1. Introduction
Sex-biased gene expression, which refers to differential expression between males and females, is a fundamental mechanism contributing to sexual dimorphism and shaping species’ evolutionary trajectories [
1,
2,
3,
4]. This sex-specific expression is shaped by multiple factors, including sexual selection, reproductive strategies, and ecological pressures, leading to evolutionary patterns distinct from those of unbiased genes [
5,
6]. Due to their species richness and ecological versatility, insects represent an ideal model for investigating the evolutionary dynamics of sex-biased gene expression [
7,
8,
9,
10,
11,
12,
13,
14,
15].
Previous studies have shown that sex-biased genes often undergo rapid evolution, largely driven by sexual selection and sex-specific ecological pressures [
16,
17,
18,
19,
20]. However, the extent to which these genes are conserved or divergent across species and evolutionary lineages remains poorly understood, especially in large-scale comparative studies across insect orders. A broad-scale analysis of sex-biased gene evolution in insects may reveal not only general principles of gene evolution but also lineage-specific ecological and evolutionary forces shaping sex-specific expression patterns [
21,
22,
23,
24].
To address these knowledge gaps, we analyzed sex-biased gene expression in 13 insect species representing four major orders: Hemiptera, Diptera, Lepidoptera, and Coleoptera. Our objectives were to (1) identify and characterize sex-biased genes in these species; (2) investigate the evolutionary conservation and divergence of sex-biased gene expression across species; and (3) assess the functional relevance of sex-biased genes exhibiting accelerated evolution in specific lineages.
A particular focus was placed on Tribolium castaneum, in which we observed a unique combination of reversed sex-biased expression and accelerated evolutionary rates. By exploring these patterns, we aim to elucidate how sex-biased gene expression contributes to trait diversification and adaptive evolution. This work provides a foundation for future studies into the molecular mechanisms underlying sexual dimorphism and ecological adaptation in insects.
2. Materials and Methods
2.1. Data Acquisition
All sex-biased genes were obtained from 13 insect species representing four orders—Hemiptera (
Acyrthosiphon pisum), Coleoptera (
Tribolium castaneum), Lepidoptera (
Bombyx mori,
Danaus plexippus), and Diptera (
Aedes aegypti,
Anopheles albimanus,
Anopheles gambiae,
Anopheles stephensi,
Drosophila melanogaster,
D. pseudoobscura,
D. simulans,
D. virilis, and
D. yakuba)—based on the InSexBase database [
25]. Genome annotation files and sex-biased gene expression profiles were retrieved from InSexBase and InsectBase 2.0 for subsequent analyses [
25,
26].
2.2. Orthogroup Clustering and Sequence Alignment
Orthologous relationships among sex-biased genes (SBGs) were inferred using OrthoFinder v2.5.5 [
27]. Protein sequences of SBG orthologs were aligned using the MUFASA.py script from Seqrutinator v1.0 [
28], incorporating MAFFT v7.525 [
29] and HMMER v3.1 [
30]. Subsequently, sequences with potential sequencing errors, gene model inconsistencies, or pseudogene origins were filtered out using the seqrutinator.py script. Both OrthoFinder and Seqrutinator were run with their default parameters (including an MCL inflation value of 1.5 in OrthoFinder), to ensure reproducibility. Biological replicates were already incorporated during the construction of the InSexBase/InsectBase expression datasets, and our analyses were based on the processed expression matrices provided by these databases. Therefore, no additional batch correction was required [
25,
26].
2.3. Phylogenetic Tree Construction and Visualization
Maximum likelihood phylogenetic analysis was conducted using IQ-TREE v2.2.2.9 [
31], with the best-fitting amino acid substitution models selected by ModelFinder (integrated in IQ-TREE v2.2.2.9) [
32]. Nodal support was estimated using the Shimodaira–Hasegawa approximate likelihood ratio test (SH-aLRT) test and ultrafast bootstrapping with 1000 replicates. The resulting phylogenetic trees were visualized using Interactive Tree of Life (iTOL) v6 [
33] and FigTree v1.4.5
http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 23 March 2025).
