Threats of Climate Change to Freshwater Ecosystems in Pakistan: eDNA Monitoring Will Be the Next-Generation Tool Used in Biodiversity, Conservation, and Management
Abstract
Simple Summary
Abstract
1. Climate Change
2. Climate Change in Pakistan
3. Drivers of Global Climate Change
4. Effects of Climate Change on Freshwater Ecosystems and Fishes
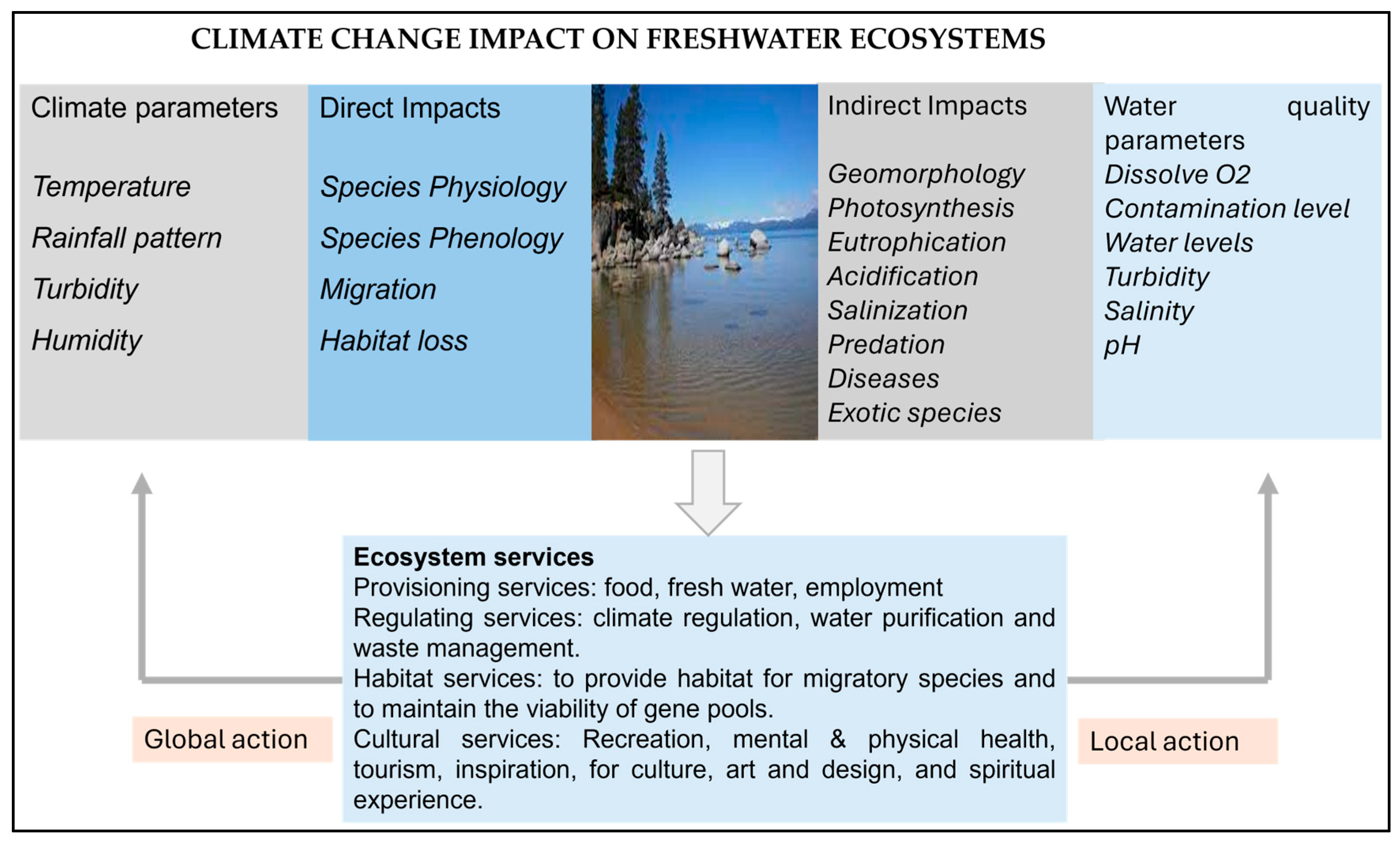
5. Significance of Freshwater Ecosystems
6. Key Threats to Freshwater Ecosystems
7. Freshwater Fishes
8. Freshwater Fishes Response to Climate Change
9. Biotic Components of Freshwater Ecosystems and Climate Change
10. Climate Change and Abiotic Components of Ecosystem
11. Climate Change and Hydrological Cycle
12. eDNA Biomonitoring a Conservation Tool
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, Y.J.; Li, C.Y.; Xiaoming, W.A.N.G.; Wang, Y.; Wang, S.X.; Chang, Y.P.; Qin, J.; Wang, S.P.; Zhao, Q.D.; Wang, Z.R. An overview of climate change impacts on the society in China. Adv. Clim. Chang. Res. 2021, 12, 210–223. [Google Scholar] [CrossRef]
- Yalew, S.G.; van Vliet, M.T.; Gernaat, D.E.; Ludwig, F.; Miara, A.; Park, C.; Byers, E.; De Cian, E.; Piontek, F.; Iyer, G.; et al. Impacts of climate change on energy systems in global and regional scenarios. Nat. Energy 2020, 5, 794–802. [Google Scholar] [CrossRef]
- Rahman, H.A. Climate change scenarios in Malaysia: Engaging the public. Int. J. Malay-Nusant. Stud. 2018, 1, 55–77. [Google Scholar]
- Sintayehu, D.W. Impact of climate change on biodiversity and associated key ecosystem services in Africa: A systematic review. Ecosyst. Health Sustain. 2018, 4, 225–239. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Ndehedehe, C.E.; Ferreira, V.G.; Agutu, N.O.; Onojeghuo, A.O.; Okwuashi, O.; Kassahun, H.T.; Dewan, A. What if the rains do not come? J. Hydrol. 2021, 595, 126040. [Google Scholar] [CrossRef]
- Haile, G.G.; Tang, Q.; Leng, G.; Jia, G.; Wang, J.; Cai, D.; Sun, S.; Baniya, B.; Zhang, Q. Long-term spatiotemporal variation of drought patterns over the Greater Horn of Africa. Sci. Total Environ. 2020, 704, 135299. [Google Scholar] [CrossRef]
- Usman, M.; Ndehedehe, C.E.; Farah, H.; Manzanas, R. Impacts of climate change on the streamflow of a large river basin in the Australian tropics using optimally selected climate model outputs. J. Clean. Prod. 2021, 315, 128091. [Google Scholar] [CrossRef]
- Lenoir, J.; Bertrand, R.; Comte, L.; Bourgeaud, L.; Hattab, T.; Murienne, J.; Grenouillet, G. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 2020, 4, 1044–1059. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Cahill, A.E.; Aiello-Lammens, M.E.; Fisher-Reid, M.C.; Hua, X.; Karanewsky, C.J.; Yeong Ryu, H.; Sbeglia, G.C.; Spagnolo, F.; Waldron, J.B.; Warsi, O.; et al. How does climate change cause extinction? Proc. R. Soc. B Biol. Sci. 2013, 280, 20121890. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar] [CrossRef]
- Benton, M.J. Hyperthermal-driven mass extinctions: Killing models during the Permian–Triassic mass extinction. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170076. [Google Scholar] [CrossRef] [PubMed]
- Dunhill, A.M.; Foster, W.J.; Azaele, S.; Sciberras, J.; Twitchett, R.J. Modelling determinants of extinction across two Mesozoic hyperthermal events. Proc. R. Soc. B 2018, 285, 20180404. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.L.; Hull, P.; Lunt, D.J.; Zachos, J.C. Placing our current ‘hyperthermal’ in the context of rapid climate change in our geological past. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170086. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.P.; Grasby, S.E. On the causes of mass extinctions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 478, 3–29. [Google Scholar] [CrossRef]
- Radchuk, V.; Reed, T.; Teplitsky, C.; van de Pol, M.; Charmantier, A.; Hassall, C.; Adamík, P.; Adriaensen, F.; Ahola, M.P.; Arcese, P.; et al. Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 2019, 10, 3109. [Google Scholar] [CrossRef]
- Roy, H.E.; Peyton, J.; Aldridge, D.C.; Bantock, T.; Blackburn, T.M.; Britton, R.; Clark, P.; Cook, E.; Dehnen-Schmutz, K.; Dines, T.; et al. Horizon scanning for invasive alien species with the potential to threaten biodiversity in Great Britain. Glob. Chang. Biol. 2014, 20, 3859–3871. [Google Scholar] [CrossRef]
- Cook, J.; Nuccitelli, D.; Green, S.A.; Richardson, M.; Winkler, B.; Painting, R.; Way, R.; Jacobs, J.; Skuce, A. Quantifying the consensus on anthropogenic global warming in the scientific literature. Environ. Res. Lett. 2013, 8, 024024. [Google Scholar] [CrossRef]
- Harrod, C. Climate change and freshwater fisheries. In Freshwater Fisheries Ecology; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 641–694. [Google Scholar]
- Collen, B.; Whitton, F.; Dyer, E.E.; Baillie, J.E.; Cumberlidge, N.; Darwall, W.R.; Pollock, C.; Richman, N.I.; Soulsby, A.M.; Böhm, M. Global patterns of freshwater species diversity, threat and endemism. Glob. Ecol. Biogeogr. 2014, 23, 40–51. [Google Scholar] [CrossRef]
- Holmlund, C.M.; Hammer, M. Ecosystem services generated by fish populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Ali, S.; Eum, H.I.; Cho, J.; Dan, L.; Khan, F.; Dairaku, K.; Shrestha, M.L.; Hwang, S.; Nasim, W.; Khan, I.A.; et al. Assessment of climate extremes in future projections downscaled by multiple statistical downscaling methods over Pakistan. Atmos. Res. 2019, 222, 114–133. [Google Scholar] [CrossRef]
- Aziz, N. Hydrological Modeling and Impact of Climate Change:(Historical Overview on Northern Areas of Pakistan). Int. J. Res. Appl. Sci. Eng. Technol. 2020, 8, 326–328. [Google Scholar] [CrossRef]
- Eckstein, D.; Künzel, V.; Schäfer, L.; Winges, M. Global Climate Risk Index 2020; Germanwatch: Bonn, Germany, 2019. [Google Scholar]
- Ritchie, H.; Roser, M.; Rosado, P. CO₂ and Greenhouse Gas Emissions. Our World in Data. 2020. Available online: https://ourworldindata.org/co2-and-greenhouse-gas-emissions (accessed on 5 January 2024).
