From Microbial Consortia to Ecosystem Resilience: The Integrative Roles of Holobionts in Stress Biology
Simple Summary
Abstract
1. Introduction
2. From Primordial Soup to Coevolved Consortia: The Deep Roots of Symbiosis
3. The Plant Microbiome
4. The Animal Microbiome: Establishment, Composition, and Host Interactions
5. On the Interaction Between Different Holobionts
6. How the Environment Influences Holobiont Colonization
7. How the Microbiome Comes to Dominate the Holobiont
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| By | Billion years ago |
| GOE | Great Oxidation Event |
| VOC | Volatile organic compound |
| OGBa | Operational group of Bacillus amyloliquefaciens |
| ABA | Abscisic acid |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| SCFA | Short-chain fatty acids |
| HPA | Hypothalamic–Pituitary–Adrenal (axis) |
| ACC | 1-aminocyclopropane-1-carboxylate |
| GABA | γ-aminobutyric acid |
| IgA | Immunoglobulin A |
References
- Rosenberg, E.; Sharon, G.; Atad, I.; Zilber-Rosenberg, I. The evolution of animals and plants via symbiosis with microorganisms. Environ. Microbiol. Rep. 2010, 2, 500–506. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Theis, K.R. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef]
- Voolstra, C.R.; Suggett, D.J.; Peixoto, R.S.; Parkinson, J.E.; Quigley, K.M.; Silveira, C.B.; Sweet, M.; Muller, E.M.; Barshis, D.J.; Bourne, D.G.; et al. Extending the natural adaptive capacity of coral holobionts. Nat. Rev. Earth Environ. 2021, 2, 747–762. [Google Scholar] [CrossRef]
- Childress, J.J.; Girguis, P.R. The metabolic demands of endosymbiotic chemoautotrophic metabolism on host physiological capacities. J. Exp. Biol. 2011, 214, 312–325. [Google Scholar] [CrossRef]
- Foster, K.R.; Schluter, J.; Coyte, K.Z.; Rakoff-Nahoum, S. The evolution of the host microbiome as an ecosystem on a leash. Nature 2017, 548, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Sudalaimuthuasari, N.; Hazzouri, K.M.; Saeed, E.E.; Shah, I.; Amiri, K.M. Tapping into plant-microbiome interactions through the lens of multi-omics techniques. Cells 2022, 11, 3254. [Google Scholar] [CrossRef]
- Koide, R.T. On holobionts, holospecies, and holoniches: The role of microbial symbioses in ecology and evolution. Microb. Ecol. 2023, 85, 1143–1149. [Google Scholar] [CrossRef]
- Margulis, L. Recombination of non-chromosomal genes in Chlamydomonas: Assortment of mitochondria and chloroplasts? J. Theor. Biol. 1970, 26, 337–342. [Google Scholar] [CrossRef]
- Douglas, A.E. Symbiosis as a general principle in eukaryotic evolution. Cold Spring Harb. Perspect. Biol. 2014, 6, a016113. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.A. Symbiosis as an adaptive process and source of phenotypic complexity. In In the Light of Evolution: Volume I: Adaptation and Complex Design; Avise, J.C., Ayala, F.J., Eds.; National Academies Press: Washington, DC, USA, 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK254296 (accessed on 18 August 2025).
- Ueno, Y.; Yamada, K.; Yoshida, N.; Maruyama, S.; Isozaki, Y. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 2006, 440, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.; Reinhard, C.; Planavsky, N. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 2014, 506, 307–315. [Google Scholar] [CrossRef]