3. Results
3.1. Summary of Insect Sex-Biased Genes
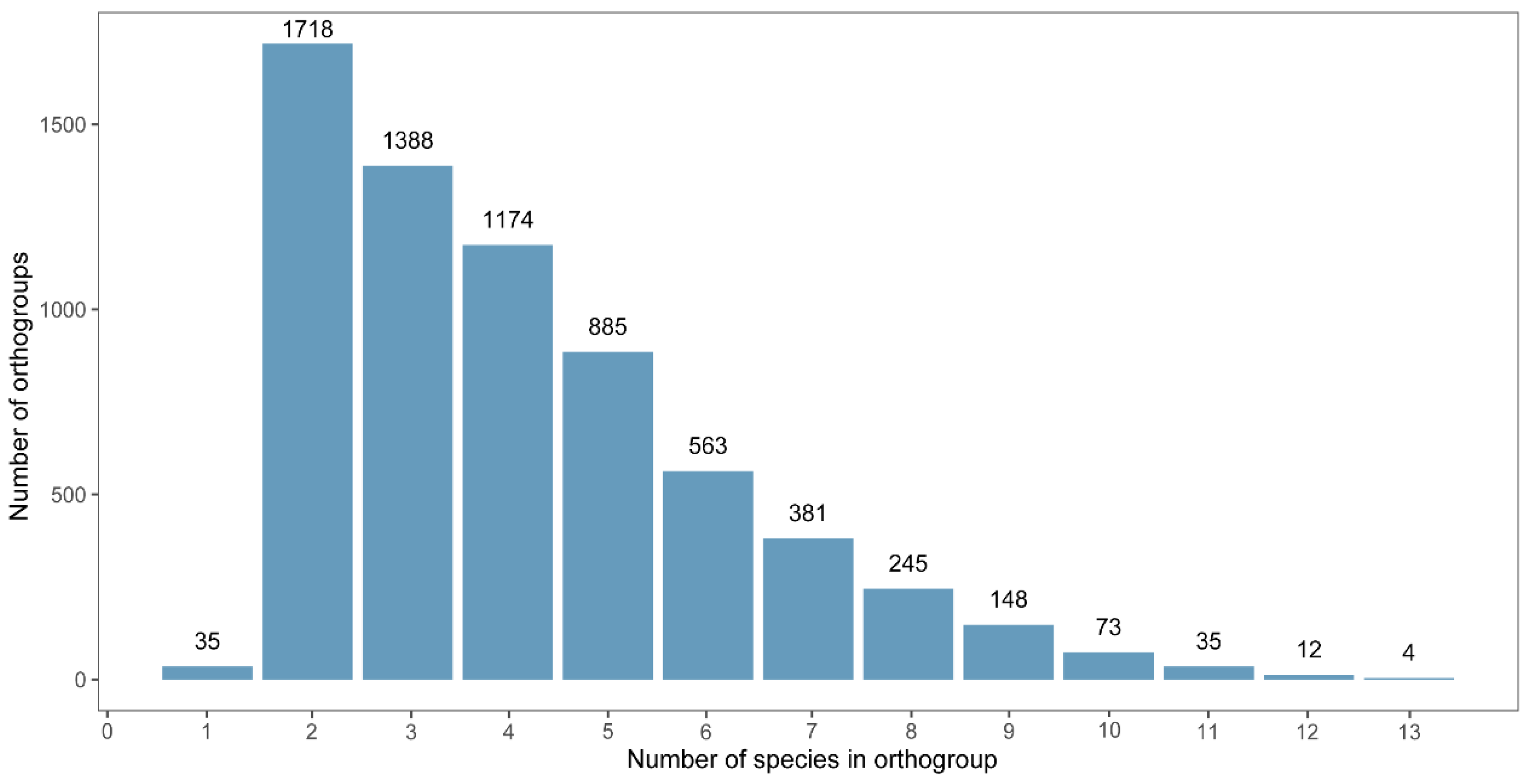
A total of 42,488 sex-biased genes (SBGs) were identified from 13 insect species. Homology-based clustering using OrthoFinder grouped these genes into 6661 orthogroups. Among them, 35 orthogroups were identified as species-specific, meaning that they contained genes from only one species (111 genes in total;
Figure 1). In contrast, four orthogroups were shared by all 13 species, representing the most conserved set across our dataset. Additionally, 181 orthogroups contained single-copy genes that were consistently conserved across all four insect orders.