- Pakistan National Policy on Climate Change. Archived from the original on 5 March 2016. Retrieved 10 May 2015. Available online: https://www.gcisc.org.pk/National_Climate_Change_Policy_2012.pdf (accessed on 5 January 2024).
- Zaheer, K.; Colom, A. How the People of Pakistan Live with Climate Change and What Communication Can Do. 2012. Available online: https://downloads.bbc.co.uk/mediaaction/pdf/climateasia/reports/ClimateAsia_PakistanReport.pdf (accessed on 17 December 2023).
- Chaudhry, Q.U.Z. Climate Change Profile of Pakistan; Asian Development Bank: Metro Manila, Philippines, 2017. [Google Scholar]
- Pakistan|UNDP Climate Change Adaptation. Available online: www.adaptation-undp.org (accessed on 25 November 2020).
- Kouser, S.; Kausar, S.; Ghani, M. COVID-19 lockdown: Current situation and challenges facing in Pakistan during lockdown. Biomedica 2020, 36, 138–144. [Google Scholar] [CrossRef]
- Rannard, G. How Pakistan floods are linked to climate change. BBC, 2 September 2022. [Google Scholar]
- Sands, L. Pakistan floods: One third of country is under water—Minister. BBC News, 29 August 2022. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Dat. 2018, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hausfather, Z.; Peters, G.P. Emissions–the ‘business as usual’ story is misleading. Nature 2020, 577, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, K.C.; Agache, I.; Jutel, M.; Annesi Maesano, I.; Akdis, M.; Sampath, V.; d’Amato, G.; Cecchi, L.; Traidl-Hoffmann, C.; Akdis, C.A. Climate change: A call to action for the United Nations. Allergy 2022, 77, 1087–1090. [Google Scholar] [CrossRef]
- Audi, M.; Ali, A.; Kassem, M. Greenhouse gases: A review of losses and benefits. Int. J. Energy Econ. Policy 2020, 10, 403–418. [Google Scholar] [CrossRef]
- Marvel, K.; Su, W.; Delgado, R.; Aarons, S.; Chatterjee, A.; Garcia, M.E.; Hausfather, Z.; Hayhoe, K.; Hence, D.A.; Jewett, E.B.; et al. Chapter 2: Climate trends. In Fifth National Climate Assessment; USGCRP (U.S. Global Change Research Program): Washington, DC, USA, 2023. [Google Scholar]
- World Metrological Organization (WMO). Greenhouse Gas Concentrations Hit Record High Again. 2024. Available online: https://wmo.int/news/media-centre/greenhouse-gas-concentrations-hit-record-high-again (accessed on 28 July 2023).
- IPCC (Intergovernmental Panel on Climate Change). Climate Change 2022—Mitigation of Climate Change. Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., Fradera, R., Pathak, M., Khourdajie, A.A., Belkacemi, M., van Diemen, R., Hasija, A., Lisboa, G., et al., Eds.; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Bolster, C.H.; Mitchell, R.; Kitts, A.; Campbell, A.; Cosh, M.; Farrigan, T.L.; Franzluebbers, A.J.; Hoover, D.L.; Jin, V.L.; Peck, D.E.; et al. Chapter 11: Agriculture, food systems, and rural communities. In Fifth National Climate Assessment; USGCRP (U.S. Global Change Research Program): Washington, DC, USA, 2023. Available online: https://nca2023.globalchange.gov/chapter/11 (accessed on 16 January 2024).
- United Nations. Climate Actions Note. 2021. Available online: https://www.unep.org/explore-topics/climate-action/what-we-do/climate-action-note/state-of-climate.html (accessed on 16 January 2024).
- Tawiah, K.; Daniyal, M.; Qureshi, M. Pakistan CO2 emission modelling and forecasting: A linear and nonlinear time series approach. J. Environ. Public Health 2023, 2023, 5903362. [Google Scholar] [CrossRef]
- Opinion: Is Pakistan Really Phasing out Coal? The Third Pole. Retrieved 4 March 2021. Available online: https://dialogue.earth/en/energy/opinion-is-pakistan-really-phasing-out-coal/ (accessed on 25 November 2023).
- Hermoso, V.; Clavero, M. Revisiting ecological integrity 30 years later: Non-native species and the misdiagnosis of freshwater ecosystem health. Fish Fish. 2013, 14, 416–423. [Google Scholar] [CrossRef]
- Crozier, L.G.; Hutchings, J.A. Plastic and evolutionary responses to climate change in fish. Evol. Appl. 2014, 7, 68–87. [Google Scholar] [CrossRef]
- Logez, M.; Pont, D. Global warming and potential shift in reference conditions: The case of functional fish-based metrics. Hydrobiologia 2013, 704, 417–436. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Jones, P.D.; Ambenje, P.; Bojariu, R.; Easterling, D.; Tank, A.K.; Parker, D.; Rahimzadeh, F.; Renwick, J.A.; Rusticucci, M.; et al. Observations: Surface and atmospheric climate change. Chapter 3. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group 1 to the 4th Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Lough, J.M.; Hobday, A.J. Observed climate change in Australian marine and freshwater environments. Mar. Freshw. Res. 2011, 62, 984–999. [Google Scholar] [CrossRef]
- Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. (Eds.) Climate Change and Water. Technical Paper of the Intergovernmental Panel on Climate Change; IPCC Secretariat: Geneva, Switzerland, 2008; 210p. [Google Scholar]
- Rosenzweig, C.; Casassa, G.; Karoly, D.J.; Imeson, A.; Liu, C.; Menzel, A.; Rawlins, S.; Root, T.L.; Seguin, B.; Tryjanowski, P.; et al. Assessment of observed changes and responses in natural and managed systems. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, N.; et al. Global climate projections. Chapter 10. In Climate Change 2007. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. (Eds.) Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Mora, C.; Frazier, A.G.; Longman, R.J.; Dacks, R.S.; Walton, M.M.; Tong, E.J.; Sanchez, J.J.; Kaiser, L.R.; Stender, Y.O.; Anderson, J.M.; et al. The projected timing of climate departure from recent variability. Nature 2013, 502, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Welcomme, R.L.; Cowx, I.G.; Coates, D.; Béné, C.; Funge-Smith, S.; Halls, A.; Lorenzen, K. Inland capture fisheries. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2881–2896. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; Gonza’lez-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.J.A.; De Meester, L.; Sondergaard, M.; Lauridsen, T.L.; Bjerring, R.; et al. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Lynch, A.J.; Myers, B.J.; Chu, C.; Eby, L.A.; Falke, J.A.; Kovach, R.P.; Krabbenhoft, T.J.; Kwak, T.J.; Lyons, J.; Paukert, C.P.; et al. Climate change effects on North American inland fish populations and assemblages. Fisheries 2016, 41, 346–361. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Trumbo, B.A.; Nislow, K.H.; Stallings, J.; Hudy, M.; Smith, E.P.; Kim, D.Y.; Wiggins, B.; Dolloff, C.A. Ranking site vulnerability to increasing temperatures in southern Appalachian brook trout streams in Virginia: An exposure-sensitivity approach. Trans. Am. Fish. Soc. 2014, 143, 173–187. [Google Scholar] [CrossRef]
- Warren, D.R.; Robinson, J.M.; Josephson, D.C.; Sheldon, D.R.; Kraft, C.E. Elevated summer temperatures delay spawning and reduce redd construction for resident brook trout (S alvelinus fontinalis). Glob. Chang. Biol. 2012, 18, 1804–1811. [Google Scholar] [CrossRef]
- Ward, E.J.; Anderson, J.H.; Beechie, T.J.; Pess, G.R.; Ford, M.J. Increasing hydrologic variability threatens depleted anadromous fish populations. Glob. Chang. Biol. 2015, 21, 2500–2509. [Google Scholar] [CrossRef]
- Poff, N.L.; Brinson, M.M.; Day, J.W. Aquatic Ecosystems and Global Climate Change; Pew Center on Global Climate Change: Arlington, VA, USA, 2002; Volume 44, pp. 1–36. [Google Scholar]
- Brooks, R.T. Potential impacts of global climate change on the hydrology and ecology of ephemeral freshwater systems of the forests of the northeastern United States. Clim. Chang. 2009, 95, 469–483. [Google Scholar] [CrossRef]
- Lawrence, D.J.; Stewart-Koster, B.; Olden, J.D.; Ruesch, A.S.; Torgersen, C.E.; Lawler, J.J.; Butcher, D.P.; Crown, J.K. The interactive effects of climate change, riparian management, and a nonnative predator on stream-rearing salmon. Ecol. Appl. 2014, 24, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Muhlfeld, C.C.; Kovach, R.P.; Jones, L.A.; Al-Chokhachy, R.; Boyer, M.C.; Leary, R.F.; Lowe, W.H.; Luikart, G.; Allendorf, F.W. Invasive hybridization in a threatened species is accelerated by climate change. Nat. Clim. Chang. 2014, 4, 620–624. [Google Scholar] [CrossRef]
- Karvonen, A.; Rintamäki, P.; Jokela, J.; Valtonen, E.T. Increasing water temperature and disease risks in aquatic systems: Climate change increases the risk of some, but not all, diseases. Int. J. Parasitol. 2010, 40, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, S. (Ed.) Impacts of Climate Change on Fish Species and Aquatic Ecosystems in the Great Lakes and Prairie Regions of Canada: A Compilation of Reports; Canaian Manuscript Report of Fisheries and Aquatic Sciences 3108; Fisheries and Oceans Canada: Québec City, QC, Canada, 2016. [Google Scholar]
