Acute Restraint Stress Impairs Aversive Memory Retention but Not Memory Formation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Experimental Procedures
2.2. Behavioural Apparatus—Plus-Maze Discriminative Avoidance Task (PMDAT)
2.3. Acute Restraint Stress (ARS) Protocol
2.4. Immunohistochemistry
2.5. Statistical Analysis
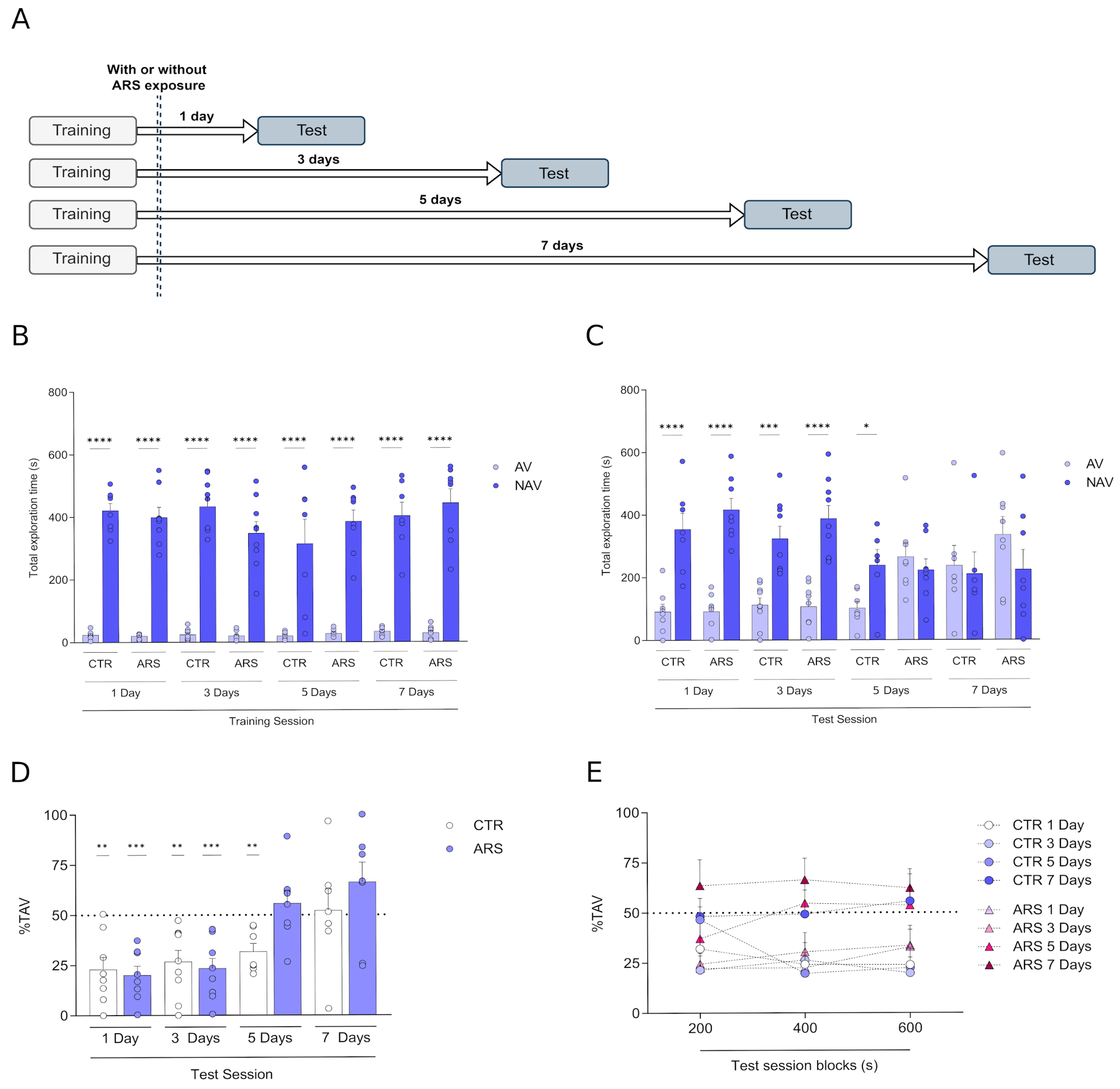
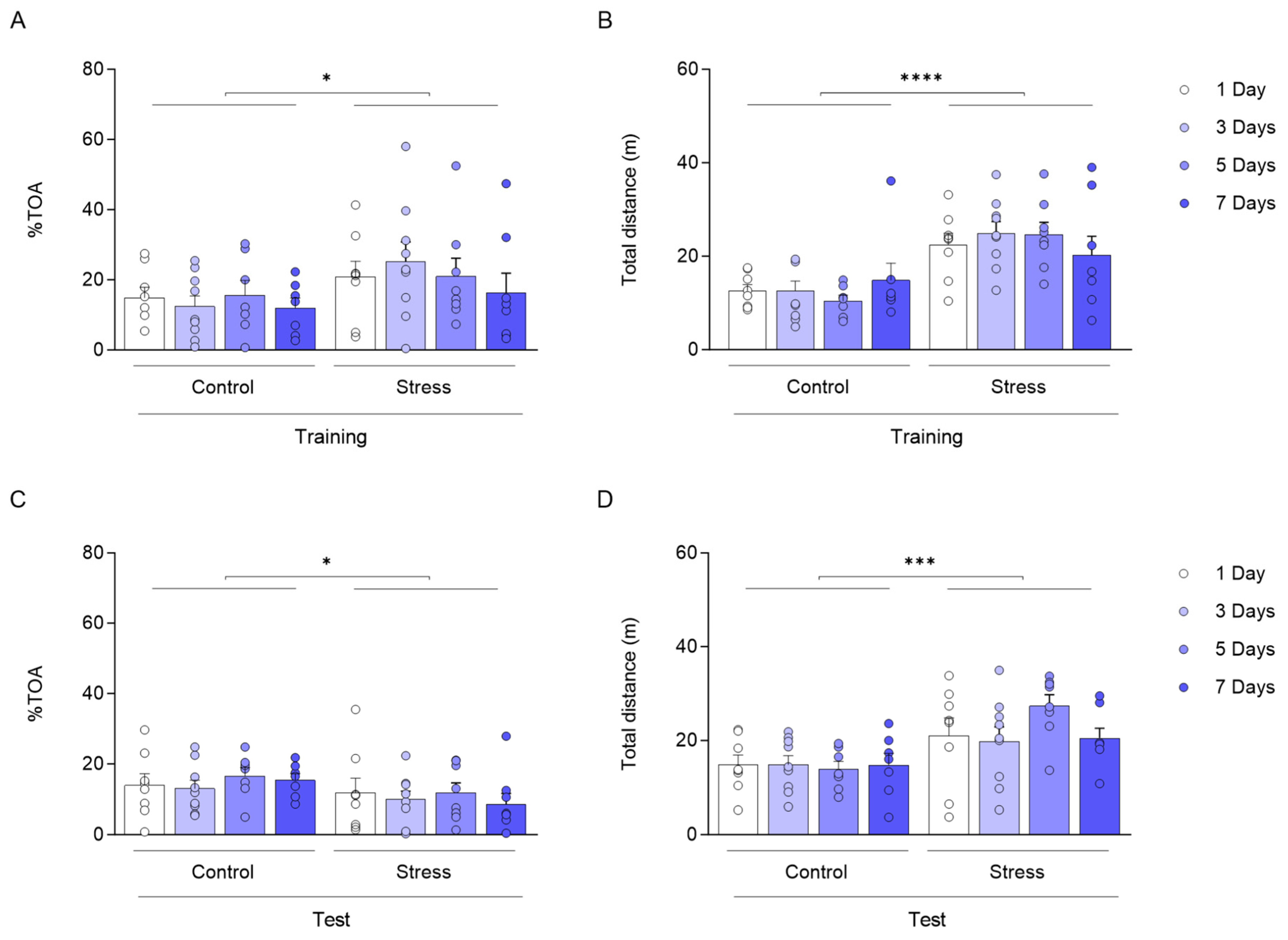
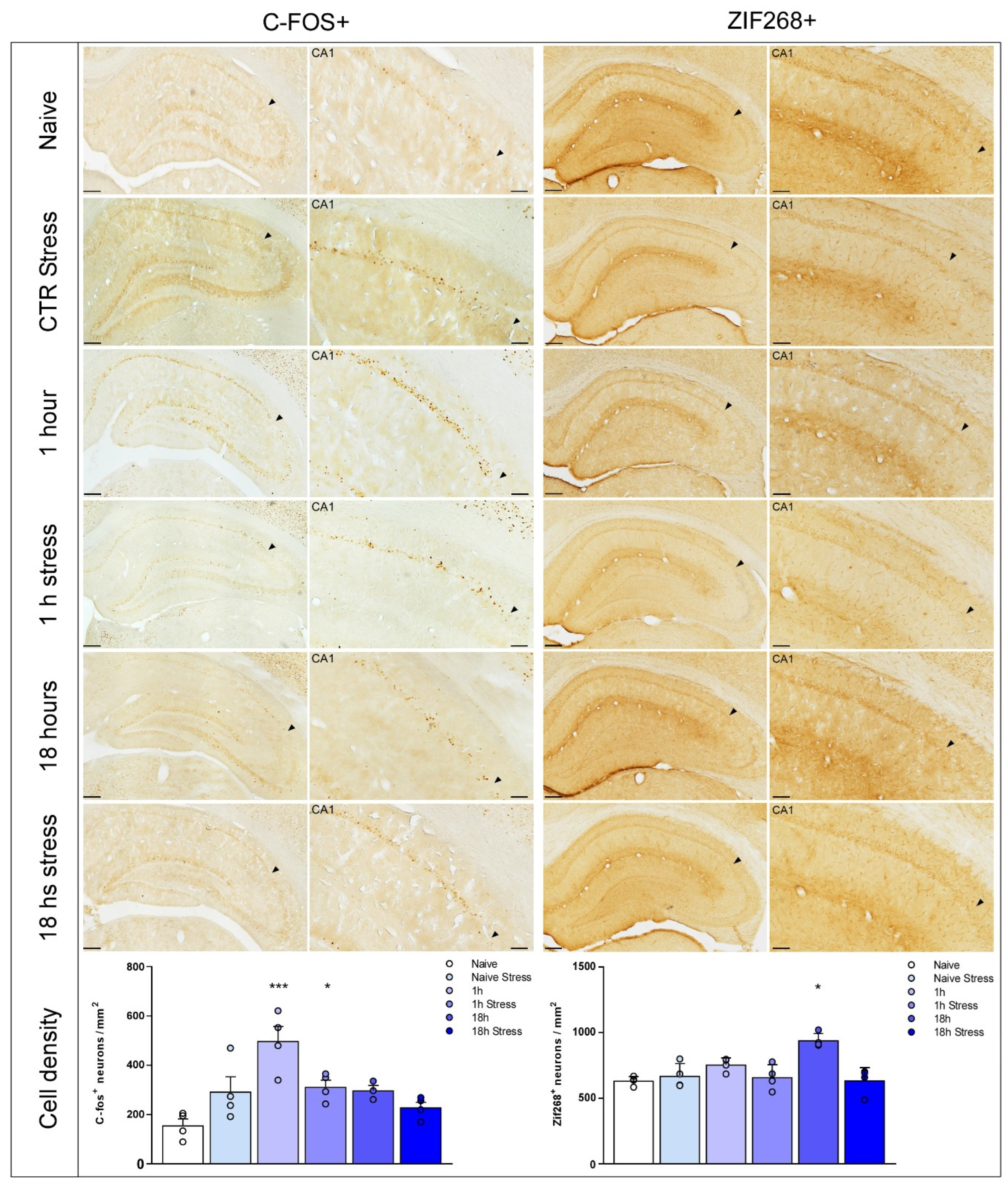
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnsten, A.F. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat. Neurosci. 2015, 18, 1376–1385. [Google Scholar] [CrossRef]
- Bittar, T.P.; Pelaez, M.C.; Hernandez Silva, J.C.; Quessy, F.; Lavigne, A.A.; Morency, D.; Blanchette, L.J.; Arsenault, E.; Cherasse, Y.; Seigneur, J.; et al. Chronic Stress Induces Sex-Specific Functional and Morphological Alterations in Corticoaccumbal and Corticotegmental Pathways. Biol. Psychiatry 2021, 90, 194–205. [Google Scholar] [CrossRef]
- Sandi, C.; Haller, J. Stress and the social brain: Behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015, 16, 290–304. [Google Scholar] [CrossRef]
- Schwabe, L.; Wolf, O.T.; Oitzl, M.S. Memory formation under stress: Quantity and quality. Neurosci. Biobehav. Rev. 2010, 34, 584–591. [Google Scholar] [CrossRef]
- Schwabe, L.; Wolf, O.T. Stress and multiple memory systems: From ‘thinking’ to ‘doing’. Trends Cogn. Sci. 2013, 17, 60–68. [Google Scholar] [CrossRef]
- Shields, G.S.; Sazma, M.A.; McCullough, A.M.; Yonelinas, A.P. The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychol. Bull. 2017, 143, 636–675. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Drexler, S.M.; Wolf, O.T.; Merz, C.J. Stress effects on memory retrieval of aversive and appetitive instrumental counterconditioning in men. Neurobiol. Learn. Mem. 2022, 196, 107697. [Google Scholar] [CrossRef] [PubMed]
- Riggenbach, M.R.; Weiser, J.N.; Mosley, B.E.; Hipskind, J.J.; Wireman, L.E.; Hess, K.L.; Duffy, T.J.; Handel, J.K.; Kaschalk, M.G.; Reneau, K.E.; et al. Immediate pre-learning stress enhances baseline startle response and fear acquisition in a fear-potentiated startle paradigm. Behav. Brain Res. 2019, 371, 111980. [Google Scholar] [CrossRef]
