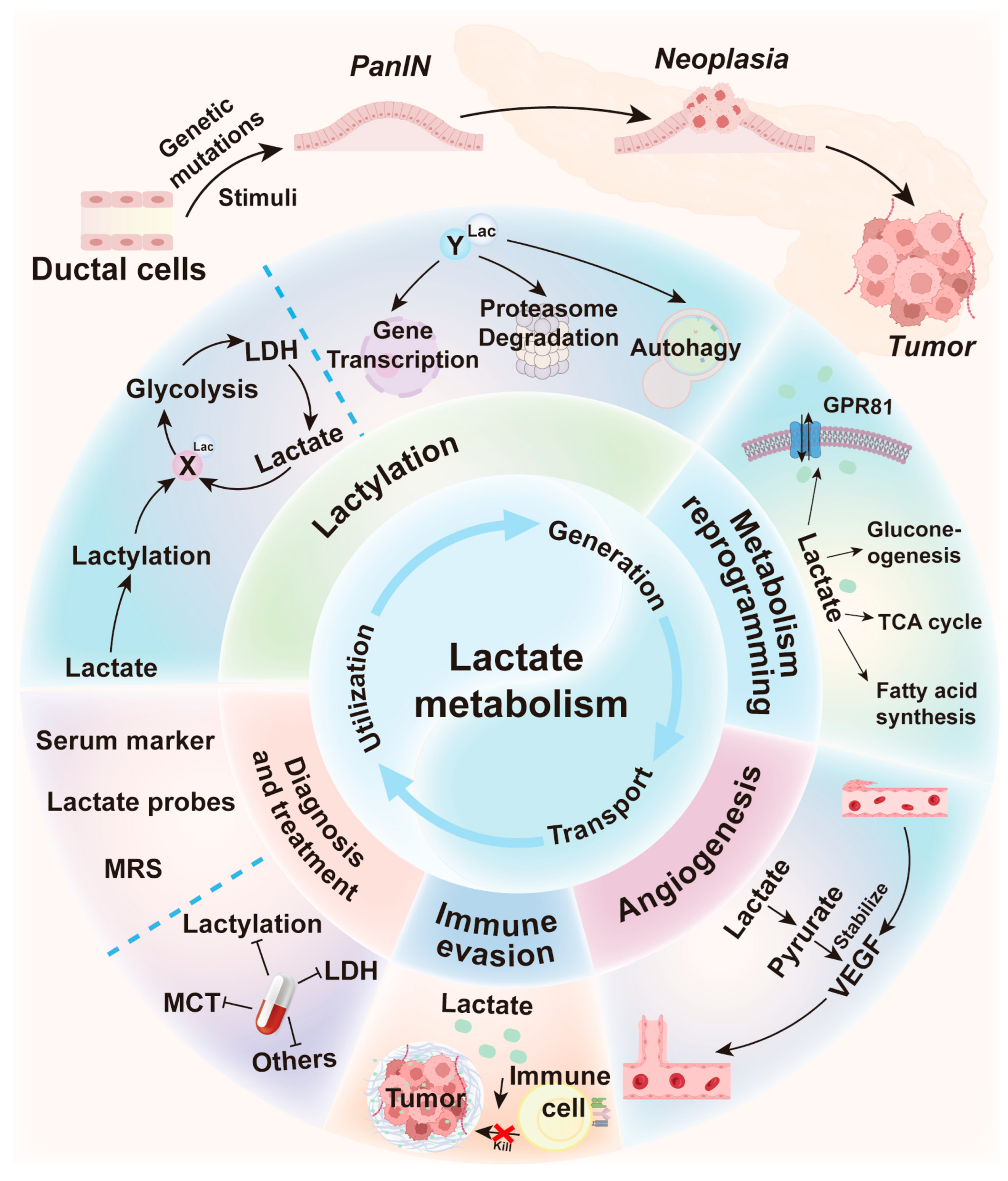
Lactate Metabolism: The String-Puller for the Development of Pancreatic Cancer
Simple Summary
Abstract
1. Introduction
2. Aberrant Lactate Production in Pancreatic Cancer
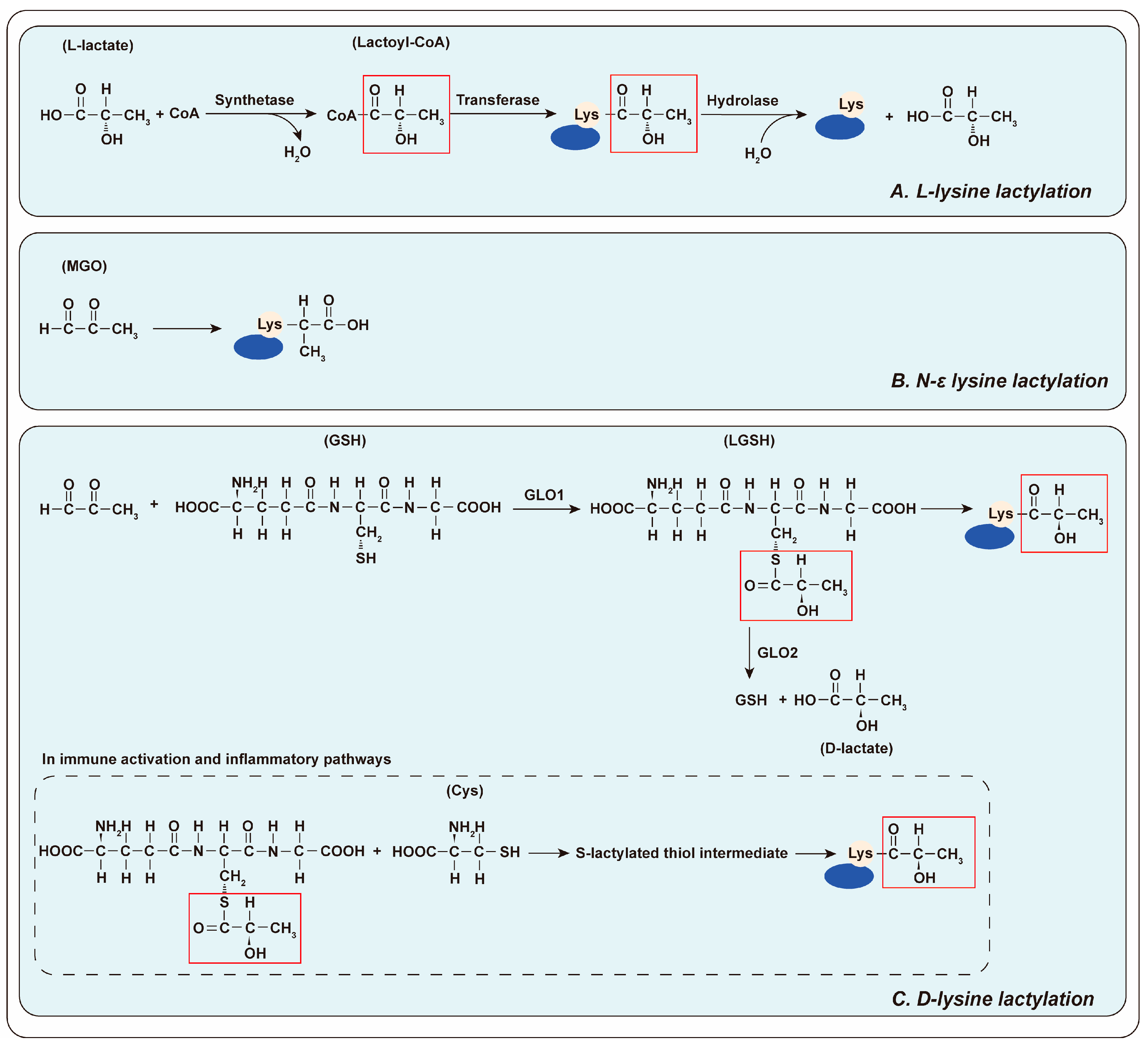
3. A Novel Protein Modification: Lactylation
3.1. The Mechanism of Lactylation
3.2. Lactylation in Pancreatic Cancer
3.3. Lactate-Regulated Other Epigenetic Modification Processes
3.4. Lactylation and Acetylation
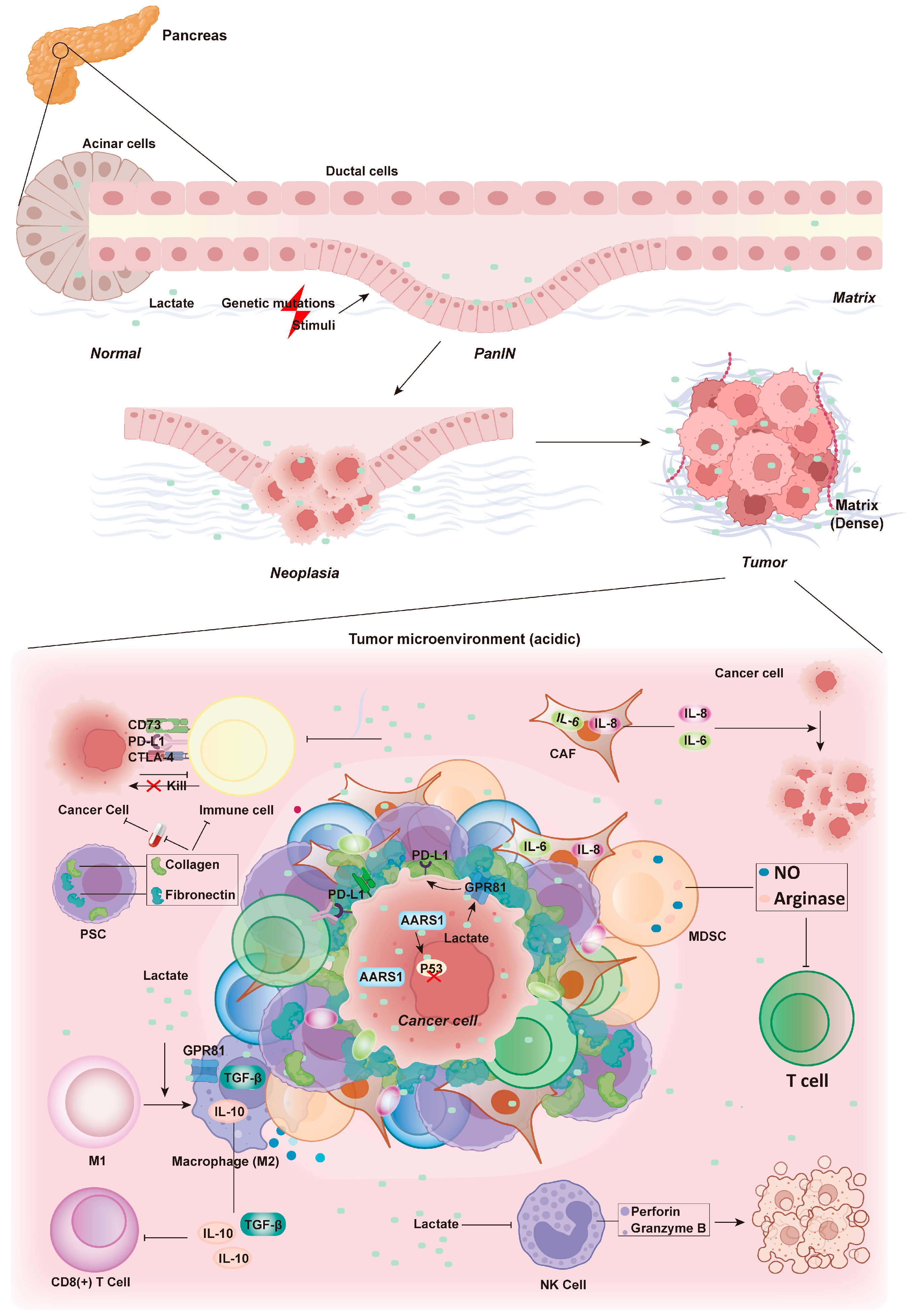
4. Effects of Lactate Metabolism on the Microenvironment of Pancreatic Cancer
4.1. Metabolism Reprogramming
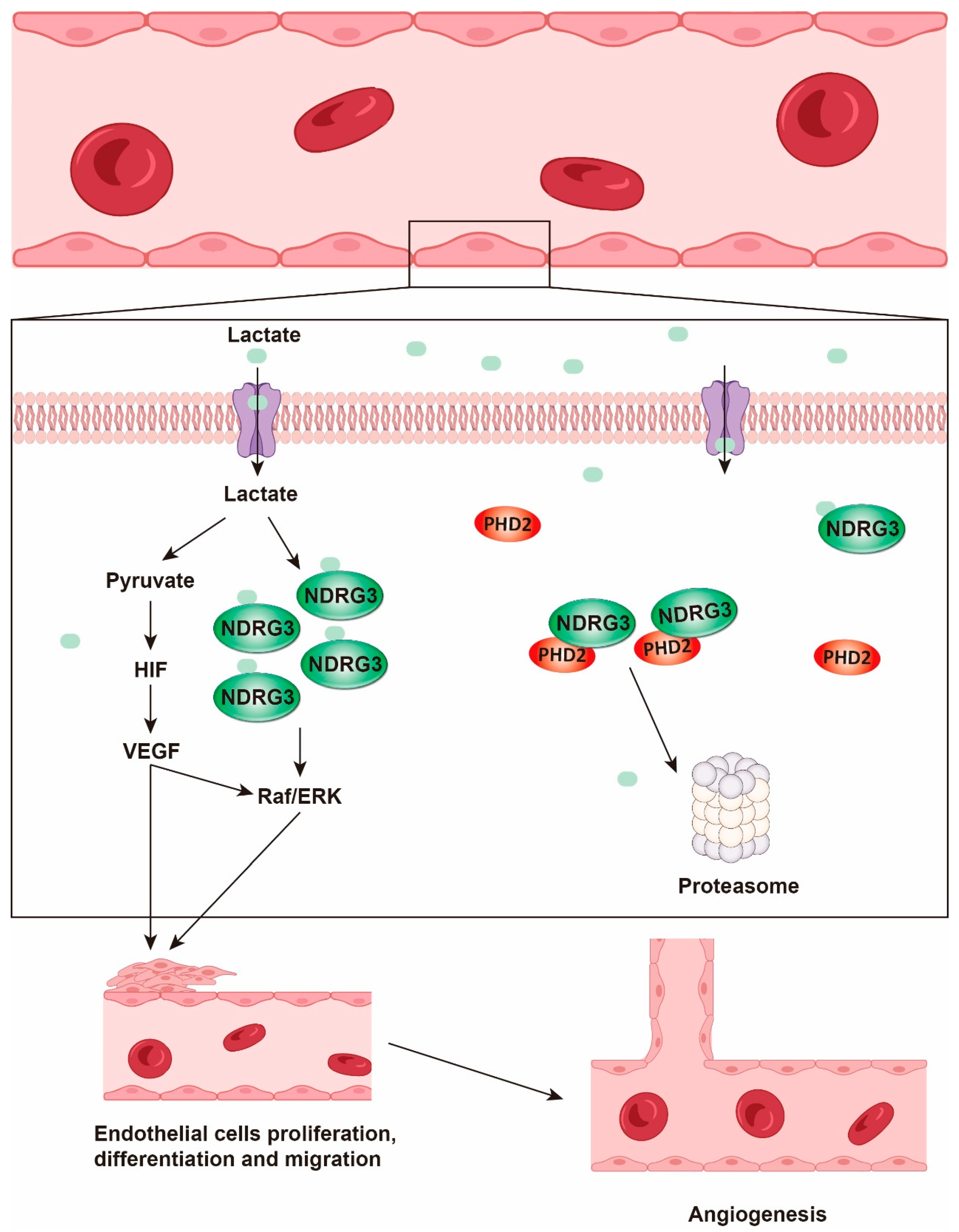
4.2. Angiogenesis
4.3. Immune Evasion
5. Diagnosis of Pancreatic Cancer Based on Lactate Metabolism
6. Targeting Lactate Metabolism in Pancreatic Cancer Therapy
6.1. Inhibiting Lactylation
6.2. Inhibiting Lactate Synthesis: Targeting LDH
6.3. Targeting Lactate Transport: The Role of MCT
6.4. Targeting Other Metabolic Pathways: An Alternative Strategy
7. Conclusions and Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso-Curbelo, D.; Ho, Y.-J.; Burdziak, C.; Maag, J.L.V.; Morris, J.P.; Chandwani, R.; Chen, H.-A.; Tsanov, K.M.; Barriga, F.M.; Luan, W.; et al. A gene-environment-induced epigenetic program initiates tumorigenesis. Nature 2021, 590, 642–648. [Google Scholar] [CrossRef]
- Danovi, S. Exploring the origins of pancreatic cancer. Nat. Genet. 2024, 56, 1038. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.; Liu, Q.; Wang, Z.; Li, S.; Liu, L.; Zhao, W.; Wang, K.; Zhang, R.; Wang, L.; et al. The LCK-14-3-3ζ-TRPM8 axis regulates TRPM8 function/assembly and promotes pancreatic cancer malignancy. Cell Death Dis. 2022, 13, 524. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, N.; Huang, H.; Li, S.; Liu, S.; Zhang, R.; Huang, Y.; Lyu, H.; Xiao, S.; Ali, D.W.; et al. Phosphorylated PTTG1 switches its subcellular distribution and promotes β-catenin stabilization and subsequent transcription activity. Oncogene 2023, 42, 2439–2455. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Deramaudt, T.; Rustgi, A.K. Mutant KRAS in the initiation of pancreatic cancer. Biochim. Biophys. Acta 2005, 1756, 97–101. [Google Scholar] [CrossRef]
- Koikawa, K.; Ohuchida, K.; Takesue, S.; Ando, Y.; Kibe, S.; Nakayama, H.; Endo, S.; Abe, T.; Okumura, T.; Horioka, K.; et al. Pancreatic stellate cells reorganize matrix components and lead pancreatic cancer invasion via the function of Endo180. Cancer Lett. 2018, 412, 143–154. [Google Scholar] [CrossRef]
- Deng, D.; Patel, R.; Chiang, C.-Y.; Hou, P. Role of the Tumor Microenvironment in Regulating Pancreatic Cancer Therapy Resistance. Cells 2022, 11, 2952. [Google Scholar] [CrossRef]
- Tao, J.; Yang, G.; Zhou, W.; Qiu, J.; Chen, G.; Luo, W.; Zhao, F.; You, L.; Zheng, L.; Zhang, T.; et al. Targeting hypoxic tumor microenvironment in pancreatic cancer. J. Hematol. Oncol. 2021, 14, 14. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Zhang, R.; Lyu, H.; Xiao, S.; Guo, D.; Ali, D.W.; Michalak, M.; Chen, X.-Z.; Zhou, C.; et al. Sense and anti-sense: Role of FAM83A and FAM83A-AS1 in Wnt, EGFR, PI3K, EMT pathways and tumor progression. Biomed. Pharmacother. 2024, 173, 116372. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Brunner, J.S.; Finley, L.W.S. Metabolic determinants of tumour initiation. Nat. Rev. Endocrinol. 2023, 19, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fu, M.; Wu, M.; Cao, Z.; Zhang, Q.; Liu, Z. Emerging mechanisms and promising approaches in pancreatic cancer metabolism. Cell Death Dis. 2024, 15, 553. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, Q.; Li, J.; Yang, Z.; Ahmad, M.; Lin, Y.; Wu, D.; Zheng, L.; Li, J.; Wang, B.; et al. A GSTP1-mediated lactic acid signaling promotes tumorigenesis through the PPP oxidative branch. Cell Death Dis. 2023, 14, 463. [Google Scholar] [CrossRef]
- Lee, D.C.; Sohn, H.A.; Park, Z.-Y.; Oh, S.; Kang, Y.K.; Lee, K.-M.; Kang, M.; Jang, Y.J.; Yang, S.-J.; Hong, Y.K.; et al. A lactate-induced response to hypoxia. Cell 2015, 161, 595–609. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Ippolito, L.; Morandi, A.; Giannoni, E.; Chiarugi, P. Lactate: A Metabolic Driver in the Tumour Landscape. Trends Biochem. Sci. 2019, 44, 153–166. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Zhou, X.; Cai, M.; Yang, F.; Huang, L.; Ling, Y.; Zhang, Y.; Nie, H.; Xing, R. Hypoxia-induced autophagy in pancreatic cancer counteracts the cytotoxicity of CD8+ T cells by inhibiting the expression of MHC-I. Genes Immun. 2024, 26, 45–53. [Google Scholar] [CrossRef]
- Singh, M.; Afonso, J.; Sharma, D.; Gupta, R.; Kumar, V.; Rani, R.; Baltazar, F.; Kumar, V. Targeting monocarboxylate transporters (MCTs) in cancer: How close are we to the clinics? Semin. Cancer Biol. 2023, 90, 1–14. [Google Scholar] [CrossRef]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef]
- Chen, M.; Cen, K.; Song, Y.; Zhang, X.; Liou, Y.-C.; Liu, P.; Huang, J.; Ruan, J.; He, J.; Ye, W.; et al. NUSAP1-LDHA-Glycolysis-Lactate feedforward loop promotes Warburg effect and metastasis in pancreatic ductal adenocarcinoma. Cancer Lett. 2023, 567, 216285. [Google Scholar] [CrossRef]
- Cui, X.-G.; Han, Z.-T.; He, S.-H.; Wu, X.-d.; Chen, T.-R.; Shao, C.-H.; Chen, D.-L.; Su, N.; Chen, Y.-M.; Wang, T.; et al. HIF1/2α mediates hypoxia-induced LDHA expression in human pancreatic cancer cells. Oncotarget 2017, 8, 24840–24852. [Google Scholar] [CrossRef]