- Shen, M.; Xiao, N.; Zhao, Z.; Guo, N.; Luo, Z.; Sun, G.; Li, J. eDNA metabarcoding as a promising conservation tool to monitor fish diversity in Beijing water systems compared with ground cages. Sci. Rep. 2022, 12, 11113. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Javed, M. Relationships among increase in biomass, planktonic productivity and physico-chemistry of ponds stocked with Zn+ Pb+ Mn mixture stressed fish. JAPS J. Anim. Plant Sci. 2019, 29, 125–131. [Google Scholar]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Almond, R.E.; Grooten, M.; Peterson, T. Living Planet Report 2020-Bending the Curve of Biodiversity Loss; World Wildlife Fund: Glans, Switzerland, 2020. [Google Scholar]
- Williams, J.E.; Neville, H.M.; Haak, A.L.; Colyer, W.T.; Wenger, S.J.; Bradshaw, S. Climate change adaptation and restoration of western trout streams: Opportunities and strategies. Fisheries 2015, 40, 304–317. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Mata, L.J.; Arnell, N.W.; Doll, P.; Kabat, P.; Jimenez, B.; Miller, K.A.; Oki, T.; Sen, Z.; Shiklomanov, I. Freshwater resources and their management. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Caissie, D. The thermal regime of rivers: A review. Freshw. Biol. 2006, 51, 1389–1406. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Stanley, E.H.; Vander Zanden, M.J. State of the World’s freshwater ecosystems: Physical, chemical, and biological changes. Annu. Rev. Environ. Resour. 2011, 36, 75–99. [Google Scholar] [CrossRef]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The impacts of climate change on fish growth: A summary of conducted studies and current knowledge. Ecol. Indic. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Ahmad, M.; Hashmi, R.S. Global Climatic Transformation: Implications For Pakistan. Pak. J. Soc. Res. 2023, 5, 1113–1123. [Google Scholar] [CrossRef]
- Fahad, S.; Wang, J. Climate change, vulnerability, and its impacts in rural Pakistan: A review. Environ. Sci. Pollut. Res. 2020, 27, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.E.; Zachariah, M.; Saeed, F.; Siddiqi, A.; Kamil, S.; Mushtaq, H.; Arulalan, T.; AchutaRao, K.; Chaithra, S.T.; Barnes, C.; et al. Climate change increased extreme monsoon rainfall, flooding highly vulnerable communities in Pakistan. Environ. Res. Clim. 2023, 2, 025001. [Google Scholar] [CrossRef]
- Talbot, C.J.; Bennett, E.M.; Cassell, K.; Hanes, D.M.; Minor, E.C.; Paerl, H.; Raymond, P.A.; Vargas, R.; Vidon, P.G.; Wollheim, W.; et al. The impact of flooding on aquatic ecosystem services. Biogeochemistry 2018, 141, 439–461. [Google Scholar] [CrossRef] [PubMed]
- Death, R.G.; Fuller, I.C.; Macklin, M.G. Resetting the river template: The potential for climate-related extreme floods to transform river geomorphology and ecology. Freshw. Biol. 2015, 60, 2477–2496. [Google Scholar] [CrossRef]
- Aldous, A.; Fitzsimons, J.; Richter, B.; Bach, L. Droughts, floods and freshwater ecosystems: Evaluating climate change impacts and developing adaptation strategies. Mar. Freshw. Res. 2011, 62, 223–231. [Google Scholar] [CrossRef]
- Siddha, S.; Sahu, P. Impact of climate change on the river ecosystem. In Ecological Significance of River Ecosystems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 79–104. [Google Scholar]
- Fausch, K.D.; Taniguchi, Y.; Nakano, S.; Grossman, G.D.; Townsend, C.R. Flood disturbance regimes influence rainbow trout invasion success among five holarctic regions. Ecol. Appl. 2001, 11, 1438–1455. [Google Scholar] [CrossRef]
- Bănăduc, D.; Curtean-Bănăduc, A.; Barinova, S.; Lozano, V.L.; Afanasyev, S.; Leite, T.; Branco, P.; Gomez Isaza, D.F.; Geist, J.; Tegos, A.; et al. Multi-Interacting Natural and Anthropogenic Stressors on Freshwater Ecosystems: Their Current Status and Future Prospects for 21st Century. Water 2024, 16, 1483. [Google Scholar] [CrossRef]
- Carosi, A.; Lorenzoni, F.; Lorenzoni, M. Synergistic Effects of Climate Change and Alien Fish Invasions in Freshwater Ecosystems: A Review. Fishes 2023, 8, 486. [Google Scholar] [CrossRef]
- Hulme, P.E. Climate change and biological invasions: Evidence, expectations, and response options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef]
- Lutz, A.F.; Immerzeel, W.W.; Kraaijenbrink, P.D.; Shrestha, A.B.; Bierkens, M.F. Climate change impacts on the upper Indus hydrology: Sources, shifts and extremes. PLoS ONE 2016, 11, e0165630. [Google Scholar] [CrossRef] [PubMed]
- Radić, V.; Hock, R. Glaciers in the Earth’s hydrological cycle: Assessments of glacier mass and runoff changes on global and regional scales. Surv. Geophys. 2014, 35, 813–837. [Google Scholar] [CrossRef]
- Nie, Y.; Pritchard, H.D.; Liu, Q.; Hennig, T.; Wang, W.; Wang, X.; Liu, S.; Nepal, S.; Samyn, D.; Hewitt, K.; et al. Glacial change and hydrological implications in the Himalaya and Karakoram. Nat. Rev. Earth Environ. 2021, 2, 91–106. [Google Scholar] [CrossRef]
- Maharana, P.; Agnihotri, R.; Dimri, A.P. Changing Indian monsoon rainfall patterns under the recent warming period 2001–2018. Clim. Dyn. 2021, 57, 2581–2593. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Zhang, X.; Adnan, M.; Badi, W.; Dereczynski, C.; Luca, A.D.; Ghosh, S.; Iskandar, I.; Kossin, J.; Lewis, S.; et al. Weather and climate extreme events in a changing climate. In Climate Change 2021: The Physical Science Basis. Working Group I contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Garcia, C.; Schumann, D.A.; Howell, J.; Graeb, B.D.; Bertrand, K.N.; Klumb, R.A. Seasonality, floods and droughts structure larval fish assemblages in prairie rivers. Ecol. Freshw. Fish 2018, 27, 389–397. [Google Scholar] [CrossRef]
- Jean de la Paix, M.; Lanhai, L.; Xi, C.; Varenyam, A.; Anming, B. Study of impacts of floods on the water quality in an arid zone: The case of the Tarim River in Northwest China. Water Sci. Technol. 2011, 64, 1973–1979. [Google Scholar] [CrossRef]
- Qin, G.; Liu, J.; Xu, S.; Sun, Y. Pollution source apportionment and water quality risk evaluation of a drinking water reservoir during flood seasons. Int. J. Environ. Res. Public Health 2021, 18, 1873. [Google Scholar] [CrossRef]
- Aldardasawi, A.M.; Eren, B. Floods and their impact on the environment. Acad. Perspect. Procedia 2021, 4, 42–49. [Google Scholar] [CrossRef]
- Mosley, L.M. Drought impacts on the water quality of freshwater systems; review and integration. Earth-Sci. Rev. 2015, 140, 203–214. [Google Scholar] [CrossRef]
- Obedzinski, M.; Nossaman Pierce, S.; Horton, G.E.; Deitch, M.J. Effects of flow-related variables on oversummer survival of juvenile Coho salmon in intermittent streams. Trans. Am. Fish. Soc. 2018, 147, 588–605. [Google Scholar] [CrossRef]
- Damseth, S.; Thakur, K.; Kumar, R.; Kumar, S.; Mahajan, D.; Kumari, H.; Sharma, D.; Sharma, A.K. Assessing the impacts of river bed mining on aquatic ecosystems: A critical review of effects on water quality and biodiversity. HydroResearch 2024, 7, 122–130. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Khani, S.; Rajaee, T. Modeling of dissolved oxygen concentration and its hysteresis behavior in rivers using wavelet transform-based hybrid models. CLEAN–Soil Air Water 2017, 45. [Google Scholar] [CrossRef]
- Rajesh, M.; Rehana, S. Impact of climate change on river water temperature and dissolved oxygen: Indian riverine thermal regimes. Sci. Rep. 2022, 12, 9222. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Zhao, L.L.; Liao, L.; Tang, X.H.; Cui, C.; Liu, Q.; He, K.; Ma, J.D.; Jin, L.; Yan, T.; et al. Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity and promotion of cell apoptosis. Fish Shellfish. Immunol. 2020, 98, 923–936. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Ilarri, M.; Souza, A.T.; Dias, E.; Antunes, C. Influence of climate change and extreme weather events on an estuarine fish community. Sci. Total Environ. 2022, 827, 154190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Q.; Lin, Y.; Zhang, J.; Xia, J.; Ni, J.; Cooke, S.J.; Best, J.; He, S.; Feng, T.; et al. River damming impacts on fish habitat and associated conservation measures. Rev. Geophys. 2023, 61, e2023RG000819. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Bibi, S.; Xiao, P.; Zhao, P.; Wang, H. Addressing current climate issues in Pakistan: An opportunity for a sustainable future. Environ. Chall. 2024, 15, 100887. [Google Scholar] [CrossRef]
- National Climate Change Policy. 2021. Available online: https://mocc.gov.pk/SiteImage/Policy/NCCP%20Report.pdf (accessed on 20 December 2023).
