects of Ten-Eleven Translocation 1 (Tet1) on DNA Methylation and Gene Expression in Chicken Primordial Germ Cells. Reprod. Fertil. Dev. 2019, 31, 509–520. [Google Scholar] [CrossRef]
- Xu, R.; Wen, D.; Yin, L.; Tang, Y.; Lu, S.; Gao, Y.; Pan, M.-H.; Han, B.; Ma, B. Estrogen Influences the Transzonal Projection Assembly of Cumulus-Oocyte Complexes through G Protein-Coupled Estrogen Receptor during Goat Follicle Development. Mol. Reprod. Dev. 2024, 91, e23763. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, H.; Xu, R.; Luo, L.; Yin, L.; Wu, H.; Zhang, Y.; Li, C.; Lu, S.; Tang, Y.; et al. Single-Cell Sequencing Reveals the Transcriptional Alternations of 17β-Estradiol Suppressing Primordial Follicle Formation in Neonatal Mouse Ovaries. Cell Prolif. 2024, 57, e13713. [Google Scholar] [CrossRef]
- Gupta, R.K.; Singh, J.M.; Leslie, T.C.; Meachum, S.; Flaws, J.A.; Yao, H.H.-C. Di-(2-Ethylhexyl) Phthalate and Mono-(2-Ethylhexyl) Phthalate Inhibit Growth and Reduce Estradiol Levels of Antral Follicles In Vitro. Toxicol. Appl. Pharmacol. 2010, 242, 224–230. [Google Scholar] [CrossRef]
- Orisaka, M.; Hattori, K.; Fukuda, S.; Mizutani, T.; Miyamoto, K.; Sato, T.; Tsang, B.K.; Kotsuji, F.; Yoshida, Y. Dysregulation of Ovarian Follicular Development in Female Rat: LH Decreases FSH Sensitivity during Preantral-Early Antral Transition. Endocrinology 2013, 154, 2870–2880. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.G.; Dornas, R.A.P.; Mahecha, G.A.B.; Oliveira, C.A. Occurrence and Cellular Distribution of Estrogen Receptors ERα and ERβ in the Testis and Epididymal Region of Roosters. Gen. Comp. Endocrinol. 2011, 170, 597–603. [Google Scholar] [CrossRef]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.-Å.; Smithies, O. Generation and Reproductive Phenotypes of Mice Lacking Estrogen Receptor β. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar] [CrossRef]
- Cheng, G.; Weihua, Z.; Mäkinen, S.; Mäkelä, S.; Saji, S.; Warner, M.; Gustafsson, J.-A.; Hovatta, O. A Role for the Androgen Receptor in Follicular Atresia of Estrogen Receptor Beta Knockout Mouse Ovary. Biol. Reprod. 2002, 66, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Emmen, J.M.; Couse, J.F.; Elmore, S.A.; Yates, M.M.; Kissling, G.E.; Korach, K.S. In Vitro Growth and Ovulation of Follicles from Ovaries of Estrogen Receptor (ER) α and ERβ Null Mice Indicate a Role for ERβ in Follicular Maturation. Endocrinology 2005, 146, 2817–2826. [Google Scholar] [CrossRef]
- Ramsey, M.; Crews, D. Steroid Signaling System Responds Differently to Temperature and Hormone Manipulation in the Red-Eared Slider Turtle (Trachemys Scripta Elegans), a Reptile with Temperature-Dependent Sex Determination. Sex. Dev. 2007, 1, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Ru, W.; Hua, L.; Wei, Y.; Li, W.; Cao, D.; Ge, Y.; Chen, H.; Lan, X.; Gong, S. The Effect of the Cyp19a1 Gene Methylation Modification on Temperature-Dependent Sex Determination of Reeves’ Turtle (Mauremys reevesii). Asian Herpetol. Res. 2017, 8, 213–220. [Google Scholar] [CrossRef]
- Ge, C.; Ye, J.; Zhang, H.; Zhang, Y.; Sun, W.; Sang, Y.; Capel, B.; Qian, G. Dmrt1 Induces the Male Pathway in a Turtle with Temperature-Dependent Sex Determination. Development 2017, 144, dev.152033. [Google Scholar] [CrossRef]