- Xu, F.; Huang, M.; Chen, Q.; Niu, Y.; Hu, Y.; Hu, P.; Chen, D.; He, C.; Huang, K.; Zeng, Z.; et al. LncRNA HIF1A-AS1 Promotes Gemcitabine Resistance of Pancreatic Cancer by Enhancing Glycolysis through Modulating the AKT/YB1/HIF1α Pathway. Cancer Res. 2021, 81, 5678–5691. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- de la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, H.; Lin, M.; Yan, Z.; An, L.; Cao, Z.; Geng, D.; Yue, J.; Tang, Y.; Tian, L.; et al. The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J. Clin. Investig. 2024, 134, e174587. [Google Scholar] [CrossRef]
- Peralta, R.M.; Xie, B.; Lontos, K.; Nieves-Rosado, H.; Spahr, K.; Joshi, S.; Ford, B.R.; Quann, K.; Frisch, A.T.; Dean, V.; et al. Dysfunction of exhausted T cells is enforced by MCT11-mediated lactate metabolism. Nat. Immunol. 2024, 25, 2297–2307. [Google Scholar] [CrossRef]
- Chen, D.; Liu, P.; Lu, X.; Li, J.; Qi, D.; Zang, L.; Lin, J.; Liu, Y.; Zhai, S.; Fu, D.; et al. Pan-cancer analysis implicates novel insights of lactate metabolism into immunotherapy response prediction and survival prognostication. J. Exp. Clin. Cancer Res. 2024, 43, 125. [Google Scholar] [CrossRef]
- Fan, X.; Dai, Y.; Mo, C.; Li, H.; Luan, X.; Wang, B.; Yang, J.; Jiao, G.; Lu, X.; Chen, Z.; et al. TRIM21 Promotes Tumor Growth and Gemcitabine Resistance in Pancreatic Cancer by Inhibiting EPHX1-Mediated Arachidonic Acid Metabolism. Adv. Sci. 2024, 12, e2413674. [Google Scholar] [CrossRef]
- Santana-Codina, N.; Roeth, A.A.; Zhang, Y.; Yang, A.; Mashadova, O.; Asara, J.M.; Wang, X.; Bronson, R.T.; Lyssiotis, C.A.; Ying, H.; et al. Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis. Nat. Commun. 2018, 9, 4945. [Google Scholar] [CrossRef]
- Butera, G.; Pacchiana, R.; Mullappilly, N.; Margiotta, M.; Bruno, S.; Conti, P.; Riganti, C.; Donadelli, M. Mutant p53 prevents GAPDH nuclear translocation in pancreatic cancer cells favoring glycolysis and 2-deoxyglucose sensitivity. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1914–1923. [Google Scholar] [CrossRef]
- Hu, H.-F.; Ye, Z.; Qin, Y.; Xu, X.-W.; Yu, X.-J.; Zhuo, Q.-F.; Ji, S.-R. Mutations in key driver genes of pancreatic cancer: Molecularly targeted therapies and other clinical implications. Acta Pharmacol. Sin. 2021, 42, 1725–1741. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, T.; Zhao, Z.; Li, X.; Song, Y.; He, Y.; Zhou, Y.; Li, P.; An, L.; Wang, F. SMAD4 Limits PARP1 dependent DNA Repair to Render Pancreatic Cancer Cells Sensitive to Radiotherapy. Cell Death Dis. 2024, 15, 818. [Google Scholar] [CrossRef]
- Qin, C.; Yang, G.; Yang, J.; Ren, B.; Wang, H.; Chen, G.; Zhao, F.; You, L.; Wang, W.; Zhao, Y. Metabolism of pancreatic cancer: Paving the way to better anticancer strategies. Mol. Cancer 2020, 19, 50. [Google Scholar] [CrossRef]
- Shiratori, R.; Furuichi, K.; Yamaguchi, M.; Miyazaki, N.; Aoki, H.; Chibana, H.; Ito, K.; Aoki, S. Glycolytic suppression dramatically changes the intracellular metabolic profile of multiple cancer cell lines in a mitochondrial metabolism-dependent manner. Sci. Rep. 2019, 9, 18699. [Google Scholar] [CrossRef]
- Suzuki, T.; Kishikawa, T.; Sato, T.; Takeda, N.; Sugiura, Y.; Seimiya, T.; Sekiba, K.; Ohno, M.; Iwata, T.; Ishibashi, R.; et al. Mutant KRAS drives metabolic reprogramming and autophagic flux in premalignant pancreatic cells. Cancer Gene Ther. 2022, 29, 505–518. [Google Scholar] [CrossRef]
- Li, F.; Si, W.; Xia, L.; Yin, D.; Wei, T.; Tao, M.; Cui, X.; Yang, J.; Hong, T.; Wei, R. Positive feedback regulation between glycolysis and histone lactylation drives oncogenesis in pancreatic ductal adenocarcinoma. Mol. Cancer 2024, 23, 90. [Google Scholar] [CrossRef]
- Ancey, P.-B.; Contat, C.; Meylan, E. Glucose transporters in cancer—from tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef]
- Lu, K.; Yang, J.; Li, D.E.C.; He, S.-B.; Zhu, D.-M.; Zhang, L.-F.; Zhang, X.U.; Chen, X.-C.; Zhang, B.; Zhou, J. Expression and clinical significance of glucose transporter-1 in pancreatic cancer. Oncol. Lett. 2016, 12, 243–249. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Lee, M.-S.; Dennis, C.; Naqvi, I.; Dailey, L.; Lorzadeh, A.; Ye, G.; Zaytouni, T.; Adler, A.; Hitchcock, D.S.; Lin, L.; et al. Ornithine aminotransferase supports polyamine synthesis in pancreatic cancer. Nature 2023, 616, 339–347. [Google Scholar] [CrossRef]
- Kovacević, Z.; Morris, H.P. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972, 32, 326–333. [Google Scholar]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Mirveis, Z.; Howe, O.; Cahill, P.; Patil, N.; Byrne, H.J. Monitoring and modelling the glutamine metabolic pathway: A review and future perspectives. Metabolomics 2023, 19, 67. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Chen, L.; Cui, H. Targeting Glutamine Induces Apoptosis: A Cancer Therapy Approach. Int. J. Mol. Sci. 2015, 16, 22830–22855. [Google Scholar] [CrossRef] [PubMed]
- Pasti, A.P.; Rossi, V.; Di Stefano, G.; Brigotti, M.; Hochkoeppler, A. Human lactate dehydrogenase A undergoes allosteric transitions under pH conditions inducing the dissociation of the tetrameric enzyme. Biosci. Rep. 2022, 42, BSR20212654. [Google Scholar] [CrossRef] [PubMed]
- Damiani, C.; Colombo, R.; Gaglio, D.; Mastroianni, F.; Pescini, D.; Westerhoff, H.V.; Mauri, G.; Vanoni, M.; Alberghina, L. A metabolic core model elucidates how enhanced utilization of glucose and glutamine, with enhanced glutamine-dependent lactate production, promotes cancer cell growth: The WarburQ effect. PLoS Comput. Biol. 2017, 13, e1005758. [Google Scholar] [CrossRef]