- Hlohowskyj, I.; Brody, M.S.; Lackey, R.T. Methods for assessing the vulnerability of African fisheries resources to climate change. Clim. Res. 1996, 6, 97–106. [Google Scholar] [CrossRef]
- McDowall, R.M. Global climate change and fish and fisheries: What might happen in a temperate oceanic archipelago like New Zealand. GeoJournal 1992, 28, 29–37. [Google Scholar] [CrossRef]
- Meisner, J.D.; Shuter, B.J. Assessing potential effects of global climate change on tropical freshwater fishes. GeoJournal 28 1992, 21–27. [Google Scholar] [CrossRef]
- Shuter, B.J.; Meisner, J.D. Tools for assessing the impact of climate change on freshwater fish populations. GeoJournal 28 1992, 7–20. [Google Scholar] [CrossRef]
- Maitland, P.S. Climatic change and fish in northern Europe: Some possible scenarios. Proc. Inst. Fish. Manag. Annu. Study Course 1991, 22, 97–110. [Google Scholar]
- Tonn, W.M. Climate change and fish communities: A conceptual framework. Trans. Am. Fish. Soc. 1990, 119, 337–352. [Google Scholar] [CrossRef]
- Magnuson, J.J.; Meisner, J.D.; Hill, D.K. Potential changes in the thermal habitat of Great Lakes fish after global climate warming. Trans. Am. Fish. Soc. 1990, 119, 254–264. [Google Scholar] [CrossRef]
- Meisner, J.D. Effect of climatic warming on the southern margins of the native range of brook trout, Salvelinus fontinalis. Can. J. Fish. Aquat. Sci. 1990, 47, 1065–1070. [Google Scholar] [CrossRef]
- Deangelis, D.L.; Cushman, R.M. Potential application of models in forecasting the effects of climate changes on fisheries. Trans. Am. Fish. Soc. 1990, 119, 224–239. [Google Scholar] [CrossRef]
- Shuter, B.J.; Post, J.R. Climate, population viability, and the zoogeography of temperate fishes. Trans. Am. Fish. Soc. 1990, 119, 314–336. [Google Scholar] [CrossRef]
- Hassan, B.; Qadri, H.; Ali, M.N.; Khan, N.A.; Yatoo, A.M. Impact of climate change on freshwater ecosystem and its sustainable management. In Fresh Water Pollution Dynamics and Remediation; Springer: Singapore, 2020; pp. 105–121. [Google Scholar]
- Mckenzie, D.; Geffroy, B.; Farrell, A. Effects of global warming on fishes and fisheries. J. Fish Biol. 2021, 98, 1489–1492. [Google Scholar] [CrossRef]
- Rogers, L.A.; Dougherty, A.B. Effects of climate and demography on reproductive phenology of a harvested marine fish population. Glob. Chang. Biol. 2019, 25, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.J.; Lynch, A.J.; Bunnell, D.B.; Chu, C.; Falke, J.A.; Kovach, R.P.; Krabbenhoft, T.J.; Kwak, T.J.; Paukert, C.P. Global synthesis of the documented and projected effects of climate change on inland fishes. Rev. Fish Biol. Fish. 2017, 27, 339–361. [Google Scholar] [CrossRef]
- Audzijonyte, A.; Richards, S.A.; Stuart-Smith, R.D.; Pecl, G.; Edgar, G.J.; Barrett, N.S.; Payne, N.; Blanchard, J.L. Fish body sizes change with temperature but not all species shrink with warming. Nat. Ecol. Evol. 2020, 4, 809–814. [Google Scholar] [CrossRef]
- Goikoetxea, A.; Sadoul, B.; Blondeau-Bidet, E.; Aerts, J.; Blanc, M.O.; Parrinello, H.; Barrachina, C.; Pratlong, M.; Geffroy, B. Genetic pathways underpinning hormonal stress responses in fish exposed to short-and long-term warm ocean temperatures. Ecol. Indic. 2021, 120, 106937. [Google Scholar] [CrossRef]
- Stillman, J.H. Heat waves, the new normal: Summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 2019, 34, 86–100. [Google Scholar] [CrossRef]
- Cochrane, K.; De Young, C.; Soto, D.; Bahri, T. (Eds.) Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; FAO Fisheries and Aquaculture Technical Paper: Rome, Italy, 2009; p. 212. [Google Scholar]
- Parry, M.L.; Canziani, O.; Palutikof, J.; Van der Linden, P.; Hanson, C. (Eds.) Climate Change 2007-Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- Nyingi, D.W.; Gichuki, N.; Ogada, M.O. Freshwater ecology of Kenyan highlands and lowlands. In Developments in Earth Surface Processes; Elsevier: Amsterdam, The Netherlands, 2013; Volume 16, pp. 199–218. [Google Scholar]
- Cooke, S.J.; Bergman, J.N.; Nyboer, E.A.; Reid, A.J.; Gallagher, A.J.; Hammerschlag, N.; Van de Riet, K.; Vermaire, J.C. Overcoming the concrete conquest of aquatic ecosystems. Biol. Conserv. 2020, 247, 108589. [Google Scholar] [CrossRef]
- Liu, J.; Kattel, G.; Arp, H.P.H.; Yang, H. Towards threshold-based management of freshwater ecosystems in the context of climate change. Ecol. Model. 2015, 318, 265–274. [Google Scholar] [CrossRef]
- Azevedo-Santos, V.M.; Frederico, R.G.; Fagundes, C.K.; Pompeu, P.S.; Pelicice, F.M.; Padial, A.A.; Nogueira, M.G.; Fearnside, P.M.; Lima, L.B.; Daga, V.S.; et al. Protected areas: A focus on Brazilian freshwater biodiversity. Divers. Distrib. 2019, 25, 442–448. [Google Scholar] [CrossRef]
- Leidy, R.A.; Moyle, P.B. Keeping up with the status of freshwater fishes: A California (USA) perspective. Conserv. Sci. Pract. 2021, 3, e474. [Google Scholar] [CrossRef]
- Usman, K.; Pervaiz, K.; Khan, H.; Rehman, H.U.; Aslam, S. Current status of ichthyofauna at Khaki site, River Siren Mansehra Khyber Pakhtunkhwa, Pakistan. J. Entomol. Zool. Stud. 2017, 5, 361–363. [Google Scholar]
- Junk, W.J.; Soares, M.G.M.; Bayley, P.B. Freshwater fishes of the Amazon River basin: Their biodiversity, fisheries, and habitats. Aquat. Ecosyst. Health Manag. 2007, 10, 153–173. [Google Scholar] [CrossRef]
- FAO Fisheries and Aquaculture Department. The State of World Fisheries and Aquaculture 2012; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Nelson, J.S. Fishes of the World; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rafique, M.; Khan, N.U.H. Distribution and status of significant freshwater fishes of Pakistan. Rec. Zool. Surv. Pak. 2012, 21, 90–95. [Google Scholar]
- IUCN. IUCN Freshwater Fish Specialist Group. 2015. Available online: http://www.iucnffsg.org/freshwaterfishes/freshwater-fish-diversity/ (accessed on 4 July 2017).
