High Salt Intake Affects Visceral Adipose Tissue Homeostasis: Beneficial Effects of GLP-1 Agonists
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Design
2.2. Systolic Blood Pressure
2.3. Sample Collection
2.3.1. Urine
2.3.2. Serum
2.3.3. Tissues
2.4. Biochemical Determinations
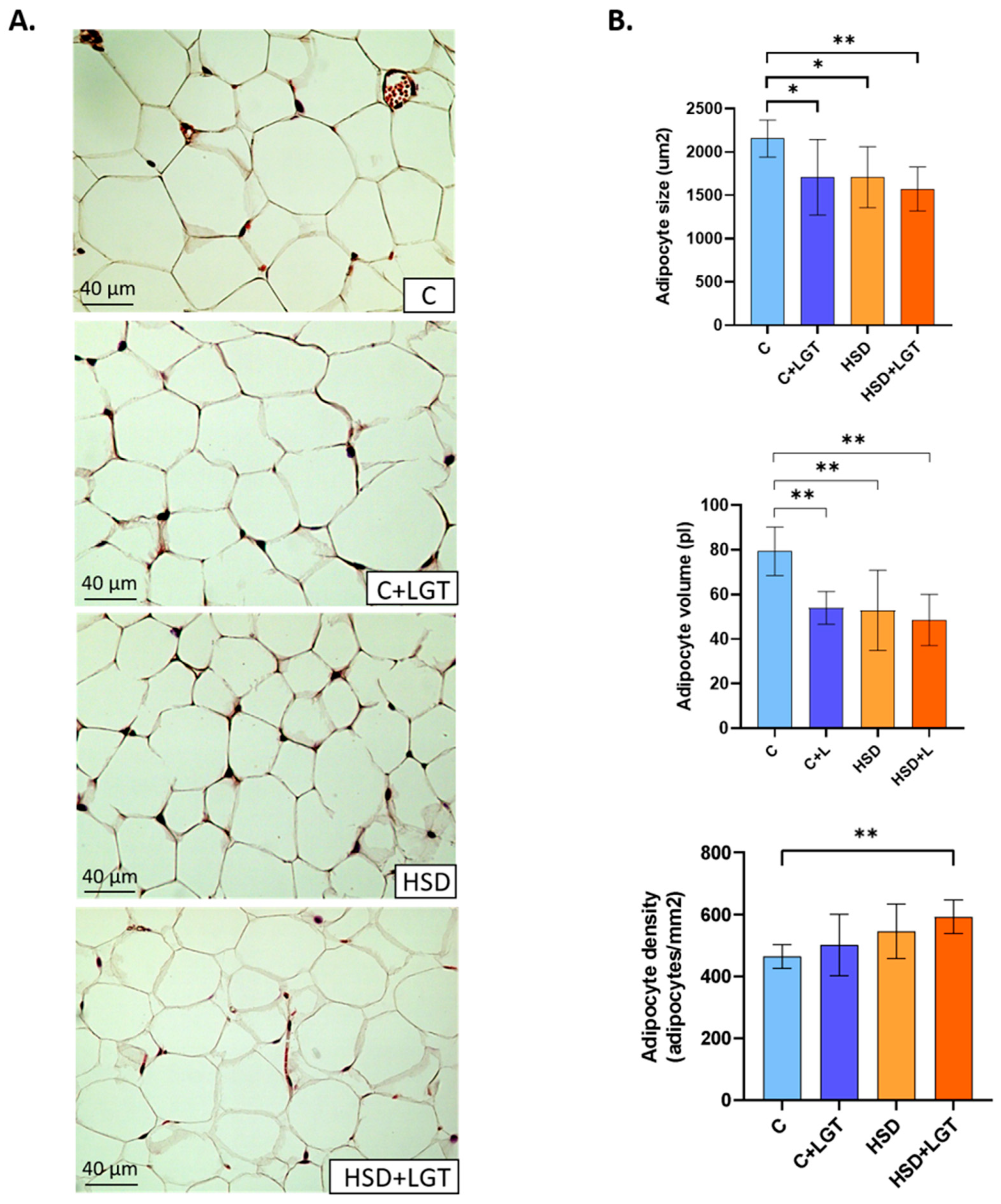
2.5. Histological Analysis
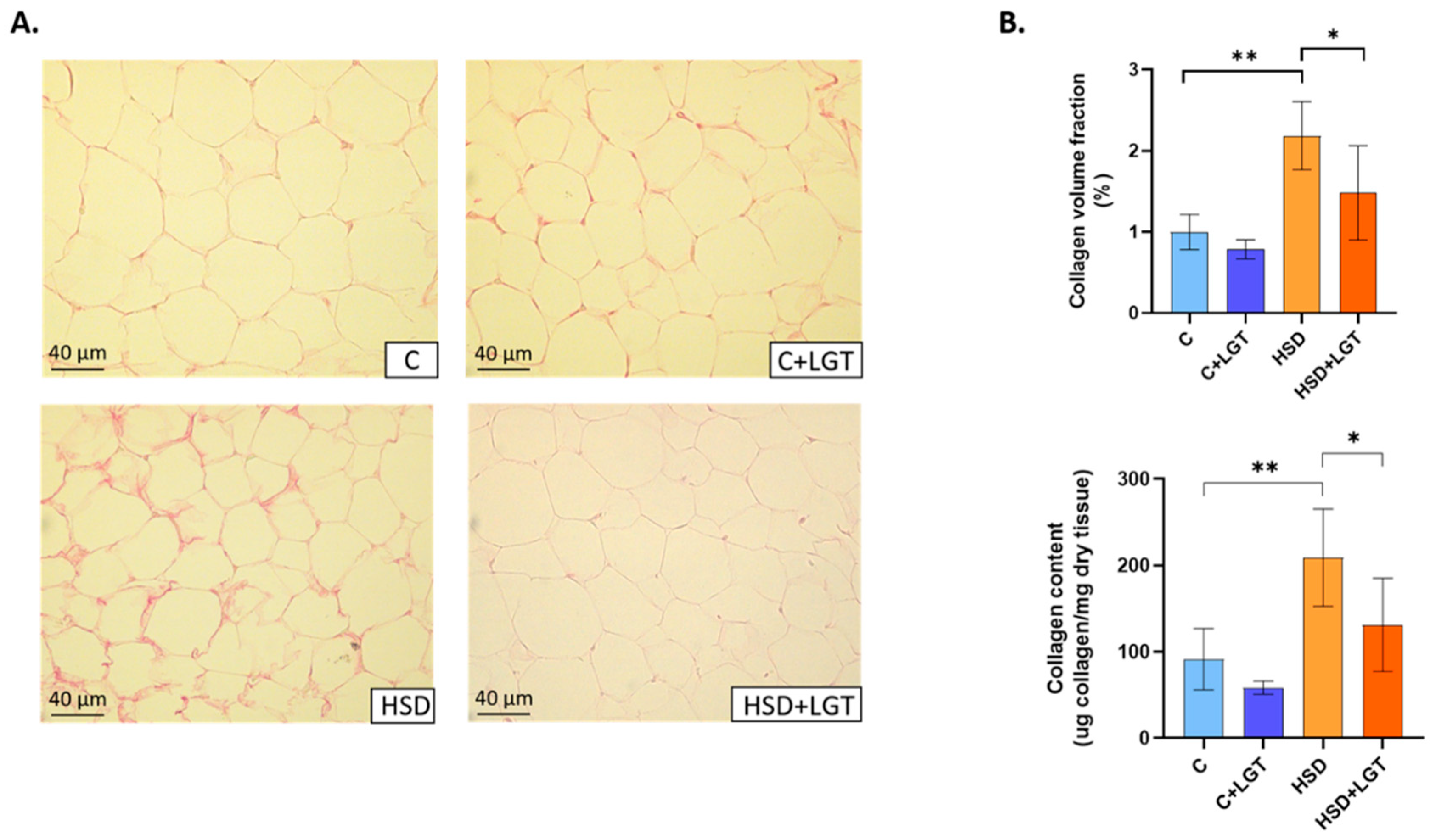
2.6. Adipose Tissue Collagen Content
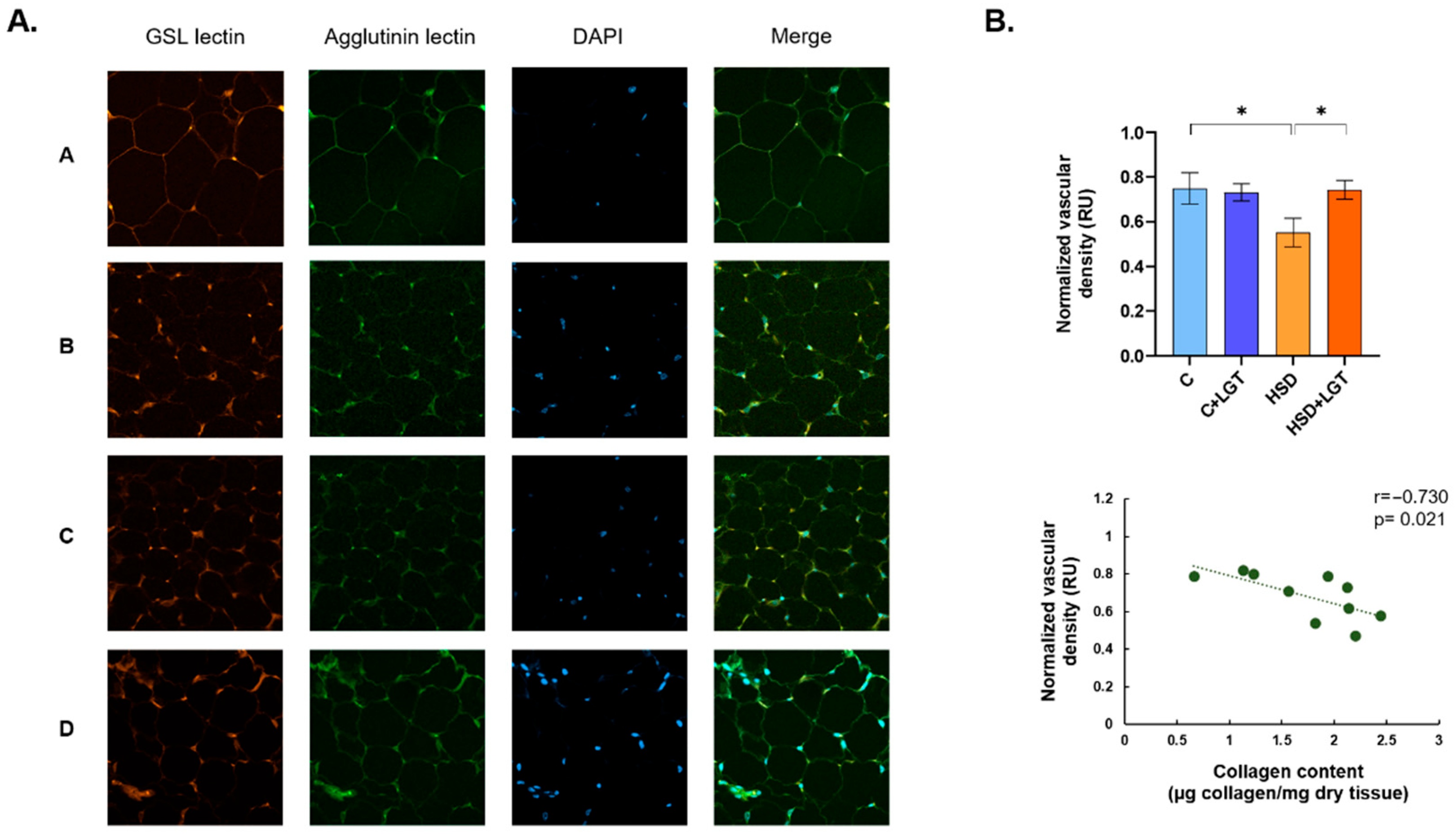
2.7. Vascular Density in EAT