- Dovmark, T.H.; Saccomano, M.; Hulikova, A.; Alves, F.; Swietach, P. Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene 2017, 36, 4538–4550. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Gaffney, D.O.; Jennings, E.Q.; Anderson, C.C.; Marentette, J.O.; Shi, T.; Schou Oxvig, A.M.; Streeter, M.D.; Johannsen, M.; Spiegel, D.A.; Chapman, E.; et al. Non-enzymatic Lysine Lactoylation of Glycolytic Enzymes. Cell Chem. Biol. 2020, 27, 206–213.e206. [Google Scholar] [CrossRef]
- Liu, H.; Ge, B. Lactylation as a post-translational regulator of cGAS and immunity. Mol. Cell 2024, 84, 4483–4485. [Google Scholar] [CrossRef]
- Sun, P.; Ma, L.; Lu, Z. Lactylation: Linking the Warburg effect to DNA damage repair. Cell Metab. 2024, 36, 1637–1639. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, J.; Zhu, Z.; Mao, Q.; Xu, Z.; Singh, P.K.; Rimayi, C.C.; Moreno-Yruela, C.; Xu, S.; Li, G.; et al. Lysine L-lactylation is the dominant lactylation isomer induced by glycolysis. Nat. Chem. Biol. 2025, 21, 91–99. [Google Scholar] [CrossRef]
- Zhu, R.; Ye, X.; Lu, X.; Xiao, L.; Yuan, M.; Zhao, H.; Guo, D.; Meng, Y.; Han, H.; Luo, S.; et al. ACSS2 acts as a lactyl-CoA synthetase and couples KAT2A to function as a lactyltransferase for histone lactylation and tumor immune evasion. Cell Metab. 2024, 37, 361–376. [Google Scholar] [CrossRef]
- Liu, R.; Ren, X.; Park, Y.E.; Feng, H.; Sheng, X.; Song, X.; AminiTabrizi, R.; Shah, H.; Li, L.; Zhang, Y.; et al. Nuclear GTPSCS functions as a lactyl-CoA synthetase to promote histone lactylation and gliomagenesis. Cell Metab. 2024, 37, 377–394.e9. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Xu, J.; Wang, P.; Wu, B.; Lu, S.; Lu, X.; You, S.; Huang, X.; Li, M.; et al. α-myosin heavy chain lactylation maintains sarcomeric structure and function and alleviates the development of heart failure. Cell Res. 2023, 33, 679–698. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Zhai, L.; Zhang, T.; Yin, H.; Gao, H.; Zhao, F.; Wang, Z.; Yang, X.; Jin, M.; et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell 2024, 187, 294–311.e21. [Google Scholar] [CrossRef]
- Niu, Z.; Chen, C.; Wang, S.; Lu, C.; Wu, Z.; Wang, A.; Mo, J.; Zhang, J.; Han, Y.; Yuan, Y.; et al. HBO1 catalyzes lysine lactylation and mediates histone H3K9la to regulate gene transcription. Nat. Commun. 2024, 15, 3561. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, M.; Li, J.; Cui, J.; Zhang, P.; Liu, F.; Wu, Y.; Deng, W.; Ma, J.; Li, X.; et al. KAT8-catalyzed lactylation promotes eEF1A2-mediated protein synthesis and colorectal carcinogenesis. Proc. Natl. Acad. Sci. USA 2024, 121, e2314128121. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Li, H.; Chen, X.; Fu, H.; Mao, D.; Chen, W.; Lan, L.; Wang, C.; Hu, K.; et al. NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature 2024, 631, 663–669. [Google Scholar] [CrossRef]
- Zong, Z.; Xie, F.; Wang, S.; Wu, X.; Zhang, Z.; Yang, B.; Zhou, F. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell 2024, 187, 2375–2392.e33. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, C.; Li, R.; Zhou, L.; Ran, Y.; Yang, Q.; Huang, H.; Lu, H.; Song, H.; Yang, B.; et al. AARS1 and AARS2 sense L-lactate to regulate cGAS as global lysine lactyltransferases. Nature 2024, 634, 1229–1237. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Z.; He, L.; Shen, Y.; Yan, Y.; Lv, X.; Zhu, X.; Li, W.; Tian, W.-Y.; Zheng, Y.; et al. Metabolic regulation of cytoskeleton functions by HDAC6-catalyzed α-tubulin lactylation. Nat. Commun. 2024, 15, 8377. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, M.; Chen, X.; He, X.; Li, X.; Wei, H.; Tan, Y.; Min, J.; Azam, T.; Xue, M.; et al. TRAP1 drives smooth muscle cell senescence and promotes atherosclerosis via HDAC3-primed histone H4 lysine 12 lactylation. Eur. Heart J. 2024, 45, 4219–4235. [Google Scholar] [CrossRef]
- Deng, C.; Zhu, J.; Fang, Z.; Yang, Y.; Zhao, Q.; Zhang, Z.; Jin, Z.; Jiang, H. Identification and analysis of microplastics in para-tumor and tumor of human prostate. EBioMedicine 2024, 108, 105360. [Google Scholar] [CrossRef]
- Du, R.; Gao, Y.; Yan, C.; Ren, X.; Qi, S.; Liu, G.; Guo, X.; Song, X.; Wang, H.; Rao, J.; et al. Sirtuin 1/sirtuin 3 are robust lysine delactylases and sirtuin 1-mediated delactylation regulates glycolysis. iScience 2024, 27, 110911. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Yang, B.; Sun, S.; Zhang, P.; Luo, Z.; Feng, T.; Cui, Z.; Zhu, T.; Li, Y.; et al. Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer. Nat. Commun. 2023, 14, 6523. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Xu, J.; Wang, K.; Hou, F.; Luan, X.; Chen, L. Aldehyde Dehydrogenase 2 Lactylation Aggravates Mitochondrial Dysfunction by Disrupting PHB2 Mediated Mitophagy in Acute Kidney Injury. Adv. Sci. 2025, 12, 2411943. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Yruela, C.; Zhang, D.; Wei, W.; Bæk, M.; Liu, W.; Gao, J.; Danková, D.; Nielsen, A.L.; Bolding, J.E.; Yang, L.; et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci. Adv. 2022, 8, eabi6696. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, Y.; Chen, M.; Tan, Y.; Min, J.; He, X.; Liu, F.; Gu, J.; Jiang, H.; Zheng, L.; et al. ASF1A-dependent P300-mediated histone H3 lysine 18 lactylation promotes atherosclerosis by regulating EndMT. Acta Pharm. Sin. B 2024, 14, 3027–3048. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Q.; Yao, Q.; Yang, Z.; Li, W.; Cheng, X.; Wen, Y.; Chen, R.; Xu, J.; Wang, X.; et al. Nonenzymatic lysine D-lactylation induced by glyoxalase II substrate SLG dampens inflammatory immune responses. Cell Res. 2025, 35, 97–116. [Google Scholar] [CrossRef]