- Ahmad, M.; Shah, A.H.; Maqbool, Z.; Khalid, A.; Khan, K.R.; Farooq, M. Ichthyofaunal diversity and conservation status in rivers of Khyber Pakhtunkhwa, Pakistan. Proc. Int. Acad. Ecol. Environ. Sci. 2020, 10, 131–143. [Google Scholar]
- Mirza, M.R.; Mirza, Z.S. Longitudinal zonation in the fish fauna of the Indus River in Pakistan. Biologia 2014, 60, 149–152. [Google Scholar]
- Allison, E.H.; Ellis, F.; Mvula, P.M.; Mathieu, L.F. Fisheries management and uncertainty: The causes and consequences of variability in inland fisheries in Africa, with special reference to Malawi. In Proceedings of the Lake Malawi Fisheries Management Symposium, Lilongwe, Malawi, 4–9 June 2001; Weyl, O.L.F., Weyl, M.V., Eds.; National Aquatic Resource Management Programme: Lilongwe, Malawi, 2001; pp. 66–79. [Google Scholar]
- Daw, T.; Adger, W.N.; Brown, K.; Badjeck, M.-C. Climate change and capture fisheries: Potential impacts, adaptation and mitigation. Chapter 2. In Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; Cochrane, K., De Young, C., Soto, D., Bahri, T., Eds.; FAO: Rome, Italy, 2009; pp. 107–150. [Google Scholar]
- Welcomme, R.L. An overview of global catch statistics for inland fish. ICES J. Mar. Sci. 2011, 68, 1751–1756. [Google Scholar] [CrossRef]
- Neiland, A.E. Artisanal Fisheries of the Chad Basin in Africa, An Overview of Research and Annotated Bibliography 1920–1990; FAO: Rome, Italy, 1992; pp. 1–89. [Google Scholar]
- Food and Agriculture Organization of the United Nations. 2024. Available online: https://www.fao.org/asiapacific/news/news-detail/FAO-Report-Global-fisheries-and-aquaculture-production-reaches-a-new-record-high-/en (accessed on 12 September 2024).
- Rountrey, A.N.; Coulson, P.G.; Meeuwig, J.J.; Meekan, M. Water temperature and fish growth: Otoliths predict growth patterns of a marine fish in a changing climate. Glob. Chang. Biol. 2014, 20, 2450–2458. [Google Scholar] [CrossRef]
- Gamperl, A.K.; Ajiboye, O.O.; Zanuzzo, F.S.; Sandrelli, R.M.; Ellen de Fátima, C.P.; Beemelmanns, A. The impacts of increasing temperature and moderate hypoxia on the production characteristics, cardiac morphology and haematology of Atlantic Salmon (Salmo salar). Aquaculture 2020, 519, 734874. [Google Scholar] [CrossRef]
- Heather, F.J.; Childs, D.Z.; Darnaude, A.M.; Blanchard, J.L. Using an integral projection model to assess the effect of temperature on the growth of gilthead seabream Sparus aurata. PLoS ONE 2018, 13, e0196092. [Google Scholar] [CrossRef]
- Tao, J.; Che, R.; He, D.; Yan, Y.; Sui, X.; Chen, Y. Trends and potential cautions in food web research from a bibliometric analysis. Scientometrics 2015, 105, 435–447. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Henrys, P.A.; Feuchtmayr, H.; Jones, I.D.; Maberly, S.C.; Winfield, I.J. Food web de-synchronization in E ngland’s largest lake: An assessment based on multiple phenological metrics. Glob. Chang. Biol. 2013, 19, 3568–3580. [Google Scholar] [CrossRef]
- Queiros, Q.; McKenzie, D.J.; Dutto, G.; Killen, S.; Saraux, C.; Schull, Q. Fish shrinking, energy balance and climate change. Sci. Total Environ. 2024, 906, 167310. [Google Scholar] [CrossRef] [PubMed]
- Carozza, D.A.; Bianchi, D.; Galbraith, E.D. Metabolic impacts of climate change on marine ecosystems: Implications for fish communities and fisheries. Glob. Ecol. Biogeogr. 2019, 28, 158–169. [Google Scholar] [CrossRef]
- Murdoch, A.; Power, M. The effect of lake morphometry on thermal habitat use and growth in Arctic charr populations: Implications for understanding climate-change impacts. Ecol. Freshw. Fish 2013, 22, 453–466. [Google Scholar] [CrossRef]
- Pilière, A.; Schipper, A.M.; Breure, A.M.; Posthuma, L.; de Zwart, D.; Dyer, S.D.; Huijbregts, M.A. Comparing responses of freshwater fish and invertebrate community integrity along multiple environmental gradients. Ecol. Indic. 2014, 43, 215–226. [Google Scholar] [CrossRef]
- Tao, J.; Kennard, M.J.; Jia, Y.; Chen, Y. Climate-driven synchrony in growth-increment chronologies of fish from the world’s largest high-elevation river. Sci. Total Environ. 2018, 645, 339–346. [Google Scholar] [CrossRef]
- Ding, C.Z.; Jiang, X.M.; Chen, L.Q.; Juan, T.; Chen, Z.M. Growth variation of Schizothorax dulongensis Huang, 1985 along altitudinal gradients: Implications for the Tibetan Plateau fishes under climate change. J. Appl. Ichthyol. 2016, 32, 729–733. [Google Scholar] [CrossRef]
- Conti, L.; Comte, L.; Hugueny, B.; Grenouillet, G. Drivers of freshwater fish colonisations and extirpations under climate change. Ecography 2015, 38, 510–519. [Google Scholar] [CrossRef]
- Manjarrés-Hernández, A.; Guisande, C.; García-Roselló, E.; Heine, J.; Pelayo-Villamil, P.; Pérez-Costas, E.; González-Vilas, L.; González-Dacosta, J.; Duque, S.R.; Granado-Lorencio, C.; et al. Predicting the effects of climate change on future freshwater fish diversity at global scale. Nat. Conserv. 2021, 43, 1–24. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Oldfather, M.F. Climate change, ecosystem processes and biological diversity responses in high elevation communities. Climate 2021, 9, 87. [Google Scholar] [CrossRef]
- Khan, A.M.; Ali, Z.; Shelly, S.Y.; Ahmad, Z.; Mirza, M.R. Aliens; a catastrophe for native fresh water fish diversity in Pakistan. J. Anim. Plant Sci. 2011, 21, 435–440. [Google Scholar]
- Cambray, J.A. Impact on indigenous species biodiversity caused by the globalisation of alien recreational freshwater fisheries. Hydrobiologia 2003, 500, 217–230. [Google Scholar] [CrossRef]
- Chen, S.; Billings, S.A. Representations of non-linear systems: The NARMAX model. Int. J. Control 1989, 49, 1013–1032. [Google Scholar] [CrossRef]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Val, A.L. Biodiversity–the hidden risks. An. Acad. Bras. Ciênc. 2020, 92, 20200699. [Google Scholar] [CrossRef] [PubMed]
- IUCN. IUCN Red List of Threatened Species; Version 2011.2; IUCN: Gland, Switzerland, 2011. [Google Scholar]
- Morrison, W.E.; Nelson, M.W.; Howard, J.F.; Teeters, E.J.; Hare, J.A.; Griffis, R.B.; Alexander, M.A. Methodology for Assessing the Vulnerability of Marine Fish and Shellfish Species to a Changing Climate; NOAA: Washington, DC, USA, 2015. [Google Scholar]
- Olusanya, H.O.; van Zyll de Jong, M. Assessing the vulnerability of freshwater fishes to climate change in Newfoundland and Labrador. PLoS ONE 2018, 13, e0208182. [Google Scholar] [CrossRef]
- Hannah, L. Protected areas and climate change. Ann. N. Y. Acad. Sci. 2008, 1134, 201–212. [Google Scholar] [CrossRef]
- Chessman, B.C. Identifying species at risk from climate change: Traits predict the drought vulnerability of freshwater fishes. Biol. Conserv. 2013, 160, 40–49. [Google Scholar] [CrossRef]
- Johnson, S.L.; Penaluna, B.E. Climate Change and Interactions With Multiple Stressors in Rivers. In Multiple Stressors in River Ecosystems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–44. [Google Scholar]