- Gumsley, A.P.; Chamberlain, K.R.; Bleeker, W.; Söderlund, U.; de Kock, M.O.; Larsson, E.R.; Bekker, A. Timing and tempo of the Great Oxidation Event. Proc. Natl. Acad. Sci. USA 2017, 114, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E.; Rosing, M.T.; Bjerrum, C. Early anaerobic metabolisms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1819–1836. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Zilber-Rosenberg, I. Microbes drive evolution of animals and plants: The hologenome concept. mBio 2016, 7, e01395-15. [Google Scholar] [CrossRef] [PubMed]
- Dubilier, N.; Bergin, C.; Lott, C. Symbiotic diversity in marine animals: The art of harnessing chemosynthesis. Nat. Rev. Microbiol. 2008, 6, 725–740. [Google Scholar] [CrossRef]
- Petersen, J.M.; Zielinski, F.U.; Pape, T.; Seifert, R.; Moraru, C.; Amann, R.; Hourdez, S.; Girguis, P.R.; Wankel, S.D.; Barbe, V.; et al. Hydrogen is an energy source for hydrothermal vent symbioses. Nature 2011, 476, 176–180. [Google Scholar] [CrossRef]
- Thomas, T.; Moitinho-Silva, L.; Lurgi, M.; Björk, J.R.; Easson, C.; Astudillo-García, C.; Olson, J.B.; Erwin, P.M.; López-Legentil, S.; Luter, H.; et al. Diversity, structure and convergent evolution of the global sponge microbiome. Nat. Commun. 2016, 7, 11870. [Google Scholar] [CrossRef]
- Moitinho-Silva, L.; Nielsen, S.; Amir, A.; Gonzalez, A.; Ackermann, G.L.; Cerrano, C.; Astudillo-García, C.; Easson, C.; Hernandez-Agreda, A.; Kotoulas, G.; et al. The sponge microbiome project. Gigascience 2017, 6, gix077. [Google Scholar] [CrossRef]
- Holland, H.D. Volcanic gases, black smokers, and the Great Oxidation Event. Geochim. Cosmochim. Acta 2002, 66, 3811–3826. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Ma, Z.; Zuo, T.; Frey, N.; Rangel-Moreno, J.; Kawamoto, S.; Putzel, G.; Chen, Z.; Cox, L.M.; Neu, J.; Yazdi, A.S.; et al. A systematic framework for understanding the microbiome in human health and disease: From basic principles to clinical translation. Signal Transduct. Target. Ther. 2024, 9, 237. [Google Scholar] [CrossRef]
- Woelfel, S.; Silva, M.S.; Stecher, B. Intestinal colonization resistance in the context of environmental, host, and microbial determinants. Cell Host Microbe 2024, 32, 820–836. [Google Scholar] [CrossRef]
- Manzanera, M.; Narváez-Reinaldo, J.J.; González-López, J.; Vílchez, J.I.; Tunnacliffe, A. Synthetic biology and the design of artificial cell-free systems for biotechnology. Front. Bioeng. Biotechnol. 2015, 3, 118. [Google Scholar] [CrossRef]
- Rangseekaew, P.; Barros-Rodríguez, A.; Pathom-aree, W.; Manzanera, M. Deep-sea actinobacteria mitigate salinity stress in tomato seedlings and their biosafety testing. Plants 2021, 10, 1687. [Google Scholar] [CrossRef]
- Kumar, A.; Dubey, A. Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. J. Adv. Res. 2020, 29, 337. [Google Scholar] [CrossRef]
- Pantigoso, H.A.; Newberger, D.; Vivanco, J.M. The rhizosphere microbiome: Plant-microbial interactions for resource acquisition. J. Appl. Microbiol. 2022, 133, 2864–2876. [Google Scholar] [CrossRef] [PubMed]