- Li, G.; Wang, D.; Zhai, Y.; Pan, C.; Zhang, J.; Wang, C.; Huang, R.; Yu, M.; Li, Y.; Liu, X.; et al. Glycometabolic reprogramming-induced XRCC1 lactylation confers therapeutic resistance in ALDH1A3-overexpressing glioblastoma. Cell Metab. 2024, 36, 1696–1710.e10. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, G.; Peng, K.; Song, Y.; Wang, Y.; Zhang, H.; Li, J.; Qiu, X.; Pu, M.; Liu, X.; et al. Lactylation stabilizes TFEB to elevate autophagy and lysosomal activity. J. Cell Biol. 2024, 223, e202308099. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, H.; Zhang, Y.; Yang, X.; Hao, S.; Liu, B.; Zhou, H.; Xu, Z.-X.; Wang, Y. Lactylation stabilizes DCBLD1 activating the pentose phosphate pathway to promote cervical cancer progression. J. Exp. Clin. Cancer Res. 2024, 43, 36. [Google Scholar] [CrossRef]
- Takata, N.; Miska, J.M.; Morgan, M.A.; Patel, P.; Billingham, L.K.; Joshi, N.; Schipma, M.J.; Dumar, Z.J.; Joshi, N.R.; Misharin, A.V.; et al. Lactate-dependent transcriptional regulation controls mammalian eye morphogenesis. Nat. Commun. 2023, 14, 4129. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Jia, G.; Liu, Y.; Lou, Y.; Li, Y.; Xia, M.; Li, H.; Li, W. PKM2 controls cochlear development through lactate-dependent transcriptional regulation. Proc. Natl. Acad. Sci. USA 2025, 122, e2410829122. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, Y.; Li, H.; Zhu, L.; Zeng, L.; Zhang, Z.; Peng, W. Liver as a new target organ in Alzheimer’s disease: Insight from cholesterol metabolism and its role in amyloid-beta clearance. Neural Regen. Res. 2025, 20, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, Z.; Qi, H.; Luo, X.; Wang, M.; Du, Z.; Guo, W. Lactate shuttling links histone lactylation to adult hippocampal neurogenesis in mice. Dev. Cell 2025, 60, 1182–1198.e8. [Google Scholar] [CrossRef]
- Feng, T.; Zhao, X.; Gu, P.; Yang, W.; Wang, C.; Guo, Q.; Long, Q.; Liu, Q.; Cheng, Y.; Li, J.; et al. Adipocyte-derived lactate is a signalling metabolite that potentiates adipose macrophage inflammation via targeting PHD2. Nat. Commun. 2022, 13, 5208. [Google Scholar] [CrossRef]
- Romero, M.; Miller, K.; Gelsomini, A.; Garcia, D.; Li, K.; Suresh, D.; Frasca, D. Immunometabolic effects of lactate on humoral immunity in healthy individuals of different ages. Nat. Commun. 2024, 15, 7515. [Google Scholar] [CrossRef]
- Ayyangar, U.; Karkhanis, A.; Tay, H.; Afandi, A.F.B.; Bhattacharjee, O.; Ks, L.; Lee, S.H.; Chan, J.; Raghavan, S. Metabolic rewiring of macrophages by epidermal-derived lactate promotes sterile inflammation in the murine skin. EMBO J. 2024, 43, 1113–1134. [Google Scholar] [CrossRef]
- Ajam-Hosseini, M.; Heydari, R.; Rasouli, M.; Akhoondi, F.; Asadi Hanjani, N.; Bekeschus, S.; Doroudian, M. Lactic acid in macrophage polarization: A factor in carcinogenesis and a promising target for cancer therapy. Biochem. Pharmacol. 2024, 222, 116098. [Google Scholar] [CrossRef]
- He, J.; Chai, X.; Zhang, Q.; Wang, Y.; Wang, Y.; Yang, X.; Wu, J.; Feng, B.; Sun, J.; Rui, W.; et al. The lactate receptor HCAR1 drives the recruitment of immunosuppressive PMN-MDSCs in colorectal cancer. Nat. Immunol. 2025, 26, 391–403. [Google Scholar] [CrossRef]
- Huang, H.; Wang, S.; Xia, H.; Zhao, X.; Chen, K.; Jin, G.; Zhou, S.; Lu, Z.; Chen, T.; Yu, H.; et al. Lactate enhances NMNAT1 lactylation to sustain nuclear NAD+ salvage pathway and promote survival of pancreatic adenocarcinoma cells under glucose-deprived conditions. Cancer Lett. 2024, 588, 216806. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, D.; Wang, Y.; Liu, M.; Zhang, Y.; Feng, T.; Xiao, C.; Song, H.; Miao, R.; Xu, L.; et al. Lactylated Apolipoprotein C-II Induces Immunotherapy Resistance by Promoting Extracellular Lipolysis. Adv. Sci. 2024, 11, e2406333. [Google Scholar] [CrossRef]
- Hong, H.; Han, H.; Wang, L.; Cao, W.; Hu, M.; Li, J.; Wang, J.; Yang, Y.; Xu, X.; Li, G.; et al. ABCF1-K430-Lactylation promotes HCC malignant progression via transcriptional activation of HIF1 signaling pathway. Cell Death Differ. 2025, 32, 613–631. [Google Scholar] [CrossRef]
- Kemp, S.B.; Carpenter, E.S.; Steele, N.G.; Donahue, K.L.; Nwosu, Z.C.; Pacheco, A.; Velez-Delgado, A.; Menjivar, R.E.; Lima, F.; The, S.; et al. Apolipoprotein E Promotes Immune Suppression in Pancreatic Cancer through NF-κB-Mediated Production of CXCL1. Cancer Res. 2021, 81, 4305–4318. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, H.; Guo, Y.; Chen, Y.; Chen, H.; Liu, Y. X-ray repair cross-complementing protein 1 (XRCC1) loss promotes β-lapachone -induced apoptosis in pancreatic cancer cells. BMC Cancer 2021, 21, 1234. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Xu, J.; Pan, H.; Wang, W.; Yu, X.; Shi, S. Histone lactylation: From tumor lactate metabolism to epigenetic regulation. Int. J. Biol. Sci. 2024, 20, 1833–1854. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, H.; Zhao, P.; Lu, W.; Song, S.; He, T.; Fan, J.; Wang, D.; Yang, P.; Jie, Q.; et al. Targeting sulfation-dependent mechanoreciprocity between matrix and osteoblasts to mitigate bone loss. Sci. Transl. Med. 2023, 15, eadg3983. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, W. Acetylation in the regulation of autophagy. Autophagy 2023, 19, 379–387. [Google Scholar] [CrossRef]