3.2. Phylogenetic Analysis
To compare the evolutionary dynamics of sex-biased genes (SBGs) with general protein-coding genes, two phylogenetic trees were constructed using single-copy orthologs. A total of 1524 SBG orthologs present in at least five species were used to construct a phylogeny of 13 insect species (
Figure 2B), while 1402 orthologs shared across all species were selected from the entire protein-coding gene set to build a corresponding reference tree (
Figure 2A). Comparative analysis of the two trees revealed a high degree of topological congruence, suggesting that SBGs largely follow evolutionary trajectories similar to those of general protein-coding genes (
Figure 2).
However, a notable topological discrepancy was observed between Drosophila virilis and D. pseudoobscura. In the protein-coding gene tree, these species formed a paraphyletic group, whereas the SBG-based tree supported a monophyletic relationship. This indicates that while SBGs may have distinct functional roles, their broader phylogenetic patterns remain largely consistent with those of general genes.
In contrast, comparison of branch lengths revealed substantial differences in evolutionary rates. In the SBG-derived tree, branch length differences among closely related species were relatively small (
Figure 2B). In contrast,
D. virilis exhibited significantly longer branch lengths in the protein-coding gene tree (
Figure 2A), suggesting that sex-biased genes in this species may have undergone accelerated evolution, potentially reflecting a lineage-specific adaptive trajectory.
3.3. Evolutional Pattens of Conserved Sex-Biased Genes
To investigate the evolutionary conservation of sex-biased gene (SBG) expression, 181 single-copy orthologous gene families were identified. Among them, 12 gene families exhibited consistently male-biased expression across all four insect orders and were defined as “consistent male-biased single-copy orthologous gene families”. Notably, no gene families showed consistent female-biased expression, suggesting that female-biased patterns may be less evolutionarily conserved than male-biased ones.
In contrast, 169 gene families exhibited reversed sex-biased expression across different insect orders, with orthologs showing male-biased expression in some lineages and female-biased expression in others. These were categorized into two groups: non-consistent male-biased and non-consistent female-biased gene families. Specifically, 27 gene families were classified as non-consistent male-biased (i.e., male-biased in three orders and female-biased in one), while 44 were defined as non-consistent female-biased (i.e., female-biased in three orders and male-biased in one). Interestingly, non-consistent female-biased gene families were 63% more frequent than their male-biased counterparts, implying greater variability in female-biased expression. However, this trend may also reflect sampling bias among the sequenced species.
Further analysis of the 44 non-consistent female-biased gene families revealed that
Tribolium castaneum exhibited sex-biased expression reversals in 18 cases, accounting for 40.9% of the total (
Table 1). This frequency was substantially higher than that observed in the second-ranked species,
Bombyx mori and
Anopheles gambiae. In many cases where orthologs in other species were female-biased,
T. castaneum showed a male-biased expression pattern. This distinct reversal trend highlights the lineage-specific dynamics of sex-biased gene expression in
T. castaneum, offering novel insights into its evolutionary divergence.
3.4. Analysis of Sex-Biased Expression Reversal Genes
To further explore the phenotypic implications of sex-biased expression reversal, 18 orthologous gene families with reversed expression patterns in T. castaneum were analyzed. These genes showed male-biased expression in T. castaneum, in contrast to female-biased expression in other insect species.