- Delherbe, N.A.; Gomez, O.; Plominsky, A.M.; Oliver, A.; Manzanera, M.; Kalyuzhnaya, M.G. Atmospheric methane consumption in arid ecosystems acts as a reverse chimney and is accelerated by plant-methanotroph biomes. ISME J. 2025, 19, wraf026. [Google Scholar] [CrossRef]
- Barros-Rodríguez, A.; Pacheco, P.; Peñas-Corte, M.; Fernández-González, A.J.; Cobo-Díaz, J.F.; Enrique-Cruz, Y.; Manzanera, M. Comparative study of Bacillus-based plant biofertilizers: A proposed index. Biology 2024, 13, 668. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.; Schellekens, H.; Cuesta-Marti, C.; Fitzgerald, P.; Cryan, J.F.; Clarke, G.; Dinan, T.G. A menu for microbes: Unraveling appetite regulation and weight dynamics through the microbiota-brain connection across the lifespan. Am. J. Physiol. Gastrointest. Liver Physiol. 2025, 328, G206–G228. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Negrini, D.; Moriondo, A. Interplay between gut lymphatic vessels and microbiota. Cells 2021, 10, 2584. [Google Scholar] [CrossRef]
- Narváez-Reinaldo, J.J.; Barba, I.; González-López, J.; Tunnacliffe, A.; Manzanera, M. Rapid method for isolation of desiccation-tolerant strains and xeroprotectants. Appl. Environ. Microbiol. 2010, 76, 5254–5262. [Google Scholar] [CrossRef]
- Rebolleda-Gómez, M.; Forrester, N.J.; Russell, A.L.; Wei, N.; Fetters, A.M.; Stephens, J.D.; Ashman, T.L. Gazing into the anthosphere: Considering how microbes influence floral evolution. New Phytol. 2019, 224, 1012–1020. [Google Scholar] [CrossRef]
- Martin, V.N.; Schaeffer, R.N.; Fukami, T. Potential effects of nectar microbes on pollinator health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20210155. [Google Scholar] [CrossRef] [PubMed]
- Maurice-Lira, J.V.; Pérez-Moreno, J.; Delgadillo-Martínez, J.; Salcedo-Vite, K. Future perspectives in the study of mutualistic interactions between insects and their microorganisms. Web Ecol. 2025, 25, 39–45. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Grube, M.; Köberl, M. The plant microbiome explored: Implications for experimental botany. J. Exp. Bot. 2016, 67, 995–1002. [Google Scholar] [CrossRef]
- Klein, M.; Stewart, J.D.; Porter, S.S.; Weedon, J.T.; Kiers, E.T. Evolution of manipulative microbial behaviors in the rhizosphere. Evol. Appl. 2022, 15, 1521–1536. [Google Scholar] [CrossRef]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance generated by plant/fungal symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef]
- Walker, V.K.; Palmer, G.R.; Voordouw, G. Freeze-thaw tolerance and clues to the winter survival of a soil community. Appl. Environ. Microbiol. 2006, 72, 1784–1792. [Google Scholar] [CrossRef]
- Kaplan, F.; Lewis, L.A.; Wastian, J.; Holzinger, A. Plasmolysis effects and osmotic potential of two phylogenetically distinct alpine strains of Klebsormidium (Streptophyta). Protoplasma 2013, 249, 789–804. [Google Scholar] [CrossRef]
- Beller, L.; Deboutte, W.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Valles-Colomer, M.; Rymenans, L.; Jansen, D.; Van Espen, L.; Papadaki, M.I.; et al. Successional stages in infant gut microbiota maturation. mBio 2021, 12, e01857-21. [Google Scholar] [CrossRef]