- Vogel, S.; Schwabe, L. Learning and memory under stress: Implications for the classroom. npj Sci. Learn. 2016, 1, 16011. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res. Rev. 1997, 24, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Espejo, P.J.; Ortiz, V.; Martijena, I.D.; Molina, V.A. GABAergic signaling within the Basolateral Amygdala Complex modulates resistance to the labilization/reconsolidation process. Neurobiol. Learn. Mem. 2017, 144, 166–173. [Google Scholar] [CrossRef]
- Hebert, M.A.; Serova, L.I.; Sabban, E.L. Single and repeated immobilization stress differentially trigger induction and phosphorylation of several transcription factors and mitogen-activated protein kinases in the rat locus coeruleus. J. Neurochem. 2005, 95, 484–498. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Endocrinology of the stress-response. In Behavioral Endocrinology, 2nd ed.; Becker, J.B., Breedlove, S.M., Crews, D., McCarthy, M.M., Eds.; MIT Press: Cambridge, MA, USA, 2002; pp. 409–450. ISBN 0-262-02511-6. [Google Scholar]
- Guzowski, J.F. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus 2002, 12, 86–104. [Google Scholar] [CrossRef]
- Katche, C.; Goldin, A.; Gonzalez, C.; Bekinschtein, P.; Medina, J.H. Maintenance of long-term memory storage is dependent on late posttraining Egr-1 expression. Neurobiol. Learn. Mem. 2012, 98, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Revest, J.M.; Di Blasi, F.; Kitchener, P.; Rougé-Pont, F.; Desmedt, A.; Turiault, M.; Piazza, P.V. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat. Neurosci. 2002, 8, 664–672. [Google Scholar] [CrossRef]
- Veyrac, A.; Besnard, A.; Caboche, J.; Davis, S.; Laroche, S. The transcription factor ZIF268/Egr1, brain plasticity, and memory. Prog. Mol. Biol. Transl. Sci. 2014, 122, 89–129. [Google Scholar] [CrossRef]