Phylogenetic trees were constructed based on these 18 gene families. In most cases, gene trees were congruent with the species-level phylogeny (
Figure 3 and
Figure 4), suggesting that the evolutionary trajectories of these genes largely mirrored species evolution. However, three gene trees exhibited distinct patterns (
Figure 4). Specifically, gene trees for OG0001228, OG0002310, and OG0003141 showed substantially longer branch lengths for
T. castaneum orthologs, indicating potential accelerated evolution in this species.
These findings provide new perspectives on the evolutionary significance of sex-biased expression reversal and offer insights into lineage-specific adaptations and evolutionary mechanisms contributing to biodiversity in T. castaneum.
3.5. Functional Analysis of Three Orthogroups with Accelerated Evolution in T. castaneum
A functional analysis was conducted for the three orthogroups exhibiting accelerated evolution in T. castaneum.
The first, OG0001228, corresponds to the proteasome activator complex subunit 3 (
PSME-3) gene (
T. castaneum, LOC663373) (
Table 2). In both nematodes and humans,
PSME-3 has been shown to be closely associated with lifespan regulation [
34]. High expression of this gene extends lifespan in nematodes and delays cellular aging in humans, whereas reduced expression shortens lifespan. Given the observed sex-biased expression of this gene in insects, we examined reported lifespan differences between sexes across various insect species. In taxa where
PSME-3 is female-biased (e.g., mosquitoes, fruit flies, pea aphids, and silkworms), females typically have longer lifespans. Conversely, in
T. castaneum, where the gene is male-biased, males tend to live longer [
35]. These observations are consistent with the hypothesis that
PSME-3 may play a conserved role in lifespan regulation and could contribute to sex-specific lifespan divergence in insects, a possibility that warrants further functional validation.
We analyzed the chromosomal locations of the OG0001228 orthogroup across various species and observed distinct patterns related to sex-specific expression and chromosome type. In species with XY sex determination, genes exhibiting female-biased expression are predominantly located on the X chromosome, as seen in A. albimanus (AALB006416), A. gambiae (AgaP_AGAP000308), A. stephensi (Aste1435_g), and *D. pseudoobscura (LOC4815668). In contrast, T. castaneum (LOC663373) shows male-biased expression, with its orthologous gene situated on an autosome, consistent with males having one fewer X chromosome than females. Similarly, orthologs in A. pisum (LOC100169004) and B. mori (KWMTBOMO04424) are also located on autosomes. These findings suggest that the evolutionary positioning of these genes may reflect a path of least resistance, adapting to the chromosomal architecture and sex-specific expression demands of each species.
The second orthogroup, OG0002310, corresponds to the non-SMC condensin I complex subunit G (
NCAPG) gene (
T. castaneum, LOC662437) (
Table 3). In several avian species,
NCAPG plays a key role in muscle development, influencing muscle mass and body size, and is a target gene in poultry breeding programs [
36,
37]. This gene may similarly contribute to sexually dimorphic traits in insects, particularly in body size and muscle development.
The third orthogroup, OG0003141, corresponds to the leucine-rich repeat containing 15 (
LRRC15) gene (
T. castaneum, LOC103314026) (
Table 4). The human ortholog of
LRRC15 has been implicated in SARS-CoV-2 susceptibility, suggesting a potential role in antiviral defense. Sex-biased expression of
LRRC15 in insects could underlie differences in immune responses and disease resistance between males and females.
4. Discussion
Our findings provide new insights into the evolution and conservation of sex-biased gene (SBG) expression across four major insect orders: Hemiptera, Diptera, Lepidoptera, and Coleoptera. The identification of 42,488 SBGs and their classification into 6661 orthogroups revealed low evolutionary conservation, with 86.5% of orthogroups present in six or fewer species. This highlights the dynamic, lineage-specific nature of sex-biased gene expression in insects, consistent with previous studies demonstrating the rapid evolution frequently associated with such genes [
38,
39].