- Chong, H.J.; D’amato, G.; Rosário Filho, N.A. Impact of the environment on the microbiome. J. Pediatr. 2022, 98, 32–37. [Google Scholar] [CrossRef]
- Sarkar, A.; Harty, S.; Johnson, K.V.A.; Moeller, A.H.; Archie, E.A.; Schell, L.D.; Carmody, R.N.; Clutton-Brock, T.H.; Dunbar, R.I.; Burnet, P.W. Microbial transmission in animal social networks and the social microbiome. Nat. Ecol. Evol. 2020, 4, 1020–1035. [Google Scholar] [CrossRef]
- Medvedev, O.; Povarova, O.; Fadeev, N.; Fatima, G.; Zolotikov, U.; Toshchakov, S. Correlation of high gut microbiota archaea methanogenesis with health characteristics of humans and animals. Microorganisms 2025, 13, 48. [Google Scholar] [CrossRef]
- Lima, R.D.; Hajiarbabi, K.; Den Ng, B.; Sood, A.; Ferreira, R.B. Skin-associated commensal microorganisms and their metabolites. J. Appl. Microbiol. 2025, 136, lxaf111. [Google Scholar] [CrossRef] [PubMed]
- Natalini, J.G.; Singh, S.; Segal, L.N. The dynamic lung microbiome in health and disease. Nat. Rev. Microbiol. 2023, 21, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Theis, B.F.; Park, J.S.; Kim, J.S.A.; Zeydabadinejad, S.; Vijay-Kumar, M.; Yeoh, B.S.; Saha, P. Gut feelings: How microbes, diet, and host immunity shape disease. Biomedicines 2025, 13, 1357. [Google Scholar] [CrossRef]
- Light, S.H.; Nagler, C.R. Regulation of immune responses to food by commensal microbes. Immunol. Rev. 2024, 326, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yu, Z.; Liu, Y.; Li, C.; Hu, H.; Hu, J.C.; Liu, M.; Zhao, Y.; Wang, X.; Feng, S.; et al. Gut-X axis. iMeta 2025, 4, e270. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, B.; Liu, X.; Zhang, X.; Zhang, W.; Tian, H.; Qi, L.; Zhang, Y.; Wang, J.; Li, X.; et al. Mesenteric lymph system constitutes the second route in gut-liver axis and transports metabolism-modulating gut microbial metabolites. J. Genet. Genom. 2022, 49, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Swaney, M.H.; Nelsen, A.; Sandstrom, S.; Kalan, L.R. Sweat and sebum preferences of the human skin microbiota. Microbiol. Spectr. 2023, 11, e04180-22. [Google Scholar] [CrossRef]
- Albuquerque, J.; Diniz, L.C.; Machado, A.M.; Lemos, L.; Dias, C.C.; Pereira, C.; Lopes, T.; Henrique, R.; Jerónimo, C.; Magalhães, A.; et al. Microbial uptake in oral mucosa–draining lymph nodes leads to rapid release of cytotoxic CD8+ T cells lacking a gut-homing phenotype. Sci. Immunol. 2022, 7, eabf1861. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A. The skin microbiome: Potential for novel diagnostic and therapeutic approaches to cutaneous disease. Semin. Cutan. Med. Surg. 2014, 33, 98–103. [Google Scholar] [CrossRef]
- Mote, R.S.; Hill, N.S.; Skarlupka, J.H.; Tran, H.M.; Walker, D.I.; Suen, G. The seed endophyte Bacillus amyloliquefaciens RTA 76 modulates the sorghum leaf microbiome. Front. Microbiol. 2020, 11, 1730. [Google Scholar] [CrossRef]
- Adams, S. Microbiome Variation in Production Phenotypes and the Role of Colonization History in Microbiome Establishment. Ph.D. Thesis, University of Nebraska-Lincoln, Lincoln, NE, USA, 2024. [Google Scholar]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; Van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol. Mol. Biol. Rev. 2019, 83, e00007-19. [Google Scholar] [CrossRef]