- Huang, H.; Ren, L.; Zhou, Y.; Chen, P.; Zhao, H.; Li, S.; Wang, H.; Li, X. KAT7-acetylated YBX1 promotes hepatocellular carcinoma proliferation by reprogramming nucleotide metabolism. BMC Cancer 2025, 25, 311. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Ren, J. Acetylation in cardiovascular diseases: Molecular mechanisms and clinical implications. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165836. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Dong, X.; Wang, M.; Qian, X.; Hu, M.; Liang, K.; Liang, Y.; Zhang, R.; Huang, Y.; Lyu, H.; et al. Phosphorylated STYK1 restrains the inhibitory role of EGFR in autophagy initiation and EGFR-TKIs sensitivity. Cell Insight. 2022, 1, 100045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, Y.; He, C.; Zhang, X.; Torraca, V.; Wang, S.; Liu, N.; Yang, J.; Liu, S.; Yuan, J.; et al. RUNDC1 inhibits autolysosome formation and survival of zebrafish via clasping ATG14-STX17-SNAP29 complex. Cell Death Differ. 2023, 30, 2231–2248. [Google Scholar] [CrossRef]
- Pittala, S.; Haspula, D.; Cui, Y.; Yang, W.-M.; Kim, Y.-B.; Davis, R.J.; Wing, A.; Rotman, Y.; McGuinness, O.P.; Inoue, A.; et al. G12/13-mediated signaling stimulates hepatic glucose production and has a major impact on whole body glucose homeostasis. Nat. Commun. 2024, 15, 9996. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Houslay, M.D. Arrestin times for compartmentalised cAMP signalling and phosphodiesterase-4 enzymes. Curr. Opin. Cell Biol. 2005, 17, 129–134. [Google Scholar] [CrossRef]
- Bie, B.; Peng, Y.; Zhang, Y.; Pan, Z.Z. cAMP-mediated mechanisms for pain sensitization during opioid withdrawal. J. Neurosci. 2005, 25, 3824–3832. [Google Scholar] [CrossRef]
- Leblais, V.; Jo, S.-H.; Chakir, K.; Maltsev, V.; Zheng, M.; Crow, M.T.; Wang, W.; Lakatta, E.G.; Xiao, R.-P. Phosphatidylinositol 3-kinase offsets cAMP-mediated positive inotropic effect via inhibiting Ca2+ influx in cardiomyocytes. Circ. Res. 2004, 95, 1183–1190. [Google Scholar] [CrossRef]
- Roland, C.L.; Arumugam, T.; Deng, D.; Liu, S.H.; Philip, B.; Gomez, S.; Burns, W.R.; Ramachandran, V.; Wang, H.; Cruz-Monserrate, Z.; et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 2014, 74, 5301–5310. [Google Scholar] [CrossRef]
- Watts, V.J.; Neve, K.A. Sensitization of adenylate cyclase by Galpha i/o-coupled receptors. Pharmacol. Ther. 2005, 106, 405–421. [Google Scholar] [CrossRef]
- Li, Z.; Sun, C.; Qin, Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics 2021, 11, 8322–8336. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, Y.; Li, D.; Wei, J.; Chen, K.; Zhang, E.; Liu, G.; Chu, X.; Liu, X.; Liu, W.; et al. Cancer associated fibroblasts and metabolic reprogramming: Unraveling the intricate crosstalk in tumor evolution. J. Hematol. Oncol. 2024, 17, 80. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Ni, Y.; Shen, P.; Han, X. Lactate shuttle: From substance exchange to regulatory mechanism. Hum. Cell 2022, 35, 1–14. [Google Scholar] [CrossRef]
- Dai, E.; Wang, W.; Li, Y.; Ye, D.; Li, Y. Lactate and lactylation: Behind the development of tumors. Cancer Lett. 2024, 591, 216896. [Google Scholar] [CrossRef]
- Le, A.; Rajeshkumar, N.V.; Maitra, A.; Dang, C.V. Conceptual framework for cutting the pancreatic cancer fuel supply. Clin. Cancer Res. 2012, 18, 4285–4290. [Google Scholar] [CrossRef]
- Hong, C.S.; Graham, N.A.; Gu, W.; Espindola Camacho, C.; Mah, V.; Maresh, E.L.; Alavi, M.; Bagryanova, L.; Krotee, P.A.L.; Gardner, B.K.; et al. MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4. Cell Rep. 2016, 14, 1590–1601. [Google Scholar] [CrossRef]
- Puri, S.; Juvale, K. Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: A review with structure-activity relationship insights. Eur. J. Med. Chem. 2020, 199, 112393. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis. Annu. Rev. Med. 2006, 57, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Ionescu, C.; Oprea, B.; Ciobanu, G.; Georgescu, M.; Bică, R.; Mateescu, G.-O.; Huseynova, F.; Barragan-Montero, V. The Angiogenic Balance and Its Implications in Cancer and Cardiovascular Diseases: An Overview. Medicine 2022, 58, 903. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, H.-X.; Wu, C.-T.; Wang, W.-Q.; Jin, W.; Gao, H.-L.; Li, H.; Zhang, S.-R.; Xu, J.-Z.; Qi, Z.-H.; et al. Angiogenesis in pancreatic cancer: Current research status and clinical implications. Angiogenesis 2019, 22, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Xelwa, N.; Candy, G.P.; Devar, J.; Omoshoro-Jones, J.; Smith, M.; Nweke, E.E. Targeting Growth Factor Signaling Pathways in Pancreatic Cancer: Towards Inhibiting Chemoresistance. Front. Oncol. 2021, 11, 683788. [Google Scholar] [CrossRef]
- Taylor, C.T.; Scholz, C.C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef]
- Zou, J.; Li, P.; Lu, F.; Liu, N.; Dai, J.; Ye, J.; Qu, X.; Sun, X.; Ma, D.; Park, J.; et al. Notch1 is required for hypoxia-induced proliferation, invasion and chemoresistance of T-cell acute lymphoblastic leukemia cells. J. Hematol. Oncol. 2013, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Xu, J.; Zhang, B.; Ji, S.; Xu, W.; Liu, J.; Jin, K.; Liang, D.; Liang, C.; Liu, L.; et al. VEGF Promotes Glycolysis in Pancreatic Cancer via HIF1α Up-Regulation. Curr. Mol. Med. 2016, 16, 394–403. [Google Scholar] [CrossRef]
- Yang, J.; Ren, B.; Yang, G.; Wang, H.; Chen, G.; You, L.; Zhang, T.; Zhao, Y. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol. Life Sci. 2020, 77, 305–321. [Google Scholar] [CrossRef]
- Park, K.C.; Lee, D.C.; Yeom, Y.I. NDRG3-mediated lactate signaling in hypoxia. BMB Rep. 2015, 48, 301–302. [Google Scholar] [CrossRef]
- Feng, C.; Tan, P.; Nie, G.; Zhu, M. Biomimetic and bioinspired nano-platforms for cancer vaccine development. Exploration 2023, 3, 20210263. [Google Scholar] [CrossRef]