Phylogenetic analysis using 1524 single-copy SBG orthologs and 1402 single-copy orthologs from general protein-coding genes confirmed that SBGs largely follow evolutionary trajectories similar to those of typical protein-coding genes. The high degree of topological congruence between the two phylogenetic trees suggests that, while SBGs may have lineage- or function-specific roles, their overall evolutionary history is not distinct from other coding genes. However, discrepancies—such as the paraphyly of D. virilis and D. pseudoobscura in the protein-coding tree versus their monophyly in the SBG tree—suggest potential differences in selective pressures acting on sex-biased genes within specific lineages.
Additionally, an accelerated evolutionary rate of SBGs was observed in
D. virilis, as evidenced by increased branch lengths. This may reflect adaptive responses to sex-specific selective pressures or ecological factors unique to the species. Similar patterns have been reported in other taxa, where sexual selection and reproductive conflict drive rapid evolution in sex-biased genes [
40,
41].
The absence of any single-copy orthogroups with consistent female-biased expression across all four orders—contrasting with twelve consistent male-biased families—supports the idea that male-biased gene expression tends to be more evolutionarily conserved. In contrast, the frequent reversals in female-biased expression, particularly in T. castaneum, may suggest relaxed selection or species-specific regulatory mechanisms affecting female-biased genes. These expression reversals may be linked to ecological or life-history traits that demand greater transcriptional plasticity in females.
In addition, dosage compensation mechanisms—by equalizing the expression of sex-linked genes between sexes—may also modulate the extent of observed sex-biased expression. Dosage compensation is well documented in
Drosophila and has also been demonstrated in
A. stephensi [
42,
43]. Differences among insect lineages in the presence or efficiency of dosage compensation may partly explain variation in the strength and chromosomal distribution of sex-biased genes. Moreover, sexual dimorphism in mosquitoes is reflected in feeding behavior, as only females are hematophagous and thus directly exposed to pathogens. This difference may shape immune responses and contribute to sex-biased gene expression in immunity-related pathways, as highlighted by previous studies [
44]. Future studies that integrate these perspectives, together with expanded sampling and functional validation, will help clarify the mechanisms underlying sex-biased gene evolution.
5. Conclusions
In conclusion, this study demonstrates the complex evolutionary dynamics of sex-biased gene expression across four major insect orders. Although sex-biased genes generally follow evolutionary trajectories similar to other protein-coding genes, they exhibit lower conservation across species and show distinct lineage-specific adaptations. Branch length differences in specific sex-biased genes of T. castaneum may suggest lineage-specific ecological or evolutionary pressures, but require further molecular-evolutionary tests to confirm. Moreover, contrasting conservation patterns between male- and female-biased genes highlight divergent selective forces shaping sex-specific traits: male-biased genes appear more conserved, which may reflect stronger purifying selection linked to male reproductive functions, whereas the lower conservation of female-biased genes could be influenced by relaxed constraints or lineage-specific ecological factors.
These findings provide hypotheses regarding the molecular basis of sexual dimorphism and its possible role in shaping genetic and phenotypic diversity in insects. Future work should broaden taxonomic sampling and integrate functional validation to elucidate the regulatory and adaptive mechanisms underlying these patterns. Such efforts will provide deeper insights into how sex-biased gene expression drives evolutionary innovation and ecological diversification across insect lineages.