- Shahbaz, F.; Muccee, F.; Mirza, A.F.; Tanveer, M.A.; Al Haddad, A.H.; Hussain, N. In-vitro assessment of probiotic potential of bacteria isolated from chicken gastrointestinal tract. Antonie Van Leeuwenhoek 2025, 118, 92. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Sinotte, V.M.; Schmidt, S.; Li, G.; Renelies-Hamilton, J.; Koné, N.G.A.; Poulsen, M. Impact of microbial symbionts on fungus-farming termites and their derived ecosystem functions. In Assessing the Microbiological Health of Ecosystems; Springer: Berlin/Heidelberg, Germany, 2022; pp. 185–210. [Google Scholar]
- Bhagwat, P.V.; Ravindran, C.; Irudayarajan, L. Beneficial properties of mucus in coral adaptations and ecological interactions. Mar. Biol. 2024, 171, 46. [Google Scholar] [CrossRef]
- Wizenty, J.; Sigal, M. Helicobacter pylori, microbiota and gastric cancer—Principles of microorganism-driven carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 296–313. [Google Scholar] [CrossRef]
- Shiver, A.L.; Sun, J.; Culver, R.; Violette, A.; Wynter, C.; Nieckarz, M.; Stewart, C.J.; Spinler, J.K.; Hoffman, K.L.; Luna, R.A.; et al. Genome-scale resources in the infant gut symbiont Bifidobacterium breve reveal genetic determinants of colonization and host-microbe interactions. Cell 2025, 188, 2003–2021. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, H. The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front. Plant Sci. 2015, 6, 774. [Google Scholar] [CrossRef]
- Haertl, T.; Owsienko, D.; Schwinn, L.; Hirsch, C.; Eskofier, B.M.; Lang, R.; Wirtz, S.; Loos, H.M. Exploring the interrelationship between the skin microbiome and skin volatiles: A pilot study. Front. Ecol. Evol. 2023, 11, 1107463. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Yu, K.; Feussner, K.; de Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.H.M.; Floková, K.; Barja, M.V.; et al. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222. [Google Scholar] [CrossRef] [PubMed]
- Warrier, M.; Shih, D.M.; Burrows, A.C.; Ferguson, D.; Gromovsky, A.D.; Brown, A.L.; Marshall, S.; McDaniel, A.; Schugar, R.C.; Wang, Z.; et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015, 10, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial volatiles: Small molecules with an important role in intra-and inter-kingdom interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, J.C.; Wang, M.R.; Li, J.W.; Fan, X.; Fazio, G.; Hurtado-Gonzales, O.P.; Postman, J.; Wang, Q.C. Application of biotechniques for in vitro virus and viroid elimination in pome fruit crops. Phytopathology 2024, 114, 930–954. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Yan, L.; Jin, Y.; Sun, L.; Yang, Y.; Zhao, Y.; Wang, Q.C.; Zhao, Z. Different eradication effects of latent viruses by combining thermotherapy with shoot tip culture or cryotherapy in four apple cultivars. Sci. Hortic. 2021, 288, 110356. [Google Scholar] [CrossRef]
- Torres-Cortés, G.; Bonneau, S.; Bouchez, O.; Genthon, C.; Briand, M.; Jacques, M.A.; Barret, M. Functional microbial features driving community assembly during seed germination and emergence. Front. Plant Sci. 2018, 9, 902. [Google Scholar] [CrossRef]