- de Souza, I.B.M.B.; Meurer, Y.D.S.R.; Tavares, P.M.; Pugliane, K.C.; Lima, R.H.; Silva, R.H.; Barbosa, F.F. Episodic-like memory impairment induced by sub-anaesthetic doses of ketamine. Behav. Brain Res. 2019, 359, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.S.; Tocco, G.; Shors, T.J.; Thompson, R.F. Activation of immediate early genes after acute stress. Neuroreport 1991, 2, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.B.; O’Callaghan, J.P. Aging, stress and the hippocampus. Ageing Res. Rev. 2005, 4, 123–140. [Google Scholar] [CrossRef]
- Silva, R.H.; Frussa-Filho, R. The plus-maze discriminative avoidance task: A new model to study memory-anxiety interactions. Effects of chlordiazepoxide and caffeine. J. Neurosci. Methods 2000, 102, 117–125. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006; ISBN 9780080475134. [Google Scholar]
- Lima, R.H.; Radiske, A.; Köhler, C.A.; Gonzalez, M.C.; Bevilaqua, L.R.; Rossato, J.I.; Cammarota, M. Nicotine modulates the long-lasting storage of fear memory. Learn. Mem. 2013, 20, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Rossato, J.I.; Bevilaqua, L.R.M.; Izquierdo, I.; Medina, J.H.; Cammarota, M. Dopamine controls persistence of long-term memory storage. Science 2009, 325, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Barbosa, F.F.; Munguba, H.; Costa, M.S.; Cavalcante, J.S.; Silva, R.H. Basolateral amygdala inactivation impairs learned (but not innate) fear response in rats. Neurobiol. Learn. Mem. 2011, 95, 433–440. [Google Scholar] [CrossRef]
- Leão, A.H.F.F.; Medeiros, A.M.; Apolinário, G.K.S.; Cabral, A.; Ribeiro, A.M.; Barbosa, F.F.; Silva, R.H. Hippocampal-dependent memory in the plus-maze discriminative avoidance task: The role of spatial cues and CA1 activity. Behav. Brain Res. 2016, 304, 24–33. [Google Scholar] [CrossRef]
- Gilbert, P.E.; Kesner, R.P. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav. Brain Res. 2006, 169, 142–149. [Google Scholar] [CrossRef]
- Gilbert, P.E.; Brushfield, A.M. The role of the CA3 hippocampal subregion in spatial memory: A process oriented behavioral assessment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 774–781. [Google Scholar] [CrossRef]
- Neher, T.; Cheng, S.; Wiskott, L. Memory Storage Fidelity in the Hippocampal Circuit: The Role of Subregions and Input Statistics. PLoS Comput. Biol. 2015, 11, e1004250. [Google Scholar] [CrossRef]