Author Contributions
Conceptualization, Z.C.; methodology, Y.L., Y.M. and H.Z.; software, H.Z.; validation, Z.C., Y.L. and X.C.; formal analysis, Z.C., Y.L. and X.C.; investigation, H.Z.; resources, Z.C., Y.L. and X.C.; data curation, Z.C. and H.Z.; writing—original draft preparation, Z.C.; writing—review and editing, Y.M. and X.C.; visualization, Z.C., Y.L. and X.C.; supervision, Y.M.; project administration, Z.C., Y.M. and X.C.; funding acquisition, X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhejiang Provincial Natural Science Foundation of China, grant numbers LZ23C140002 and LQZQN25H250003. The APC was funded by the Zhejiang Provincial Natural Science Foundation of China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be provided as requested.
Acknowledgments
We would like to express our sincere gratitude to the organizations and re-searchers who provided access to the public genomic datasets used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Whittle, C.A.; Extavour, C.G. Selection Shapes Turnover and Magnitude of Sex-Biased Expression in Drosophila gonads. BMC Evol. Biol. 2019, 19, 60. [Google Scholar] [CrossRef]
- Rodríguez-Montes, L.; Ovchinnikova, S.; Yuan, X.; Studer, T.; Sarropoulos, I.; Anders, S.; Kaessmann, H.; Cardoso-Moreira, M. Sex-Biased Gene Expression across Mammalian Organ Development and Evolution. Science 2023, 382, eadf1046. [Google Scholar] [CrossRef]
- Ellegren, H.; Parsch, J. The Evolution of Sex-Biased Genes and Sex-Biased Gene Expression. Nat. Rev. Genet. 2007, 8, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Parsch, J.; Ellegren, H. The Evolutionary Causes and Consequences of Sex-Biased Gene Expression. Nat. Rev. Genet. 2013, 14, 83–87. [Google Scholar] [CrossRef]
- Catalán, A.; Hutter, S.; Parsch, J. Population and Sex Differences in Drosophila melanogaster Brain Gene Expression. BMC Genom. 2012, 13, 654. [Google Scholar] [CrossRef]
- Huylmans, A.K.; Parsch, J. Population- and Sex-Biased Gene Expression in the Excretion Organs of Drosophila melanogaster. G3 Genes Genomes Genet. 2014, 4, 2307–2315. [Google Scholar] [CrossRef]
- Perry, J.C.; Harrison, P.W.; Mank, J.E. The Ontogeny and Evolution of Sex-Biased Gene Expression in Drosophila melanogaster. Mol. Biol. Evol. 2014, 31, 1206–1219. [Google Scholar] [CrossRef]
- Wei, D.; Jia, H.-T.; Zhang, M.-Y.; Li, R.; Smagghe, G.; Wang, J.-J. Comparative Analysis of Differential Gene Expression Profiling of Sex-Bias Fat Body of Bactrocera dorsalis (Diptera: Tephritidae) Identifying a New Vitellogenin Gene. Ann. Entomol. Soc. Am. 2018, 111, 43–54. [Google Scholar] [CrossRef]
- Deng, D.; Xing, S.; Liu, X.; Ji, Q.; Zhai, Z.; Peng, W. Transcriptome Analysis of Sex-Biased Gene Expression in the Spotted-Wing Drosophila, Drosophila suzukii (Matsumura). G3 Genes Genomes Genet. 2022, 12, jkac127. [Google Scholar] [CrossRef]
- Wu, C.; Twort, V.G.; Crowhurst, R.N.; Newcomb, R.D.; Buckley, T.R. Assembling Large Genomes: Analysis of the Stick Insect (Clitarchus hookeri) Genome Reveals a High Repeat Content and Sex-Biased Genes Associated with Reproduction. BMC Genom. 2017, 18, 884. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Dumas, Z.; Robinson-Rechavi, M.; Schwander, T.; Parker, D.J. Dynamics of Sex-Biased Gene Expression during Development in the Stick Insect Timema californicum. Heredity 2022, 129, 113–122. [Google Scholar] [CrossRef]