- Scarafoni, A.; Ronchi, A.; Prinsi, B.; Espen, L.; Assante, G.; Venturini, G.; Duranti, M. The proteome of exudates from germinating Lupinus albus seeds is secreted through a selective dual-step process and contains proteins involved in plant defence. FEBS J. 2013, 280, 1443–1459. [Google Scholar] [CrossRef]
- Martins, S.J.; Medeiros, F.H.V.; Lakshmanan, V.; Bais, H.P. Impact of seed exudates on growth and biofilm formation of Bacillus amyloliquefaciens ALB629 in common bean. Front. Microbiol. 2018, 8, 2631. [Google Scholar] [CrossRef]
- Echenique-Subiabre, I.; Jackrel, S.L.; McCarren, J.; James, C.C.; Perez-Coronel, E.; Tran, C.; Glasl, B.; Webster, N.S.; Thomas, T.; Blackall, L.L. Traits determine dispersal and colonization abilities of microbes. Appl. Environ. Microbiol. 2025, 91, e02055-24. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, D.; Maruyama, F. Unveiling microbial highways in the free troposphere. Proc. Natl. Acad. Sci. USA 2024, 121, e2414774121. [Google Scholar] [CrossRef]
- Jansa, J.; Hodge, A. Swimming, gliding, or hyphal riding? On microbial migration along the arbuscular mycorrhizal hyphal highway and functional consequences thereof. New Phytol. 2021, 230, 14–16. [Google Scholar] [CrossRef]
- Tian, M.; Li, Q.; Zheng, T.; Yang, S.; Chen, F.; Guan, W.; Zhang, S. Maternal microbe-specific modulation of the offspring microbiome and development during pregnancy and lactation. Gut Microbes 2023, 15, 2206505. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Huang, L.; Lu, Y.; Jiang, Y.; Luo, Q.; Zhu, J.; Wang, J.; Chen, J.; Hu, X.; Chen, H.; et al. The placental microbiome: A systematic review and meta-analysis. Microorganisms 2025, 13, 95. [Google Scholar] [CrossRef]
- Chanda, D.; De, D. Meta-analysis reveals obesity associated gut microbial alteration patterns and reproducible contributors of functional shift. Gut Microbes 2024, 16, 2304900. [Google Scholar] [CrossRef] [PubMed]
- van Oppen, M.J.H.; Oliver, J.K.; Putnam, H.M.; Gates, R.D. Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 2307–2313. [Google Scholar] [CrossRef]
- Peixoto, R.S.; Rosado, P.M.; Leite, D.C.D.A.; Rosado, A.S.; Bourne, D.G. Beneficial microorganisms for corals (BMC): Proposed mechanisms for coral health and resilience. Front. Microbiol. 2017, 8, 341. [Google Scholar] [CrossRef]
- Liebner, S.; Zeyer, J.; Wagner, D.; Schubert, C.; Pfeiffer, E.M.; Knoblauch, C. Methane oxidation associated with submerged brown mosses reduces methane emissions from Siberian polygonal tundra. J. Ecol. 2011, 99, 914–922. [Google Scholar] [CrossRef]
- Tveit, A.T.; Schwacke, R.; Svenning, M.M.; Urich, T. Organic carbon transformations in high-Arctic peat soils: Key functions and microorganisms. ISME J. 2013, 7, 299–311. [Google Scholar] [CrossRef]