- Shakir, A.S.; Ehsan, S. Climate change impact on river flows in Chitral watershed. Pak. J. Eng. Appl. Sci. 2010, 7, 12–23. [Google Scholar]
- Salma, S.; Rehman, S.; Shah, M.A. Rainfall trends in different climate zones of Pakistan. Pak. J. Meteorol. 2012, 9, 37–47. [Google Scholar]
- Altaf, M.; Javid, A.; Umair, M. Biodiversity of Ramsar sites in Pakistan; LAP: Saarbrucken, Germany, 2014; pp. 1–88. [Google Scholar]
- Camara, M.; Jamil, N.R.; Abdullah, A.F.B. Impact of land uses on water quality in Malaysia: A review. Ecol. Process. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef] [PubMed]
- Setia, R.; Dhaliwal, S.S.; Kumar, V.; Singh, R.; Kukal, S.S.; Pateriya, B. Impact assessment of metal contamination in surface water of Sutlej River (India) on human health risks. Environ. Poll. 2020, 265, 114907. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Tech. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Li, W.P.; Liu, Z.F.; Guo, T.; Chen, H.; Xie, X. Using Optimal Environmental DNA Method to Improve the Fish Diversity Survey—From Laboratory to Aquatic Life Reserve. Water 2021, 13, 1468. [Google Scholar] [CrossRef]
- Nørgaard, L.; Olesen, C.R.; Trøjelsgaard, K.; Pertoldi, C.; Nielsen, J.L.; Taberlet, P.; Ruiz-González, A.; De Barba, M.; Iacolina, L. EDNA metabarcoding for biodiversity assessment, generalist predators as sampling assistants. Sci. Rep. 2021, 11, 6820. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Yasuhara, M.; Wei, C.L.; Kucera, M.; Costello, M.J.; Tittensor, D.P.; Kiessling, W.; Bonebrake, T.C.; Tabor, C.R.; Feng, R.; Baselga, A.; et al. Past and future decline of tropical pelagic biodiversity. Proc. Natl. Acad. Sci. USA 2020, 117, 12891–12896. [Google Scholar] [CrossRef]
- Stafford, R.; Jones, P.J. Viewpoint–Ocean plastic pollution: A convenient but distracting truth? Mar. Policy 2019, 103, 187–191. [Google Scholar] [CrossRef]
- Trathan, P.N.; García-Borboroglu, P.; Boersma, D.; Bost, C.A.; Crawford, R.J.; Crossin, G.T.; Cuthbert, R.J.; Dann, P.; Davis, L.S.; De La Puente, S.; et al. Pollution, habitat loss, fishing, and climate change as critical threats to penguins. Conserv. Biol. 2015, 29, 31–41. [Google Scholar] [CrossRef]
- Kaimuddin, A.H.; Laë, R.; Tito De Morais, L. Fish species in a changing world: The route and timing of species migration between tropical and temperate ecosystems in Eastern Atlantic. Front. Mar. Sci. 2016, 3, 162. [Google Scholar] [CrossRef]
- Desiderà, E.; Guidetti, P.; Panzalis, P.; Navone, A.; Valentini-Poirrier, C.A.; Boissery, P.; Gervaise, C.; Di Iorio, L. Acoustic fish communities: Sound diversity of rocky habitats reflects fish species diversity. Mar. Ecol. Prog. Ser. 2019, 608, 183–197. [Google Scholar] [CrossRef]
- Staehr, P.A.; Dahl, K.; Buur, H.; Göke, C.; Sapkota, R.; Winding, A.; Panova, M.; Obst, M.; Sundberg, P. Environmental DNA Monitoring of Biodiversity Hotspots in Danish Marine Waters. Front. Mar. Sci. 2022, 8, 800474. [Google Scholar] [CrossRef]
- Carvalho, C.O.; Gromstad, W.; Dunthorn, M.; Karlsen, H.E.; Schrøder-Nielsen, A.; Ready, J.S.; Haugaasen, T.; Sørnes, G.; de Boer, H.; Mauvisseau, Q. Harnessing eDNA metabarcoding to investigate fish community composition and its seasonal changes in the Oslo fjord. Sci. Rep. 2024, 14, 10154. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Wu, H.; Liu, F.; Peng, W.; Zhang, X.; Chang, F.; Xie, P.; Zhang, H. A review and perspective of EDNA application to eutrophication and HAB control in freshwater and marine ecosystems. Microorganisms 2020, 8, 417. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Miaud, C.; Pompanon, F.; Taberlet, P. Species detection using environmental DNA from water samples. Biol. Lett. 2008, 4, 423–425. [Google Scholar] [CrossRef]
- Nagai, S.; Hida, K.; Urushizaki, S.; Onitsuka, G.; Yasuike, M.; Nakamura, Y.; Fujiwara, A.; Tajimi, S.; Kimoto, K.; Kobayashi, T.; et al. Influences of diurnal sampling bias on fixed-point monitoring of plankton biodiversity determined using a massively parallel sequencing-based technique. Gene 2016, 576, 667–675. [Google Scholar] [CrossRef]
- Sildever, S.; Kawakami, Y.; Kanno, N.; Kasai, H.; Shiomoto, A.; Katakura, S.; Nagai, S. Toxic HAB species from the Sea of Okhotsk detected by a metagenetic approach, seasonality and environmental drivers. Harmful Algae 2019, 87, 101631. [Google Scholar] [CrossRef]
- Iacolina, L.; Lukassen, M.B.; Fløjgaard, C.; Buttenschøn, R.; Nielsen, J.L.; Pertoldi, C. EDNA and metabarcoding for rewilding projects monitoring, a dietary approach. Mamm. Biol. 2020, 100, 411–418. [Google Scholar] [CrossRef]
- Taberlet, P.; Bonin, A.; Zinger, L.; Coissac, E. Environmental DNA for Biodiversity Research and Monitoring; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Rees, H.C.; Maddison, B.C.; Middleditch, D.J.; Patmore, J.R.; Gough, K.C. The detection of aquatic animal species using environmental DNA–a review of EDNA as a survey tool in ecology. J. Appl. Ecol. 2014, 51, 1450–1459. [Google Scholar] [CrossRef]
- Xu, N.; Zhu, B.; Shi, F.; Shao, K.; Que, Y.; Li, W.; Li, W.; Jiao, W.; Tian, H.; Xu, D.; et al. Monitoring seasonal distribution of an endangered anadromous sturgeon in a large river using environmental DNA. Sci. Nat. 2018, 105, 62. [Google Scholar] [CrossRef] [PubMed]
- Laramie, M.B.; Pilliod, D.S.; Goldberg, C.S. Characterizing the distribution of an endangered salmonid using environmental DNA analysis. Biol. Conserv. 2015, 183, 29–37. [Google Scholar] [CrossRef]
- Xing, Y.; Gao, W.; Shen, Z.; Zhang, Y.; Bai, J.; Cai, X.; Ouyang, J.; Zhao, Y. Review of Environmental DNA Field and Laboratory Protocols Applied in Fish Ecology and Environmental Health. Front. Environ. Sci. 2022, 10, 725360. [Google Scholar] [CrossRef]
- Qu, C.; Stewart, K.A.; Clemente-Carvalho, R.; Zheng, J.; Wang, Y.; Gong, C.; Ma, L.; Zhao, J.; Lougheed, S.C. Comparing fish prey diversity for a critically endangered aquatic mammal in a reserve and the wild using EDNA metabarcoding. Sci. Rep. 2020, 10, 16715. [Google Scholar] [CrossRef] [PubMed]
- Muha, T.P.; Rodriguez-Barreto, D.; O’Rorke, R.; Garcia de Leaniz, C.; Consuegra, S. Using EDNA metabarcoding to monitor changes in fish community composition after barrier removal. Front. Ecol. Evol. 2021, 9, 629217. [Google Scholar] [CrossRef]
- Valdivia-Carrillo, T.; Rocha-Olivares, A.; Reyes-Bonilla, H.; Domínguez-Contreras, J.F.; Munguia-Vega, A. Integrating EDNA metabarcoding and simultaneous underwater visual surveys to describe complex fish communities in a marine biodiversity hotspot. Mol. Ecol. Resour. 2021, 21, 1558–1574. [Google Scholar] [CrossRef]
- Sales, N.G.; Wangensteen, O.S.; Carvalho, D.C.; Deiner, K.; Præbel, K.; Coscia, I.; McDevitt, A.D.; Mariani, S. Space-time dynamics in monitoring neotropical fish communities using EDNA metabarcoding. Sci. Total Environ. 2021, 754, 142096. [Google Scholar] [CrossRef]