- Liu, C.; He, D.; Li, L.; Zhang, S.; Wang, L.; Fan, Z.; Wang, Y. Extracellular vesicles in pancreatic cancer immune escape: Emerging roles and mechanisms. Pharmacol. Res. 2022, 183, 106364. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, Y.; Zhang, J.; Chen, C.; Gao, L.; Shi, C.; Fu, Z.; Han, M.; Tang, C.; Sun, P.; et al. Reversing immune evasion using a DNA nano-orchestrator for pancreatic cancer immunotherapy. Acta Biomater. 2023, 166, 512–523. [Google Scholar] [CrossRef]
- Karamitopoulou, E. Tumour microenvironment of pancreatic cancer: Immune landscape is dictated by molecular and histopathological features. Br. J. Cancer 2019, 121, 5–14. [Google Scholar] [CrossRef]
- Bengsch, F.; Knoblock, D.M.; Liu, A.; McAllister, F.; Beatty, G.L. CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunol. Immunother. 2017, 66, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Huang, X.; Lu, M.; Zhang, G.; Han, X.; Liang, T. Transcriptional control of pancreatic cancer immunosuppression by metabolic enzyme CD73 in a tumor-autonomous and -autocrine manner. Nat. Commun. 2023, 14, 3364. [Google Scholar] [CrossRef]
- Farhangnia, P.; Khorramdelazad, H.; Nickho, H.; Delbandi, A.-A. Current and future immunotherapeutic approaches in pancreatic cancer treatment. J. Hematol. Oncol. 2024, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, H.; Zhang, Y.; Wei, H.; Zhu, Z.; Zhu, B.; Yang, M.; Cao, W.; Wang, L.; Wu, Z. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene 2017, 36, 5829–5839. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Chen, S.; Chen, X.; Wu, T.; Yang, Y.; Li, S.; Ma, J.; Zhao, J.; Lin, W.; Li, W.; et al. M2-TAM subsets altered by lactic acid promote T-cell apoptosis through the PD-L1/PD-1 pathway. Oncol. Rep. 2020, 44, 1885–1894. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, J.; Lu, L.; Deng, D.; Huang, J.; Tang, Z.; Shi, X.; Lo, P.-C.; Lovell, J.F.; Zheng, Y.; et al. Tumor cell membrane-based vaccines: A potential boost for cancer immunotherapy. Exploration 2024, 4, 20230171. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.; Xu, Y.; Zhao, X.; Zhang, Y.; Yang, X.; Wang, Y.; Zhao, R.; Anderson, G.J.; Zhao, Y.; et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat. Commun. 2018, 9, 3390. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Jiang, A.; Lin, A.; Liu, Z.; Cheng, X.; Wang, W.; Cheng, Q.; Zhang, J.; Wei, T.; et al. Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: A review. J. Transl. Med. 2024, 22, 293. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Z.; Yu, X.; Huang, T.; Chen, J.; Wang, J.; Wilhelm, J.; Li, S.; Song, J.; Li, W.; et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat. Commun. 2022, 13, 4981. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Zhou, J.; Lyu, N.; Wang, Q.; Yang, M.; Kimchi, E.T.; Cheng, K.; Joshi, T.; Tukuli, A.R.; Staveley-O’Carroll, K.F.; Li, G. A novel role of TGFBI in macrophage polarization and macrophage-induced pancreatic cancer growth and therapeutic resistance. Cancer Lett. 2023, 578, 216457. [Google Scholar] [CrossRef] [PubMed]
- Kerzel, T.; Giacca, G.; Beretta, S.; Bresesti, C.; Notaro, M.; Scotti, G.M.; Balestrieri, C.; Canu, T.; Redegalli, M.; Pedica, F.; et al. In vivo macrophage engineering reshapes the tumor microenvironment leading to eradication of liver metastases. Cancer Cell 2023, 41, 1892–1910.e10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, H.-W.; Xing, Z.-Q.; Tang, B.; Zhou, X. Lactate induces alternative polarization (M2) of macrophages under lipopolysaccharide stimulation in vitro through G-protein coupled receptor 81. Chin. Med. J. 2020, 133, 1761–1763. [Google Scholar] [CrossRef]
- Qiao, T.; Xiong, Y.; Feng, Y.; Guo, W.; Zhou, Y.; Zhao, J.; Jiang, T.; Shi, C.; Han, Y. Inhibition of LDH-A by Oxamate Enhances the Efficacy of Anti-PD-1 Treatment in an NSCLC Humanized Mouse Model. Front. Oncol. 2021, 11, 632364. [Google Scholar] [CrossRef]
- Tao, H.; Zhong, X.; Zeng, A.; Song, L. Unveiling the veil of lactate in tumor-associated macrophages: A successful strategy for immunometabolic therapy. Front. Immunol. 2023, 14, 1208870. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, J.; Guo, Z. Targeting metabolic pathway enhance CAR-T potency for solid tumor. Int. Immunopharmacol. 2024, 143, 113412. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Hu, Y.; He, G.; Huang, L.; Liu, Y.; Wang, Z.L.; Jiang, P. Enhancing CAR-T cell therapy against solid tumor by drug-free triboelectric immunotherapy. Biomaterials 2025, 314, 122871. [Google Scholar] [CrossRef]
- Goswami, K.K.; Banerjee, S.; Bose, A.; Baral, R. Lactic acid in alternative polarization and function of macrophages in tumor microenvironment. Hum. Immunol. 2022, 83, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Payer, Á.R.; Gonzalez, S.; López-Soto, A. Mechanisms of Apoptosis Resistance to NK Cell-Mediated Cytotoxicity in Cancer. Int. J. Mol. Sci. 2020, 21, 3726. [Google Scholar] [CrossRef]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol. Immunol. 2023, 20, 432–447. [Google Scholar] [CrossRef]
- Jiang, W.; Qiao, L.; Zuo, D.; Qin, D.; Xiao, J.; An, H.; Wang, Y.; Zhang, X.; Jin, Y.; Ren, L. Aberrant lactate dehydrogenase A signaling contributes metabolic signatures in pancreatic cancer. Ann. Transl. Med. 2021, 9, 358. [Google Scholar] [CrossRef]
- Wu, X.; Li, T.; Jiang, R.; Yang, X.; Guo, H.; Yang, R. Targeting MHC-I molecules for cancer: Function, mechanism, and therapeutic prospects. Mol. Cancer 2023, 22, 194. [Google Scholar] [CrossRef]