- Mimee, M.; Nadeau, P.; Hayward, A.; Carim, S.; Flanagan, S.; Jerger, L.; Collins, J.; McDonnell, S.; Shuler, R.; Hillson, N.; et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 2016, 360, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, E.S.; DeLoache, W.C.; Pruss, K.M.; Whitaker, W.R.; Sonnenburg, J.L. An exclusive metabolic niche enables strain engraftment in the gut microbiota. Nature 2018, 557, 434–438. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Torres-Barceló, C. The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg. Microbes Infect. 2018, 7, 168. [Google Scholar] [CrossRef]
- Cabral, B.G.; Fernández-González, A.J.; Martín-Platero, A.M.; Valverde-Corredor, A.; Ramos-Solano, B.; García-Villaraco, A.; Gutiérrez-Mañero, F.J.; Manzanera, M. The rhizosphere microbiome: Significance in agriculture and biotechnology. Microorganisms 2020, 8, 1760. [Google Scholar] [CrossRef]
- Yang, L.; Ding, J.; Zhang, C.; Jia, Z.; Zou, H.; Qin, S.; Li, F. Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep. 2005, 23, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Hsiao, E.Y. Microbiomes as sources of emergent host phenotypes. Science 2019, 365, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Curry, K.D.; Wang, Q.; Nute, M.G.; Tyshaieva, A.; Reeves, E.; Soriano, S.; Wu, Q.; Graeber, E.; Finzer, P.; Mendling, W.; et al. Emu: Species-level microbial community profiling of full-length 16S rRNA Oxford Nanopore sequencing data. Nat. Methods 2022, 19, 845–853. [Google Scholar] [CrossRef]
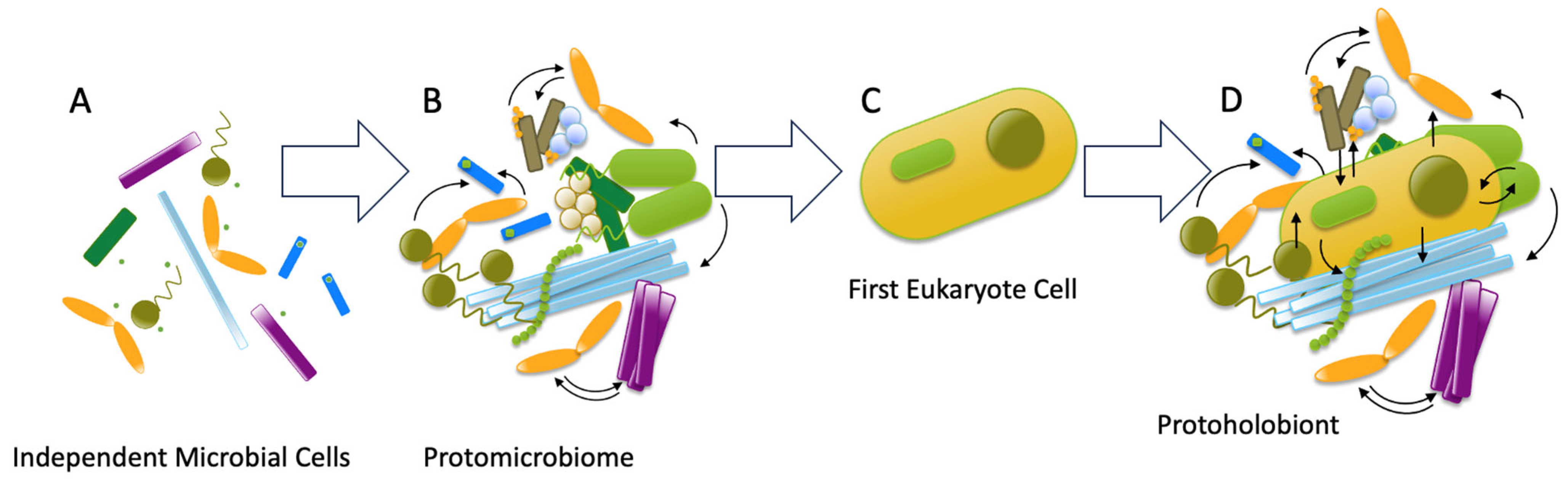

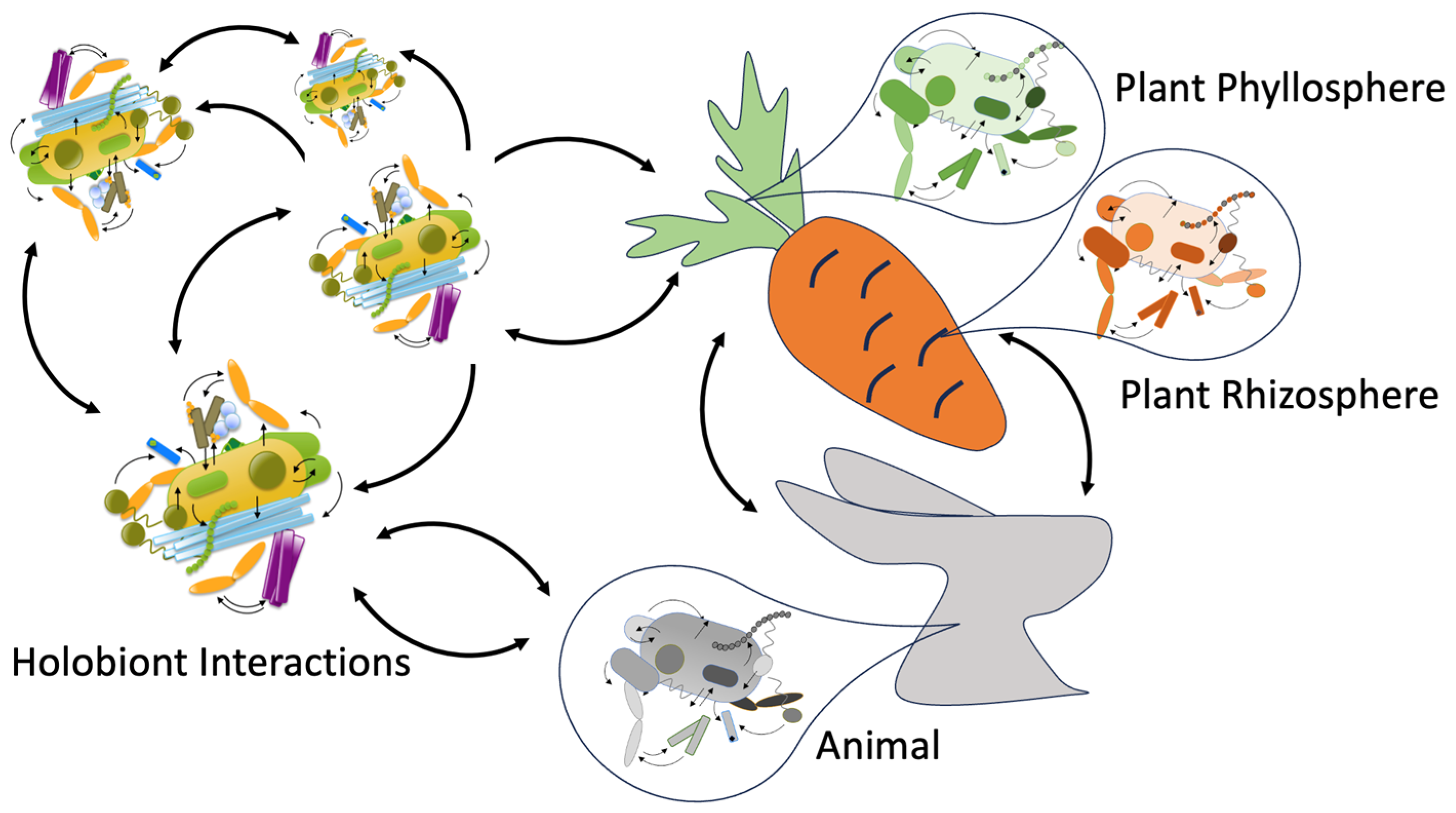
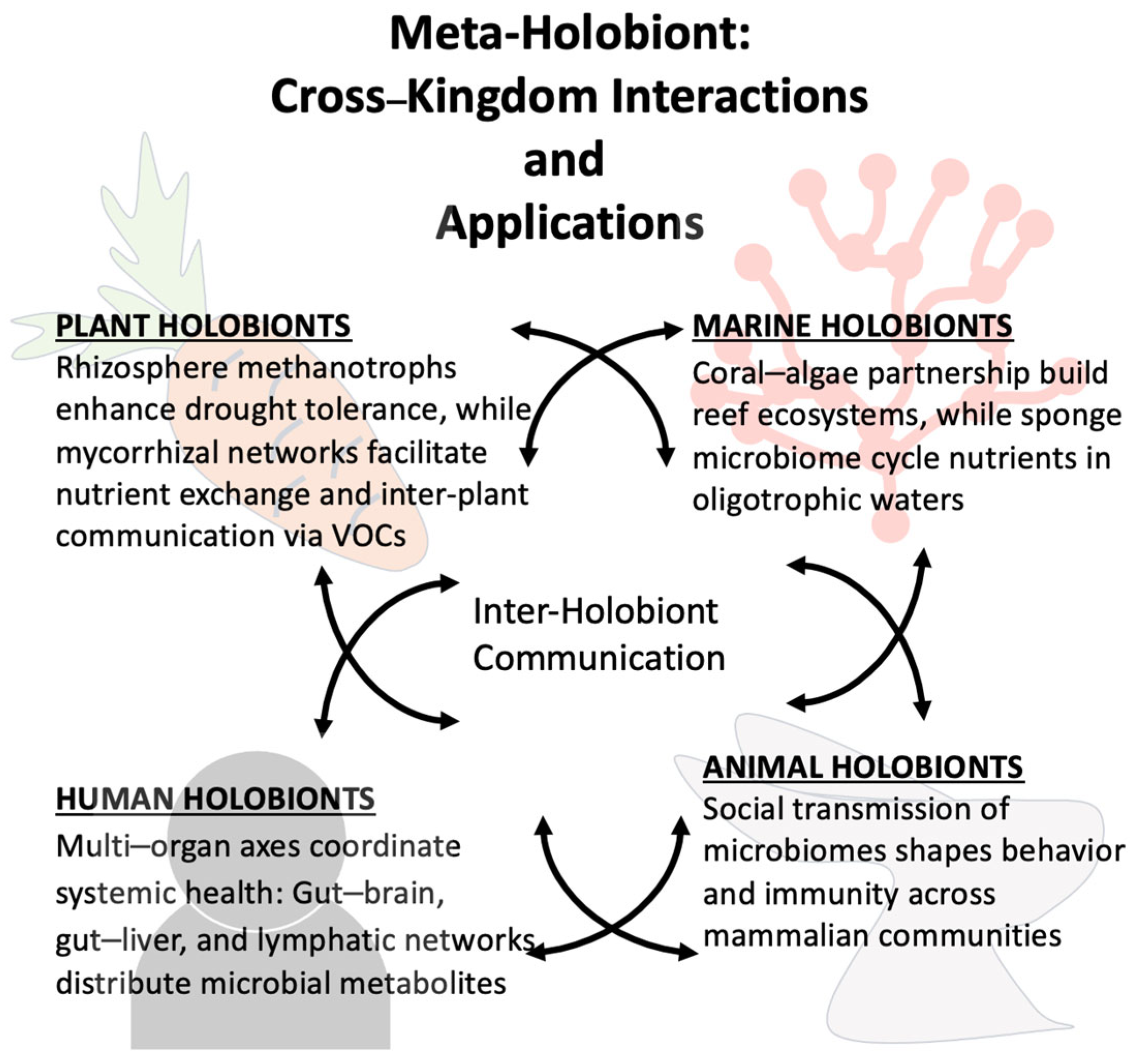
| Stress Type | Host System | Microbiome Role | Mechanism | Outcome | Reference in Text |
|---|---|---|---|---|---|
| Drought | Plants | Methanotrophs (e.g., Methylobacterium) | Oxidize CH4 → CO2 + H2O; methanol production reshapes rhizosphere | Enhanced water retention, stress-responsive genes | Delherbe et al. (2025) [31] |
| Pathogen Defense | Plants | Bacillus velezensis A6 | Antibiotic production, VOC signaling, induction of systemic resistance | Reduced pathogen load, improved survival | Barros-Rodríguez et al. (2024) [32] |
| Nutrient Limitation | Plants | Rhizosphere microbiota (e.g., Pseudomonas) | Phosphate solubilization, siderophore production | Improved nutrient uptake | Manzanera et al. (2015) [27] |
| Obesity | Humans | Gut microbiota (e.g., Alistipes, Bifidobacterium) | SCFA production, gut-barrier integrity, modulation of satiety signals | Metabolic homeostasis | Ribeiro et al. (2025) [33] |
| Neurodegeneration | Humans | Gut–brain axis microbes (e.g., Lactobacillus) | Neurotransmitter synthesis (e.g., GABA, s serotonin), immune modulation | Mood regulation, reduced neuroinflammation | Solari et al. (2021) [34] |
| Type of Interaction | Representative Examples | Mechanisms | Benefits/Costs for Host | Benefits/Costs for Microbe |
|---|---|---|---|---|
| Mutualism (obligate) | Mycorrhizal fungi–plant; Rhizobia–legumes | Nutrient exchange (P solubilization, N2 fixation) | Enhanced nutrient uptake, growth promotion, stress resilience | Access to carbon, stable habitat |
| Mutualism (facultative) | Plant growth-promoting rhizobacteria (Azospirillum, Bacillus) | Hormone production (auxins, cytokinins), ACC deaminase activity | Improved root growth, tolerance to abiotic stress (drought, extreme temperatures) | Rhizosphere colonization, metabolic byproducts used |