- Rohtehrá, S.; Tournayre, O.; Windle, M.J.; Cormier, E.; Schwartz, K.; Wylie-Arbic, M.; Rundle, E.; Perron, M.A.; Francis, A.; Lougheed, S.C. Nearshore fish diversity changes with sampling method and human disturbance: Comparing eDNA metabarcoding and seine netting along the Upper St. Lawrence River. J. Great Lakes Res. 2024, 50, 102317. [Google Scholar]
- Valdez-Moreno, M.; Ivanova, N.V.; Elías-Gutiérrez, M.; Pedersen, S.L.; Bessonov, K.; Hebert, P.D. Using EDNA to biomonitor the fish community in a tropical oligotrophic lake. PLoS ONE 2019, 14, e0215505. [Google Scholar] [CrossRef]
- Beng, K.C.; Corlett, R.T. Applications of environmental DNA (eDNA) in ecology and conservation: Opportunities, challenges and prospects. Biodivers. Conserv. 2020, 29, 2089–2121. [Google Scholar]
- Wee, A.K.; Salmo Iii, S.G.; Sivakumar, K.; Then, A.Y.; Basyuni, M.; Fall, J.; Habib, K.A.; Isowa, Y.; Leopardas, V.; Peer, N.; et al. Prospects and challenges of environmental DNA (eDNA) metabarcoding in mangrove restoration in Southeast Asia. Front. Mar Sci. 2023, 10, 1033258. [Google Scholar] [CrossRef]
- Ahmed, N.; Lü, H.; Ahmed, S.; Adeyeri, O.E.; Ali, S.; Hussain, R.; Shah, S. Transboundary River Water Availability to Ravi Riverfront under Changing Climate: A Step towards Sustainable Development. Sustainability 2023, 15, 3526. [Google Scholar] [CrossRef]
- Qazlbash, S.K.; Zubair, M.; Manzoor, S.A.; ul Haq, A.; Baloch, M.S. Socioeconomic determinants of climate change adaptations in the flood-prone rural community of Indus Basin, Pakistan. Environ. Dev. 2021, 37, 100603. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Willerslev, E. Environmental DNA–An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Deiner, K.; Fronhofer, E.A.; Mächler, E.; Walser, J.C.; Altermatt, F. Environmental DNA reveals that rivers are conveyer belts of biodiversity information. Nat. Commun. 2016, 7, 12544. [Google Scholar] [CrossRef]
- Addy, S.; Cooksley, S.; Dodd, N.; Waylen, K.; Stockan, J.; Byg, A.; Holstead, K. River Restoration and Biodiversity; IUCN: Gland, Switzerland, 2016; pp. 1–52. [Google Scholar]
- Nakamichi, T.; Ono, M.; Hayashi, M.; Okamura, T.; Wada, T.; Saitoh, K. Environmental DNA Analysis in a River Detected a Possible Distribution of Fish Species Difficult to Capture. Fishes 2023, 8, 496. [Google Scholar] [CrossRef]
- Boyd, S.H.; Niemiller, K.D.K.; Dooley, K.E.; Nix, J.; Niemiller, M.L. Using environmental DNA methods to survey for rare groundwater fauna: Detection of an endangered endemic cave crayfish in northern Alabama. PLoS ONE 2020, 15, e0242741. [Google Scholar] [CrossRef]
- Yamanaka, H.; Minamoto, T. The use of environmental DNA of fishes as an efficient method of determining habitat connectivity. Ecol. Indic. 2016, 62, 147–153. [Google Scholar] [CrossRef]
- Coster, S.S.; Dillon, M.N.; Moore, W.; Merovich, G.T., Jr. The update and optimization of an eDNA assay to detect the invasive rusty crayfish (Faxonius rusticus). PLoS ONE 2021, 16, e0259084. [Google Scholar] [CrossRef]
- Wiggins, J.J.; Tobias, V.D.; Holcombe, E.F.; Karpenko, K.; Huber, E.R.; Goodman, A.C. Leveraging environmental DNA (eDNA) to optimize targeted removal of invasive fishes. J. Freshw. Ecol. 2024, 39, 2378841. [Google Scholar] [CrossRef]
- Miller, A.D.; Roxburgh, S.H.; Shea, K. How frequency and intensity shape diversity–disturbance relationships. Proc. Natl. Acad. Sci. USA 2011, 108, 5643–5648. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Hou, L.; Wang, J.; Dong, X.; Han, S. Flood changed the community composition and increased the importance of stochastic process of vegetation and seed bank in a riparian ecosystem of the Yellow River. Ecol. Indic. 2023, 154, 110505. [Google Scholar] [CrossRef]
- Lacoursière-Roussel, A.; Côté, G.; Leclerc, V.; Bernatchez, L. Quantifying relative fish abundance with eDNA: A promising tool for fisheries management. J. Appl. Ecol. 2016, 53, 1148–1157. [Google Scholar] [CrossRef]
- Rourke, M.L.; Fowler, A.M.; Hughes, J.M.; Broadhurst, M.K.; DiBattista, J.D.; Fielder, S.; Wilkes Walburn, J.; Furlan, E.M. Environmental DNA (eDNA) as a tool for assessing fish biomass: A review of approaches and future considerations for resource surveys. Environ. DNA 2022, 4, 9–33. [Google Scholar] [CrossRef]
- Takahara, T.; Minamoto, T.; Yamanaka, H.; Doi, H.; Kawabata, Z.I. Estimation of fish biomass using environmental DNA. PLoS ONE 2012, 7, e35868. [Google Scholar] [CrossRef]
- Cristescu, M.E.; Hebert, P.D. Uses and misuses of environmental DNA in biodiversity science and conservation. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 209–230. [Google Scholar] [CrossRef]
- Yang, N.; Jin, D.; Govindarajan, A.F. Applying environmental DNA approaches to inform marine biodiversity conservation: The case of the Ocean Twilight Zone. Mar. Policy 2024, 165, 106151. [Google Scholar] [CrossRef]
- Papaioannou, C.; Geladakis, G.; Kommata, V.; Batargias, C.; Lagoumintzis, G. Insights in Pharmaceutical Pollution: The Prospective Role of eDNA Metabarcoding. Toxics 2023, 11, 903. [Google Scholar] [CrossRef]
- Rishan, S.T.; Kline, R.J.; Rahman, M.S. Exploitation of environmental DNA (eDNA) for ecotoxicological research: A critical review on eDNA metabarcoding in assessing marine pollution. Chemosphere 2024, 351, 141238. [Google Scholar] [CrossRef]




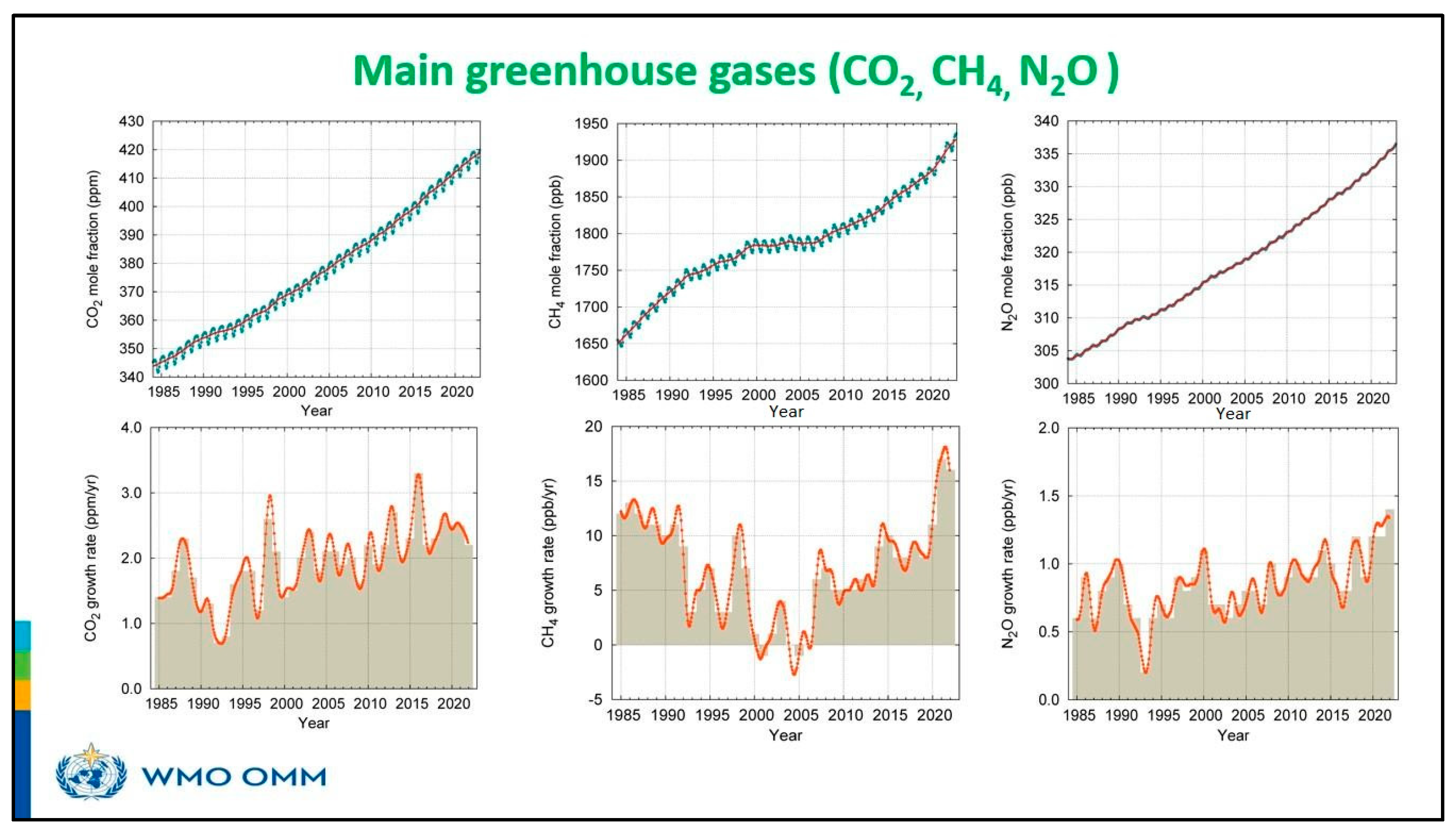

| CO2 | CH4 | N2O | |
|---|---|---|---|
| 2022 global mean abundance | 417.9 ± 0.2 ppm | 1923 ± 2 ppb | 335.8 ± 0.1 ppb |
| 2022 abundance relative to 1750 a | 150% | 264% | 124% |
| 2021–2022 absolute increase | 2.2 ppm | 16 ppb | 1.4 ppb |
| 2021–2022 relative increase | 0.53% | 0.84% | 0.42% |
| Mean annual absolute increase over the past 10 years | 2.46 ppm yr−1 | 10.2 ppb yr−1 | 1.05 ppb yr−1 |
| Name of Fish | IUCN Status | Distributional Status | Commercial Value |
|---|---|---|---|
| Butter catfish (Ompok bimaculatus) | Near Threatened | Indigenous | NA |
| Pabdah catfish (Ompok panda) | Near Threatened | Indigenous | NA |
| Freshwater shark (Wallago attu) | Near Threatened | Indigenous | Very high |
| Gangetic ailia (Ailia coila) | Near Threatened | Indigenous | NA |
| Humped featherback (Chitala chitala) | Near Threatened | Indigenous | High |
| Gangetic goonch (Bagarius bagarius) | Near Threatened | Indigenous | High |
| Himalayan snowtrout (Schizothorax plagiostomus) | Vulnerable | Indigenous | High |
| Hilsa Shad (Tenualosa ilisha) | Not Evaluated | Indigenous | Very high |
| Mirgal (Cirrhinus mrigala) | Least Concern | Indigenous | Very high |
| Catla (Gibelion catla) | Least Concern | Indigenous | Very high |
| Thicklip labeo (Labeo dyocheilus pakistanicus) | Least Concern | Indigenous | High |
| Orangefin labeo (Labeo calbasu) | Least Concern | Indigenous | High |
| Kuria labeo (Labeo gonius) | Least Concern | Indigenous | High |
| Rahu (Labeo rohita) | Least Concern | Indigenous | Very high |
| Scaly Osman (Diptychus maculatus) | Not evaluated | Indigenous | High |
| Indus snowtrout (Ptychobarbus conirostris) | Not evaluated | Indigenous | High |
| Kunar snowtrout (Racoma labiate) | Vulnerable | Indigenous | High |