- Engle, D.D.; Tiriac, H.; Rivera, K.D.; Pommier, A.; Whalen, S.; Oni, T.E.; Alagesan, B.; Lee, E.J.; Yao, M.A.; Lucito, M.S.; et al. The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. Science 2019, 364, 1156–1162. [Google Scholar] [CrossRef]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 2013, 123, 3685–3692. [Google Scholar] [CrossRef]
- Walenta, S.; Wetterling, M.; Lehrke, M.; Schwickert, G.; Sundfør, K.; Rofstad, E.K.; Mueller-Klieser, W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000, 60, 916–921. [Google Scholar]
- Heid, I.; Münch, C.; Karakaya, S.; Lueong, S.S.; Winkelkotte, A.M.; Liffers, S.T.; Godfrey, L.; Cheung, P.F.Y.; Savvatakis, K.; Topping, G.J.; et al. Functional noninvasive detection of glycolytic pancreatic ductal adenocarcinoma. Cancer Metab. 2022, 10, 24. [Google Scholar] [CrossRef]
- Truszkiewicz, A.; Bartusik-Aebisher, D.; Zalejska-Fiolka, J.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Lactate Spectroscopy Using 1.5 Tesla Clinical Apparatus. Int. J. Mol. Sci. 2022, 23, 11355. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Rodriguez-Cruz, V.; Morris, M.E. Cellular Uptake of MCT1 Inhibitors AR-C155858 and AZD3965 and Their Effects on MCT-Mediated Transport of L-Lactate in Murine 4T1 Breast Tumor Cancer Cells. AAPS J. 2019, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Benyahia, Z.; Blackman, M.C.N.M.; Hamelin, L.; Zampieri, L.X.; Capeloa, T.; Bedin, M.L.; Vazeille, T.; Schakman, O.; Sonveaux, P. In Vitro and In Vivo Characterization of MCT1 Inhibitor AZD3965 Confirms Preclinical Safety Compatible with Breast Cancer Treatment. Cancers 2021, 13, 569. [Google Scholar] [CrossRef]
- Fu, Z.; Deng, M.; Zhou, Q.; Li, S.; Liu, W.; Cao, S.; Zhang, L.; Deng, Y.; Xi, S. Arsenic activated GLUT1-mTORC1/HIF-1α-PKM2 positive feedback networks promote proliferation and migration of bladder epithelial cells. Sci. Total Env. 2024, 947, 174538. [Google Scholar] [CrossRef]
- Mondal, S.; Roy, D.; Sarkar Bhattacharya, S.; Jin, L.; Jung, D.; Zhang, S.; Kalogera, E.; Staub, J.; Wang, Y.; Xuyang, W.; et al. Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. Int. J. Cancer 2019, 144, 178–189. [Google Scholar] [CrossRef]
- Encarnación-Rosado, J.; Sohn, A.S.W.; Biancur, D.E.; Lin, E.Y.; Osorio-Vasquez, V.; Rodrick, T.; González-Baerga, D.; Zhao, E.; Yokoyama, Y.; Simeone, D.M.; et al. Targeting pancreatic cancer metabolic dependencies through glutamine antagonism. Nat. Cancer 2024, 5, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Di Magno, L.; Coluccia, A.; Bufano, M.; Ripa, S.; La Regina, G.; Nalli, M.; Di Pastena, F.; Canettieri, G.; Silvestri, R.; Frati, L. Discovery of novel human lactate dehydrogenase inhibitors: Structure-based virtual screening studies and biological assessment. Eur. J. Med. Chem. 2022, 240, 114605. [Google Scholar] [CrossRef]
- Shi, M.; Cui, J.; Du, J.; Wei, D.; Jia, Z.; Zhang, J.; Zhu, Z.; Gao, Y.; Xie, K. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clin. Cancer Res. 2014, 20, 4370–4380. [Google Scholar] [CrossRef]
- Alferiev, I.S.; Guerrero, D.T.; Soberman, D.; Guan, P.; Nguyen, F.; Kolla, V.; Fishbein, I.; Pressly, B.B.; Brodeur, G.M.; Chorny, M. Nanocarrier-Based Delivery of SN22 as a Tocopheryl Oxamate Prodrug Achieves Rapid Tumor Regression and Extends Survival in High-Risk Neuroblastoma Models. Int. J. Mol. Sci. 2022, 23, 1752. [Google Scholar] [CrossRef]
- Mohammad, G.H.; Vassileva, V.; Acedo, P.; Olde Damink, S.W.M.; Malago, M.; Dhar, D.K.; Pereira, S.P. Targeting Pyruvate Kinase M2 and Lactate Dehydrogenase A Is an Effective Combination Strategy for the Treatment of Pancreatic Cancer. Cancers 2019, 11, 1372. [Google Scholar] [CrossRef]
- Manerba, M.; Vettraino, M.; Fiume, L.; Di Stefano, G.; Sartini, A.; Giacomini, E.; Buonfiglio, R.; Roberti, M.; Recanatini, M. Galloflavin (CAS 568-80-9): A novel inhibitor of lactate dehydrogenase. ChemMedChem 2012, 7, 311–317. [Google Scholar] [CrossRef]
- Javaeed, A.; Ghauri, S.K. MCT4 has a potential to be used as a prognostic biomarker—A systematic review and meta-analysis. Oncol. Rev. 2019, 13, 403. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Cerqueira, M.C.; Rosa, B.; Sobral, C.; Pinto-Ribeiro, F.; Costa, M.F.; Baltazar, F.; Afonso, J. Prognostic Value of Monocarboxylate Transporter 1 Overexpression in Cancer: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 5141. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Bryniarski, M.A.; Morris, M.E. In Vitro and In Vivo Efficacy of the Monocarboxylate Transporter 1 Inhibitor AR-C155858 in the Murine 4T1 Breast Cancer Tumor Model. AAPS J. 2018, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Morris, M.E. In Vitro and In Vivo Efficacy of AZD3965 and Alpha-Cyano-4-Hydroxycinnamic Acid in the Murine 4T1 Breast Tumor Model. AAPS J. 2020, 22, 84. [Google Scholar] [CrossRef]
- Beloueche-Babari, M.; Casals Galobart, T.; Delgado-Goni, T.; Wantuch, S.; Parkes, H.G.; Tandy, D.; Harker, J.A.; Leach, M.O. Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br. J. Cancer 2020, 122, 895–903. [Google Scholar] [CrossRef]
- Benjamin, D.; Robay, D.; Hindupur, S.K.; Pohlmann, J.; Colombi, M.; El-Shemerly, M.Y.; Maira, S.-M.; Moroni, C.; Lane, H.A.; Hall, M.N. Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin due to NAD+ Depletion in Cancer Cells. Cell Rep. 2018, 25, 3047–3058.e4. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Shi, Z.; Hou, H.; Yu, Y.; Pan, F.; Ji, J.; Chen, Z. Syrosingopine and UK5099 synergistically suppress non-small cell lung cancer by activating the integrated stress response. Cell Death Dis. 2024, 15, 431. [Google Scholar] [CrossRef]