- Amaral, D.G.; Witter, M.P. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 1989, 31, 571–591. [Google Scholar] [CrossRef]
- Andersen, P.; Morris, R.; Amaral, D.; Bliss, T.; O’Keefe, J. The Hippocampus Book; Andersen, P., Ed.; Oxford University Press: Oxford, UK, 2009; ISBN 9780195100273. [Google Scholar]
- Marrone, D.F.; Adams, A.A.; Satvat, E. Increased pattern separation in the aged fascia dentata. Neurobiol. Aging 2011, 33, 2317.e23–2317.e32. [Google Scholar] [CrossRef] [PubMed]
- Satvat, E.; Schmidt, B.; Argraves, M.; Marrone, D.F.; Markus, E.J. Changes in Task Demands Alter the Pattern of ZIF268 Expression in the Dentate Gyrus. J. Neurosci. 2011, 31, 7163–7167. [Google Scholar] [CrossRef]
- Vazdarjanova, A.; Guzowski, J.F. Differences in Hippocampal Neuronal Population Responses to Modifications of an Environmental Context: Evidence for Distinct, Yet Complementary, Functions of CA3 and CA1 Ensembles. J. Neurosci. 2004, 24, 6489–6496. [Google Scholar] [CrossRef]
- Abraham, W.C.; Dragunow, M.; Tate, W.P. The role of immediate early genes in the stabilization of long-term potentiation. Mol. Neurobiol. 1991, 5, 297–314. [Google Scholar] [CrossRef]
- Bozon, B.; Davis, S.; Laroche, S. Regulated transciption of the immediate-early gene ZIF268: Mechanisms and gene dosage-dependent function in synaptic plasticity and memory formation. Hippocampus 2002, 12, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.T.; Katche, C.; Morici, J.F.; Medina, J.H.; Weisstaub, N.V. Immediate Early Genes, Memory and Psychiatric Disorders: Focus on C-FOS, Egr1 and Arc. Front. Behav. Neurosci. 2018, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Um, J.W. Synapse development organized by neuronal activity-regulated immediate-early genes. Exp. Mol. Med. 2018, 50, 1–17. [Google Scholar] [CrossRef]
- Minatohara, K.; Akiyoshi, M.; Okuno, H. Role of Immediate-Early Genes in Synaptic Plasticity and Neuronal Ensembles Underlying the Memory Trace. Front. Mol. Neurosci. 2016, 8, 78. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Igaz, L.M.; Bevilaqua, L.R.; Izquierdo, I.; Medina, J.H. Persistence of Long-Term Memory Storage Requires a Late Protein Synthesis- and BDNF- dependent phase in the hippocampus. Neuron 2007, 53, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Bozon, B.; Laroche, S. How necessary is the activation of the immediate early gene ZIF268 in synaptic plasticity and learning? Behav. Brain Res. 2003, 142, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Katche, C.; Bekinschtein, P.; Slipczuk, L.; Goldin, A.; Izquierdo, I.A.; Cammarota, M.; Medina, J.H. Delayed wave of C-FOS expression in the dorsal hippocampus involved specifically in persistence of long-term memory storage. Proc. Natl. Acad. Sci. USA 2010, 107, 349–354. [Google Scholar] [CrossRef]
- Beckner, V.E.; Tucker, D.M.; Delville, Y.; Mohr, D.C. Stress facilitates consolidation of verbal memory for a film but does not affect retrieval. Behav. Neurosci. 2006, 120, 518–527. [Google Scholar] [CrossRef]
- Buchanan, T.W.; Tranel, D.; Adolphs, R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn. Mem. 2006, 13, 382–387. [Google Scholar] [CrossRef]
- Buchanan, T.W.; Tranel, D. Stress and emotional memory retrieval: Effects of sex and cortisol response. Neurobiol. Learn. Mem. 2008, 89, 134–141. [Google Scholar] [CrossRef]
- Buss, C.; Wolf, O.T.; Witt, J.; Hellhammer, D.H. Autobiographic memory impairment following acute cortisol administration. Psychoneuroendocrinology 2004, 29, 1093–1096. [Google Scholar] [CrossRef]
- Cahill, L.; McGaugh, J.L. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998, 21, 294–299. [Google Scholar] [CrossRef]
- De Quervain, D.J.F.; Roozendaal, B.; McGaugh, J.L. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 1998, 394, 787–790. [Google Scholar] [CrossRef]
- Diamond, D.M.; Bennett, M.C.; Fleshner, M.; Rose, G.M. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus 1992, 2, 421–430. [Google Scholar] [CrossRef]
- Hui, I.R.; Hui, G.K.; Roozendaal, B.; McGaugh, J.L.; Weinberger, N.M. Posttraining handling facilitates memory for auditory-cue fear conditioning in rats. Neurobiol. Learn. Mem. 2006, 86, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, S.; Kirschbaum, C.; Wolf, O.T. Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiol. Learn. Mem. 2005, 83, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Park, C.R.; Zoladz, P.R.; Conrad, C.D.; Fleshner, M.; Diamond, D.M. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn. Mem. 2008, 15, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Preuß, D.; Wolf, O.T. Post-learning psychosocial stress enhances consolidation of neutral stimuli. Neurobiol. Learn. Mem. 2009, 92, 318–326. [Google Scholar] [CrossRef]
- Smeets, T.; Otgaar, H.; Candel, I.; Wolf, O.T. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology 2008, 33, 1378–1386. [Google Scholar] [CrossRef]
- Tollenaar, M.S.; Elzinga, B.M.; Spinhoven, P.; Everaerd, W. Long-Term Outcomes of Memory Retrieval Under Stress. Behav. Neurosci. 2008, 122, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Rocher, C.; Spedding, M.; Munoz, C.; Jay, T.M. Acute Stress-induced Changes in Hippocampal/Prefrontal Circuits in Rats: Effects of Antidepressants. Cereb. Cortex 2004, 14, 224–229. [Google Scholar] [CrossRef]
- Grønli, J.; Bramham, C.; Murison, R.; Kanhema, T.; Fiske, E.; Bjorvatn, B.; Portas, C.M. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol. Biochem. Behav. 2006, 85, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Seo, Y.J.; Choi, S.M.; Choi, H.W.; Jung, J.S.; Park, S.H.; Suh, H.W. The differential effects of single or repeated restraint stress on kainic acid-induced neuronal death in the hippocampal CA3 region: The role of glucocorticoid and various signal molecules. J. Neurochem. 2007, 103, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Imbe, H.; Morikawa, Y.; Kubo, C.; Senba, E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res. 2005, 53, 129–139. [Google Scholar] [CrossRef]
- Jones, M.W.; Errington, M.L.; French, P.J.; Fine, A.; Bliss, T.V.P.; Garel, S.; Davis, S. A requirement for the immediate early gene ZIF268 in the expression of late LTP and long-term memories. Nat. Neurosci. 2001, 4, 289–296. [Google Scholar] [CrossRef]
- Antoine, B.; Serge, L.; Jocelyne, C. Comparative dynamics of MAPK/ERK signalling components and immediate early genes in the hippocampus and amygdala following contextual fear conditioning and retrieval. Brain Struct. Funct. 2014, 219, 415–430. [Google Scholar] [CrossRef]