- Shen, J.; Sun, W.; Wu, K.; Zhuang, T.; Lei, J.; Ma, Q.; Xiao, L.; Ge, C. Loss-and Gain-of-Function Analyses Reveal the Essential Role of Cyp19a1 in Ovarian Determination of the Red-Eared Slider Turtle. Genetics 2023, 224, iyad041. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Zhao, A.; Cai, X.; Yu, A.; Xu, Q.; Wang, P.; Yao, J.; Wang, Q.; Wang, W. Arsenic Exposure Diminishes Ovarian Follicular Reserve and Induces Abnormal Steroidogenesis by DNA Methylation. Ecotoxicol. Environ. Saf. 2022, 241, 113816. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Asada, H.; Kizuka, F.; Tamura, I.; Maekawa, R.; Taketani, T.; Sato, S.; Yamagata, Y.; Tamura, H.; Sugino, N. Changes in Histone Modification and DNA Methylation of the StAR and Cyp19a1 Promoter Regions in Granulosa Cells Undergoing Luteinization during Ovulation in Rats. Endocrinology 2013, 154, 458–470. [Google Scholar] [CrossRef]
- Durlinger, A.L.L.; Gruijters, M.J.G.; Kramer, P.; Karels, B.; Ingraham, H.A.; Nachtigal, M.W.; Uilenbroek, J.T.J.; Grootegoed, J.A.; Themmen, A.P.N. Anti-Müllerian Hormone Inhibits Initiation of Primordial Follicle Growth in the Mouse Ovary. Endocrinology 2002, 143, 1076–1084. [Google Scholar] [CrossRef]
- van Rooij, I.A.J.; Broekmans, F.J.M.; te Velde, E.R.; Fauser, B.C.J.M.; Bancsi, L.F.J.M.M.; de Jong, F.H.; Themmen, A.P.N. Serum Anti-Müllerian Hormone Levels: A Novel Measure of Ovarian Reserve. Hum. Reprod. 2002, 17, 3065–3071. [Google Scholar] [CrossRef]
- Holt, J.E.; Jackson, A.; Roman, S.D.; Aitken, R.J.; Koopman, P.; McLaughlin, E.A. CXCR4/SDF1 Interaction Inhibits the Primordial to Primary Follicle Transition in the Neonatal Mouse Ovary. Dev. Biol. 2006, 293, 449–460. [Google Scholar] [CrossRef]
- Shimizu, K.; Nakamura, T.; Bayasula; Nakanishi, N.; Kasahara, Y.; Nagai, T.; Murase, T.; Osuka, S.; Goto, M.; Iwase, A.; et al. Molecular Mechanism of FSHR Expression Induced by BMP15 in Human Granulosa Cells. J. Assist. Reprod. Genet. 2019, 36, 1185–1194. [Google Scholar] [CrossRef]
- Wei, L.; Huang, R.; Li, L.; Fang, C.; Li, Y.; Liang, X. Reduced and Delayed Expression of GDF9 and BMP15 in Ovarian Tissues from Women with Polycystic Ovary Syndrome. J. Assist. Reprod. Genet. 2014, 31, 1483–1490. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Z.; Liu, P.; Xu, D.; Tang, L.; Fan, L.; Sun, X.; Li, J.; Wu, Q.; Li, Z.; et al. Aberrant BMP15/HIF-1α/SCF Signaling Pathway in Human Granulosa Cells Is Involved in the PCOS Related Abnormal Follicular Development. Taylor Fr. 2022, 38, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Bestor, T.H. The DNA Methyltransferases of Mammals. Hum. Mol. Genet. 2000, 9, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Gehring, M.; Reik, W.; Henikoff, S. DNA Demethylation by DNA Repair. Trends Genet. TIG 2009, 25, 82–90. [Google Scholar] [CrossRef]
- Zeitoun, K.; Takayama, K.; Michael, M.D.; Bulun, S.E. Stimulation of Aromatase P450 Promoter (II) Activity in Endometriosis and Its Inhibition in Endometrium Are Regulated by Competitive Binding of Steroidogenic Factor-1 and Chicken Ovalbumin Upstream Promoter Transcription Factor to the Same Cis-Acting Element. Mol. Endocrinol. 1999, 13, 239–253. [Google Scholar] [CrossRef][Green Version]