- Buyse, C.; Joudiou, N.; Warscotte, A.; Richiardone, E.; Mignion, L.; Corbet, C.; Gallez, B. Evaluation of Syrosingopine, an MCT Inhibitor, as Potential Modulator of Tumor Metabolism and Extracellular Acidification. Metabolites 2022, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.; Colombi, M.; Hindupur, S.K.; Betz, C.; Lane, H.A.; El-Shemerly, M.Y.M.; Lu, M.; Quagliata, L.; Terracciano, L.; Moes, S.; et al. Syrosingopine sensitizes cancer cells to killing by metformin. Sci. Adv. 2016, 2, e1601756. [Google Scholar] [CrossRef] [PubMed]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yu, X.; Liang, Y.; Zhu, Y.; He, Y.; Liao, L.; Wang, D.; Yang, Y.; Yin, X.; Li, A.; et al. Targeting PFKL with penfluridol inhibits glycolysis and suppresses esophageal cancer tumorigenesis in an AMPK/FOXO3a/BIM-dependent manner. Acta Pharm. Sin. B 2022, 12, 1271–1287. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Zhu, W.; Ye, L.; Zhang, J.; Yu, P.; Wang, H.; Ye, Z.; Tian, J. PFK15, a Small Molecule Inhibitor of PFKFB3, Induces Cell Cycle Arrest, Apoptosis and Inhibits Invasion in Gastric Cancer. PLoS ONE 2016, 11, e0163768. [Google Scholar] [CrossRef]
- Angiari, S.; Runtsch, M.C.; Sutton, C.E.; Palsson-McDermott, E.M.; Kelly, B.; Rana, N.; Kane, H.; Papadopoulou, G.; Pearce, E.L.; Mills, K.H.G.; et al. Pharmacological Activation of Pyruvate Kinase M2 Inhibits CD4+ T Cell Pathogenicity and Suppresses Autoimmunity. Cell Metab. 2020, 31, 391–405.e8. [Google Scholar] [CrossRef]
- Meng, X.; Lu, Z.; Lv, Q.; Jiang, Y.; Zhang, L.; Wang, Z. Tumor metabolism destruction via metformin-based glycolysis inhibition and glucose oxidase-mediated glucose deprivation for enhanced cancer therapy. Acta Biomater. 2022, 145, 222–234. [Google Scholar] [CrossRef]
- He, Y.-L.; Chen, M.-T.; Wang, T.; Zhang, M.-M.; Li, Y.-X.; Wang, H.-Y.; Ding, N. Development of FABP4/5 inhibitors with potential therapeutic effect on type 2 Diabetes Mellitus. Eur. J. Med. Chem. 2021, 224, 113720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pan, T.; Lee, J.; Goldberg, S.; King, S.A.; Tang, E.; Hu, Y.; Chen, L.; Hoover, A.; Zhu, L.; et al. Enabling tumor-specific drug delivery by targeting the Warburg effect of cancer. Cell Rep. Med. 2025, 6, 101920. [Google Scholar] [CrossRef] [PubMed]

| Function | Name | Reference |
|---|---|---|
| Lactyl-CoA Synthetase | ACSS2 | [61] |
| GTPSCS | [62] | |
| Lactyltransferase | P300 | [63,77] |
| CBP | [64] | |
| GCN5/KAT2A | [61] | |
| MYST1/MOF/KAT8 | [66] | |
| MYST2/HBO1/KAT7 | [65] | |
| TIP60/KAT5 | [67] | |
| AARS1 | [32,68] | |
| AARS2 | [69] | |
| HDAC6 | [70] | |
| Delactylase | HDAC 1-3 | [67,71,76] |
| SIRT 1-3 | [63,73] |
| Therapeutic Approach | Target | Representative Inhibitors | Mechanism | Advantages | Challenges |
|---|---|---|---|---|---|
| Inhibit lactate production | LDHA | Oxamate [163], FX11 [146] | Inhibits LDHA, reducing pyruvate-to-lactate conversion. | Targets the core of glycolytic flux, multiple inhibitors available. | Off-target effects, potential compensatory mechanisms by other LDH isoforms. |
| Inhibit lactate transport | MCT1/4 | AZD3965 [111], Syrosingopine [166] | Blocks lactate efflux, causing intracellular acidification and glycolysis inhibition. | Target lactate export in both cancer and stromal cells, some inhibitors are in clinical trials. | MCT4 affinity issues for some inhibitors, potential hematological toxicity. |
| Target lactylation | AARS1 (Lactyl-transferase), | β-alanine (potential) [32] | Inhibits lactyl-transferase activity, reducing protein lactylation. | Novel epigenetic targeting, potential for high specificity. | Early stage of research, lack of potent and specific clinical inhibitors. |
| HDACs (De-lactylases) | Sodium butyrate [32], TSA [32] | Inhibits de-lactylase activity, potentially hyper-stabilizing lactylated proteins. | Uses existing HDAC inhibitor, dual function (deacetylase/delactylase). | Lack of specificity, complex and unpredictable outcomes. | |
| Target upstream pathways | Glycolysis | 2-DG [167] WZB117 [168] PFK158 [169] | Inhibits key glycolytic enzymes (HK, GLUT1, PFKFB3), reducing lactate source. | Broad impact on cancer metabolism, some compounds in clinical trials. | Affect normal tissues with high glycolytic demand, compensatory metabolism. |
| Glutaminolysis | DRP-104 [170] | Inhibits glutamine metabolism, reducing a key substrate for lactate production. | Targets “glutamine addiction” of PDAC, impacts multiple metabolic pathways. | Systemic glutamine depletion has side effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Guo, D.; Wu, K.; Li, Y.; Xi, Y.; Qin, W.; Chen, X.; Zhou, C.; Tang, J. Lactate Metabolism: The String-Puller for the Development of Pancreatic Cancer. Biology 2025, 14, 1213. https://doi.org/10.3390/biology14091213
Yang L, Guo D, Wu K, Li Y, Xi Y, Qin W, Chen X, Zhou C, Tang J. Lactate Metabolism: The String-Puller for the Development of Pancreatic Cancer. Biology. 2025; 14(9):1213. https://doi.org/10.3390/biology14091213
Chicago/Turabian StyleYang, Lan, Dong Guo, Kangli Wu, Yiqi Li, Yue Xi, Wenying Qin, Xingzhen Chen, Cefan Zhou, and Jingfeng Tang. 2025. "Lactate Metabolism: The String-Puller for the Development of Pancreatic Cancer" Biology 14, no. 9: 1213. https://doi.org/10.3390/biology14091213
APA StyleYang, L., Guo, D., Wu, K., Li, Y., Xi, Y., Qin, W., Chen, X., Zhou, C., & Tang, J. (2025). Lactate Metabolism: The String-Puller for the Development of Pancreatic Cancer. Biology, 14(9), 1213. https://doi.org/10.3390/biology14091213