- Cheval, H.; Chagneau, C.; Levasseur, G.; Veyrac, A.; Faucon-Biguet, N.; Laroche, S.; Davis, S. Distinctive features of Egr transcription factor regulation and DNA binding activity in CA1 of the hippocampus in synaptic plasticity and consolidation and reconsolidation of fear memory. Hippocampus 2012, 22, 631–642. [Google Scholar] [CrossRef]
- Gutierrez-Mecinas, M.; Trollope, A.F.; Collins, A.; Morfett, H.; Hesketh, S.A.; Kersante, F.; Reul, J.M.H.M. Long-lasting behavioral responses to stress involve a direct interaction of glucocorticoid receptors with ERK1/2-MSK1-Elk-1 signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 13806–13811. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dierschnabel, A.L.; Nôga, D.A.; Brandão, L.E.M.; de Mesquita, C.C.; Câmara, D.d.A.; Meurer, Y.d.S.R.; Fiuza, F.P.; Engelberth, R.C.G.J.; da Silva, R.H.; Cavalcante, J.S.; et al. Acute Restraint Stress Impairs Aversive Memory Retention but Not Memory Formation. Biology 2025, 14, 1204. https://doi.org/10.3390/biology14091204
Dierschnabel AL, Nôga DA, Brandão LEM, de Mesquita CC, Câmara DdA, Meurer YdSR, Fiuza FP, Engelberth RCGJ, da Silva RH, Cavalcante JS, et al. Acute Restraint Stress Impairs Aversive Memory Retention but Not Memory Formation. Biology. 2025; 14(9):1204. https://doi.org/10.3390/biology14091204
Chicago/Turabian StyleDierschnabel, Aline Lima, Diana Aline Nôga, Luiz Eduardo Mateus Brandão, Catherine Caldas de Mesquita, Diego de Aquino Câmara, Ywlliane da Silva Rodrigues Meurer, Felipe Porto Fiuza, Rovena Clara Galvão Januário Engelberth, Regina Helena da Silva, Jeferson Souza Cavalcante, and et al. 2025. "Acute Restraint Stress Impairs Aversive Memory Retention but Not Memory Formation" Biology 14, no. 9: 1204. https://doi.org/10.3390/biology14091204
APA StyleDierschnabel, A. L., Nôga, D. A., Brandão, L. E. M., de Mesquita, C. C., Câmara, D. d. A., Meurer, Y. d. S. R., Fiuza, F. P., Engelberth, R. C. G. J., da Silva, R. H., Cavalcante, J. S., & Lima, R. H. (2025). Acute Restraint Stress Impairs Aversive Memory Retention but Not Memory Formation. Biology, 14(9), 1204. https://doi.org/10.3390/biology14091204