- Rago, A.; Werren, J.H.; Colbourne, J.K. Sex Biased Expression and Co-Expression Networks in Development, Using the Hymenopteran Nasonia vitripennis. PLoS Genet. 2020, 16, e1008518. [Google Scholar] [CrossRef]
- Hu, Q.-L.; Ye, Y.-X.; Zhuo, J.-C.; Huang, H.-J.; Li, J.-M.; Zhang, C.-X. Chromosome-Level Assembly, Dosage Compensation and Sex-Biased Gene Expression in the Small Brown Planthopper, Laodelphax striatellus. Genome Biol. Evol. 2022, 14, evac160. [Google Scholar] [CrossRef]
- Liu, P.-C.; Hao, D.-J.; Hu, H.-Y.; Wei, J.-R. Sexual Dimorphism and Sex-Biased Gene Expression in an Egg Parasitoid Species, Anastatus disparis. BMC Genom. 2020, 21, 492. [Google Scholar] [CrossRef] [PubMed]
- Khodursky, S.; Svetec, N.; Durkin, S.M.; Zhao, L. The Evolution of Sex-Biased Gene Expression in the Drosophila Brain. Genome Res. 2020, 30, 874–884. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wang, H.-L.; Wang, X.-W.; Liu, S.-S. Evolutionary Patterns of Sex-Biased Genes in Three Species of Haplodiploid Insects. Insects 2020, 11, 326. [Google Scholar] [CrossRef]
- Baral, S.; Arumugam, G.; Deshmukh, R.; Kunte, K. Genetic Architecture and Sex-Specific Selection Govern Modular, Male-Biased Evolution of Doublesex. Sci. Adv. 2019, 5, eaau3753. [Google Scholar] [CrossRef] [PubMed]
- Meiklejohn, C.D.; Parsch, J.; Ranz, J.M.; Hartl, D.L. Rapid Evolution of Male-Biased Gene Expression in Drosophila. Proc. Natl. Acad. Sci. USA 2003, 100, 9894–9899. [Google Scholar] [CrossRef]
- Grath, S.; Parsch, J. Rate of Amino Acid Substitution Is Influenced by the Degree and Conservation of Male-Biased Transcription over 50 Myr of Drosophila Evolution. Genome Biol. Evol. 2012, 4, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Liu, L.; Wang, J.; Wei, H.; Li, Y.; Zhang, L.; Guo, Z.; Li, Y.; Liu, T.; Zeng, Q.; et al. Ancient Homomorphy of Molluscan Sex Chromosomes Sustained by Reversible Sex-Biased Genes and Sex Determiner Translocation. Nat. Ecol. Evol. 2022, 6, 1891–1906. [Google Scholar] [CrossRef] [PubMed]
- Galouzis, C.C.; Prud’homme, B. Transvection Regulates the Sex-Biased Expression of a Fly X-Linked Gene. Science 2021, 371, 396–400. [Google Scholar] [CrossRef]
- Laslo, M.; Just, J.; Angelini, D.R. Theme and Variation in the Evolution of Insect Sex Determination. J. Exp. Zoolog. B Mol. Dev. Evol. 2023, 340, 162–181. [Google Scholar] [CrossRef]
- Chikami, Y.; Okuno, M.; Toyoda, A.; Itoh, T.; Niimi, T. Evolutionary History of Sexual Differentiation Mechanism in Insects. Mol. Biol. Evol. 2022, 39, msac145. [Google Scholar] [CrossRef]
- Pal, A.; Vicoso, B. The X Chromosome of Hemipteran Insects: Conservation, Dosage Compensation and Sex-Biased Expression. Genome Biol. Evol. 2015, 7, 3259–3268. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mei, Y.; Chen, M.; Jing, D.; He, Y.; Liu, F.; He, K.; Li, F. InSexBase: An Annotated Genomic Resource of Sex Chromosomes and Sex-Biased Genes in Insects. Database J. Biol. Databases Curation 2021, 2021, baab001. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Jing, D.; Tang, S.; Chen, X.; Chen, H.; Duanmu, H.; Cong, Y.; Chen, M.; Ye, X.; Zhou, H.; et al. InsectBase 2.0: A Comprehensive Gene Resource for Insects. Nucleic Acids Res. 2022, 50, D1040–D1045. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving Fundamental Biases in Whole Genome Comparisons Dramatically Improves Orthogroup Inference Accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef]