| Zig-zag eel (Mastacembelus armatus) | Least Concern | Indigenous | High |
| Sperata seenghala | Least Concern | Indigenous | Very high |
| Naziri bachcha (Clupisoma naziri) | Not evaluated | Indigenous | Very high |
| Rita Catfish (Rita rita) | Least Concern | Indigenous | Very high |
| Garua bachcha (Clupisoma garua) | Least Concern | Indigenous | Very high |
| Walking Catfish (Clarias batrachus) | Least Concern | Indigenous | High |
| Great snakehead (Channa marulius) | Least Concern | Indigenous | Very high |
| Chirruh snowtrout (Schizopyge esocinus) | Not evaluated | Indigenous | High |
| Ladakh snowtrout (Schizopygopsis stoliczkai) | Not evaluated/very rare | Indigenous | NA |
| Stoliczka triplophysaloach (Triplophysa stoliczkai) | Not evaluated/very rare | Indigenous | NA |
| Zebra Fish (Danio rerio) | Least Concern/very rare | Indigenous | NA |
| Whiptail Catfish (Sisor rabdophorus) | Not evaluated/very rare | Indigenous | NA |
| Bengala barb (Megarasbora elonga) | Least Concern/very rare | Indigenous | NA |
| Gangetic leaf fish (Nandus nandus) | Not evaluated/very rare | Indigenous | NA |
| Chameleon fish (Badis badis) | Not evaluated/very rare | Indigenous | NA |
| Gangetic mud eel (Monopterus cuchia) | Not evaluated/very rare | Indigenous | NA |
| One-stripe spiny eel (Macrognathus aral) | Vulnerable | Indigenous | NA |
| Punjab razorbellyminnow (Salmophasia punjabensis) | Not evaluated | Endemic | NA |
| Naseeri baril (Barilius naseeri) | Not evaluated | Endemic | NA |
| Pakistani baril (Barilius Pakistanicus) | Least Concern | Endemic | NA |
| Blue rahu (Labeo caeruleus) | Least Concern | Endemic | NA |
| Days’ labeo (Labeo nigripinnis) | Not evaluated | Endemic | NA |
| Balochistan labeo (Labeo gedrosicus) | Not evaluated | Endemic | NA |
| Macmahons’ labeo (Labeo macmahoni) | Not evaluated | Endemic | NA |
| Zhob mahasheer (Naziritor zhobensis) | Not evaluated | Endemic | NA |
| Punjab barb (Puntius punjabensis) | Least Concern | Endemic | NA |
| Salt Range barb (Puntius waageni) | Vulnerable | Endemic | NA |
| Javeds’ loach (Botia javedi) | Not evaluated | Endemic | NA |
| Swat loach (Schistura alepidota) | Not evaluated | Endemic | NA |
| Anambar loach (Schistura anambarensis) | Least Concern | Endemic | NA |
| Arifs’ loach (Schistura arifi) | Least Concern | Endemic | NA |
| Panjgur loach (Schistura baluchiorum) | Not evaluated | Endemic | NA |
| Kurram loach (Schistura curtistigma) | Vulnerable | Endemic | NA |
| Hangu loach (Schistura fascimaculata) | Not evaluated | Endemic | NA |
| Pishin loach (Schistura kessleri) | Not evaluated | Endemic | NA |
| Parachinar loach (Schistura lepidocaulis) | Not evaluated | Endemic | NA |
| Kohat loach (Schistura kohatensis) | Not evaluated | Endemic | NA |
| Mach loach (Schistura machensis) | Vulnerable | Endemic | NA |
| Khyber loach (Schistura microlabra) | Vulnerable | Endemic | NA |
| Rawlakot loach (Schistura nalbanti) | Not evaluated | Endemic | NA |
| Zhob loach (Schistura pakistanica) | Vulnerable | Endemic | NA |
| Pakhtunkhwa loach (Schistura parashari) | Near Threatened | Endemic | NA |
| Chenab loach (Schistura shadiwalensis) | Least Concern | Endemic | NA |
| Hazara Loach (Triplophysa hazaraensis) | Vulnerable | Endemic | NA |
| Nazir triplophysaloach (Triplophysa naziri) | Least Concern | Endemic | NA |
| Yasin triplophysaloach (Triplophysa yasinensis) | Least Concern | Endemic | NA |
| Horas’ mystus (Mystus horai) | Vulnerable | Endemic | NA |
| Pakistani gagata (Gagata pakistanica) | Not evaluated | Endemic | NA |
| Naziri catfish (Glyptothorax naziri) | Near Threatened | Endemic | NA |
| Kalabagh nangra (Nangra robusta) | Not evaluated | Endemic | NA |
| Sindh catfish (Ompok Sindhensis) | Not evaluated | Endemic | NA |
| Species | Occurrence | IUCN Status | Commercial Value |
|---|---|---|---|
| Goldfish (Carassius auratus) | Introduced | Least Concern | High |
| Grass carp (Ctenopharyngodon idella) | Introduced | Not evaluated | Very high |
| Common carp (Cyprinus carpio) | Introduced | Least Concern | Very high |
| Mosquito fish (Gambusia affinis) | Introduced | Least Concern | Minor commercial |
| Silver carp (Hypophthalmichthys molitrix) | Introduced | Not evaluated | Very high |
| Bighead carp (Hypophthalmichthys nobilis) | Introduced | Not evaluated | Very high |
| Rainbow trout (Oncorhynchus mykiss) | Introduced | Least Concern | Very high |
| Blue tilapia (Oreochromis aureus) | Introduced | Not evaluated | Very high |
| Mozambique tilapia (Oreochromis mossambicus) | Introduced | Vulnerable | Highly commercial |
| Nile tilapia (Oreochromis niloticus) | Introduced | Least Concern | Highly commercial |
| Sea trout (Salmo trutta) | Introduced | Least Concern | Commercial |
| Common Name | Scientific Name | IUCN Status | Occurrence | Commercial Value |
|---|---|---|---|---|
| Kashmir catfish | Glyptothorax kashmirensis | Critically Endangered | Endemic | NA |
| Verinag triplophysaloach | Triplophysa kashmirensis | Critically Endangered | Endemic | NA |
| Golden mahasheer | Tor putitora | Endangered | Indigenous | Commercial |
| Wana garra | Garra wanae | Endangered | Endemic | NA |
| Havelian loach | Schistura afasciata | Endangered | Endemic | NA |
| Harnai loach | Schistura harnaiensis | Endangered | Endemic | NA |
| Dera loach | Schistura macrolepis | Endangered | Endemic | NA |
| Pakistans’ batasio | Batasio pakistanicus | Endangered | Endemic | NA |
| Punjab catfish | Glyptothorax punjabensis | Endangered | Endemic | NA |
| Bhed catfish | Glyptothorax stocki | Endangered | Endemic | NA |
| Glyptothorax punjabensis | Endangered | Endemic | NA | |
| Harnai käärtrull | Schistura harnaiensis | Endangered | Endemic | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, G.; Abbas, S.; Nagai, S.; Mohd Arshad, N.; Bhassu, S. Threats of Climate Change to Freshwater Ecosystems in Pakistan: eDNA Monitoring Will Be the Next-Generation Tool Used in Biodiversity, Conservation, and Management. Biology 2025, 14, 1191. https://doi.org/10.3390/biology14091191
Ali G, Abbas S, Nagai S, Mohd Arshad N, Bhassu S. Threats of Climate Change to Freshwater Ecosystems in Pakistan: eDNA Monitoring Will Be the Next-Generation Tool Used in Biodiversity, Conservation, and Management. Biology. 2025; 14(9):1191. https://doi.org/10.3390/biology14091191
Chicago/Turabian StyleAli, Ghazanfer, Sidra Abbas, Satoshi Nagai, Norhafiza Mohd Arshad, and Subha Bhassu. 2025. "Threats of Climate Change to Freshwater Ecosystems in Pakistan: eDNA Monitoring Will Be the Next-Generation Tool Used in Biodiversity, Conservation, and Management" Biology 14, no. 9: 1191. https://doi.org/10.3390/biology14091191
APA StyleAli, G., Abbas, S., Nagai, S., Mohd Arshad, N., & Bhassu, S. (2025). Threats of Climate Change to Freshwater Ecosystems in Pakistan: eDNA Monitoring Will Be the Next-Generation Tool Used in Biodiversity, Conservation, and Management. Biology, 14(9), 1191. https://doi.org/10.3390/biology14091191