- Shozu, M.; Sumitani, H.; Segawa, T.; Yang, H.-J.; Murakami, K.; Kasai, T.; Inoue, M. Overexpression of Aromatase P450 in Leiomyoma Tissue Is Driven Primarily through Promoter I.4 of the Aromatase P450 Gene (CYP19). J. Clin. Endocrinol. Metab. 2002, 87, 2540–2548. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, J.; Song, Z.; Zhang, T.; Chu, K.; Li, J.; Zhou, J.; Lin, J. Upregulation of H-TERT and Ki-67 in Ectopic Endometrium Is Associated with Recurrence of Endometriosis. Pubmed 2022, 23, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guo, Z.; Peng, X.; Wu, B.; Meng, Q.; Lu, X.; Feng, L.; Guo, T. Chrysotoxine Regulates Ferroptosis and the PI3K/AKT/mTOR Pathway to Prevent Cervical Cancer. J. Ethnopharmacol. 2025, 338, 119126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Song, C.; Yin, M.; Liu, L.; Zhang, Y.; Li, Y.; Zhang, J.; Guo, M.; Li, C. TRAPT: A Multi-Stage Fused Deep Learning Framework for Predicting Transcriptional Regulators Based on Large-Scale Epigenomic Data. Nat. Commun. 2025, 16, 3611. [Google Scholar] [CrossRef]
| Species | Optimal Choline Level | Main Effects |
|---|---|---|
| Japanese quail (Coturnix japonica) | 0.126% (≈1260 mg/kg) diet; 1500 mg/kg to maintain laying performance and egg quality; 3500 mg/kg to enhance yolk antioxidant capacity | Improved egg weight and feed conversion; maintained egg quality; increased yolk antioxidant activity [66,67] |
| Laying geese | 784–913 mg/kg diet | Enhanced reproductive performance, egg production, and sex hormone levels; promoted ovarian development and hormone synthesis [68] |
| Gestating sows | ≈1910 mg/kg diet | Increased feed intake; improved piglet birth weight and litter uniformity; enhanced maternal antioxidant capacity and gut microbiota composition [69] |
| Pig | 500–1000 mg/kg (diet) | Improves ovarian function, promotes corpus luteum formation, regulates reproduction-related gene expression, and enhances reproductive performance [70] |
| Transition Holstein cows | 60 g/day rumen-protected choline (ReaShure®) | Reduced inflammatory gene expression in follicular cells; supported a healthier follicular environment during early postpartum [71] |
| Ewes (periconceptional and late gestation) | 1.60 g/ewe/day rumen-protected choline | Reduced embryonic loss; increased lamb birth weight; improved maternal antioxidant status [72] |
| Lactating women | 930 mg/day | Increased concentrations of PEMT-derived choline metabolites in breast milk; enhanced infant choline supply [73] |
| Pregnant women | 550 mg/day | Increased maternal plasma choline metabolites; promoted fetal phospholipid synthesis via the PEMT pathway; supported maternal–fetal health [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zheng, X.; Yang, H.; Wang, Z. Choline-Mediated Regulation of Follicular Growth: Interplay Between Steroid Synthesis, Epigenetics, and Oocyte Development. Biology 2025, 14, 1220. https://doi.org/10.3390/biology14091220
Liu W, Zheng X, Yang H, Wang Z. Choline-Mediated Regulation of Follicular Growth: Interplay Between Steroid Synthesis, Epigenetics, and Oocyte Development. Biology. 2025; 14(9):1220. https://doi.org/10.3390/biology14091220
Chicago/Turabian StyleLiu, Wenfeng, Xucheng Zheng, Haiming Yang, and Zhiyue Wang. 2025. "Choline-Mediated Regulation of Follicular Growth: Interplay Between Steroid Synthesis, Epigenetics, and Oocyte Development" Biology 14, no. 9: 1220. https://doi.org/10.3390/biology14091220
APA StyleLiu, W., Zheng, X., Yang, H., & Wang, Z. (2025). Choline-Mediated Regulation of Follicular Growth: Interplay Between Steroid Synthesis, Epigenetics, and Oocyte Development. Biology, 14(9), 1220. https://doi.org/10.3390/biology14091220