- Amalfitano, A.; Stocchi, N.; Atencio, H.M.; Villarreal, F.; Have, A. Seqrutinator: Scrutiny of Large Protein Superfamily Sequence Datasets for the Identification and Elimination of Non-Functional Homologues. Genome Biol. 2024, 25, 230. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Lee, H.J.; Alirzayeva, H.; Koyuncu, S.; Rueber, A.; Noormohammadi, A.; Vilchez, D. Cold Temperature Extends Longevity and Prevents Disease-Related Protein Aggregation through PA28γ-Induced Proteasomes. Nat. Aging 2023, 3, 546–566. [Google Scholar] [CrossRef]
- Pointer, M.D.; Spurgin, L.G.; McMullan, M.; Butler, S.; Richardson, D.S. Life History Correlations and Trade-Offs Resulting from Selection for Dispersal in Tribolium castaneum. J. Evol. Biol. 2024, 37, voae041. [Google Scholar] [CrossRef]
- Hu, X.; Xing, Y.; Fu, X.; Yang, Q.; Ren, L.; Wang, Y.; Li, Q.; Li, J.; Zhang, L. NCAPG Dynamically Coordinates the Myogenesis of Fetal Bovine Tissue by Adjusting Chromatin Accessibility. Int. J. Mol. Sci. 2020, 21, 1248. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Chen, Q.; Wang, D.; Zhang, X.; Huang, X.; Xu, L. Genome-Wide Association Study on Body Conformation Traits in Xinjiang Brown Cattle. Int. J. Mol. Sci. 2024, 25, 10557. [Google Scholar] [CrossRef] [PubMed]
- Baines, J.F.; Sawyer, S.A.; Hartl, D.L.; Parsch, J. Effects of X-Linkage and Sex-Biased Gene Expression on the Rate of Adaptive Protein Evolution in Drosophila. Mol. Biol. Evol. 2008, 25, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.P. Towards a More Nuanced Understanding of the Relationship between Sex-Biased Gene Expression and Rates of Protein-Coding Sequence Evolution. Mol. Biol. Evol. 2011, 28, 1893–1900. [Google Scholar] [CrossRef]
- Harrison, P.W.; Wright, A.E.; Zimmer, F.; Dean, R.; Montgomery, S.H.; Pointer, M.A.; Mank, J.E. Sexual Selection Drives Evolution and Rapid Turnover of Male Gene Expression. Proc. Natl. Acad. Sci. USA 2015, 112, 4393–4398. [Google Scholar] [CrossRef] [PubMed]
- Muyle, A. How Different Is the Evolution of Sex-Biased Gene Expression between Plants and Animals? A Commentary on: Sexual Dimorphism and Rapid Turnover in Gene Expression in Pre-Reproductive Seedlings of a Dioecious Herb. Ann. Bot. 2019, 123, iv–v. [Google Scholar] [CrossRef]
- Lucchesi, J.C.; Kelly, W.G.; Panning, B. Chromatin Remodeling in Dosage Compensation. Annu. Rev. Genet. 2005, 39, 615–651. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Biedler, J.K.; Qi, Y.; Hall, A.B.; Tu, Z. Complete Dosage Compensation in Anopheles stephensi and the Evolution of Sex-Biased Genes in Mosquitoes. Genome Biol. Evol. 2015, 7, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Kajla, M.M.; Gupta, K.; Kakani, P. Identification of an Anopheles Lineage-Specific Unique Heme Peroxidase HPX15: A Plausible Candidate for Arresting Malaria Parasite Development. J. Phylogenet. Evol. Biol. 2015, 3. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).