| Commesalism | Endophytic bacteria (Enterobacter spp.) | Colonization without major host effect | Neutral; sometimes, indirect growth promotion | Shelter; nutrient leakage |
| Context-dependent symbiosis | Pseudomonas spp. (beneficial vs. opportunistic) | Gene regulation depending on host stress | Can shift from growth promotion to pathogenicity | Survival flexibility under changing host conditions |
| Antagonism (pathogenicity) | Phytophtora infestans (late blight in potatoes) | Tissue invasion, effector secretion | Tissue damage, yield loss | Nutrient extraction, proliferation |
| Cooperation (microbe–microbe) | Pseudomonas–Trichoderma synergism | Cross-feeding, quorum sensing, co-biofilm formation | Enhanced pathogen defense, stress resilience | Expanded niche, survival advantage |
| Environmental engineering | Methanotrophs in arid soils with plants | Oxidation of CH4 → CO2 + H2O | Improved water and carbon availability | Stable ecological niche, energy source |
| Anatomical Region | Microbial Reservoirs (Examples) | Associated Lymph Node | Representative Mechanisms | Potential Physiological Outcomes |
|---|---|---|---|---|
| Gut/Mesentery | Intestinal microbiota (Bacteroides, Lactobacillus, Clostridia) | Mesenteric lymph nodes | Microbial metabolite transport via lymph and portal vein; IgA production | Modulation of hepatic metabolism, immune tolerance, systemic inflammation control |
| Cervical region | Tonsillar crypts (Fusobacterium, Prevotella), nasopharyngeal mucosa (Streptococcus pneumoniae, Neisseria), salivary glands (Streptococcus, Veillonella) | Cervical lymph nodes | Sampling of oral/nasal antigens; induction of mucosal IgA | Local mucosal immunity, regulation of airway inflammation, systemic immune priming |
| Axillary region | Apocrine glands (Corynebacterium, Staphylococcus epidermidis), hair follicles (Cutibacterium acnes, Malassezia), sebaceous glands (Staphylococcus hominidis) | Axillary lymph nodes | Antigen drainage from skin; microbial metabolite processing | Regulation of cutaneous immunity, modulation of host odor cues, pathogen defense (e.g., S. aureus) |
| Inguinal region | Perineal and genital microbiota (Lactobacillus, Gardnerella) | Inguinal lymph nodes | Antigen sampling from mucocutaneous junctions | Maintenance of genital tract immune balance, protection against urogenital pathogens |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzanera, M. From Microbial Consortia to Ecosystem Resilience: The Integrative Roles of Holobionts in Stress Biology. Biology 2025, 14, 1203. https://doi.org/10.3390/biology14091203
Manzanera M. From Microbial Consortia to Ecosystem Resilience: The Integrative Roles of Holobionts in Stress Biology. Biology. 2025; 14(9):1203. https://doi.org/10.3390/biology14091203
Chicago/Turabian StyleManzanera, Maximino. 2025. "From Microbial Consortia to Ecosystem Resilience: The Integrative Roles of Holobionts in Stress Biology" Biology 14, no. 9: 1203. https://doi.org/10.3390/biology14091203
APA StyleManzanera, M. (2025). From Microbial Consortia to Ecosystem Resilience: The Integrative Roles of Holobionts in Stress Biology. Biology, 14(9), 1203. https://doi.org/10.3390/biology14091203