2.8. Oxidative Stress Evaluation
2.8.1. Lipid Peroxidation
2.8.2. Antioxidant Enzyme Activity
2.9. Mitochondrial Morphology Evaluation
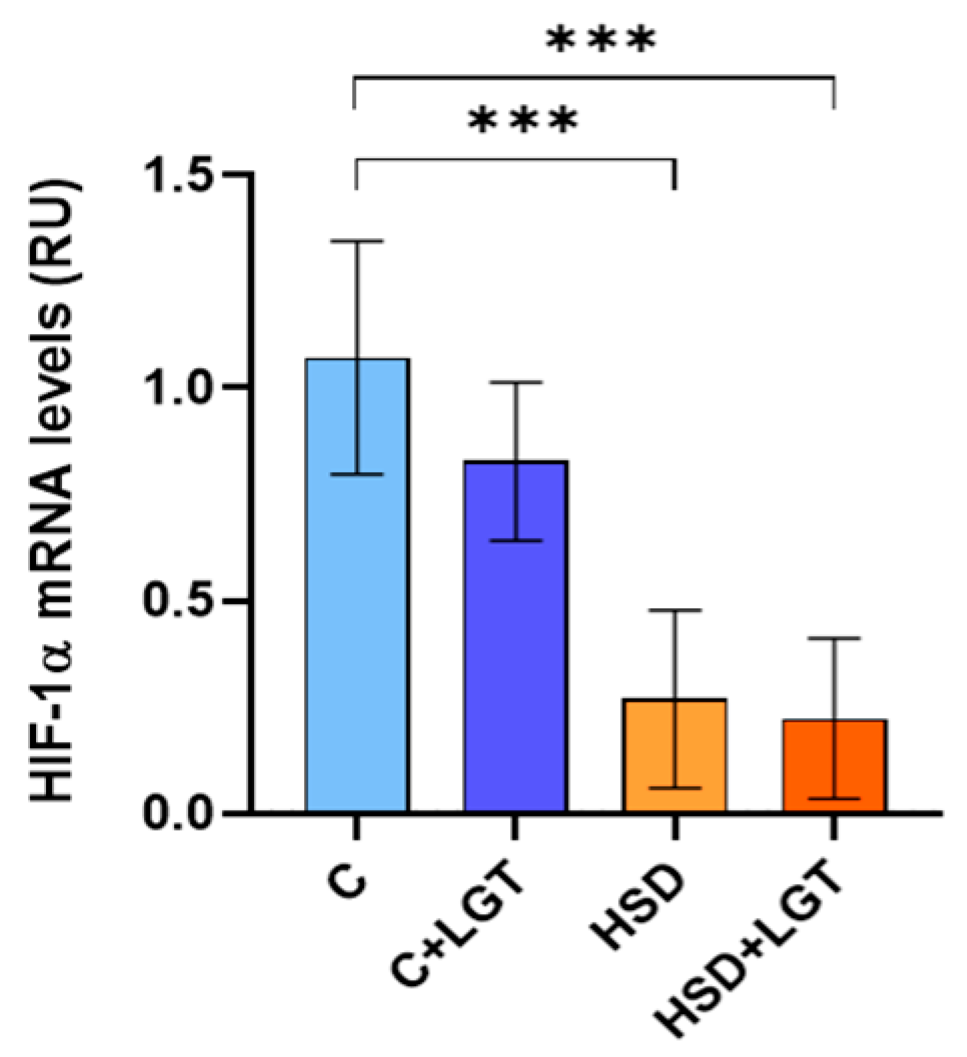
2.10. Hypoxia-Inducible Factor-1α (HIF-1α), Uncoupling Protein-1 (UCP-1), Leptin, and Adiponectin Expression
2.11. Statistical Analysis
3. Results
3.1. General Characteristics of the Animal Model
3.2. Circulating and Renal Functional Parameters
3.3. Histological Analysis of EAT
3.4. Adipose Tissue Collagen Content
3.5. Vascular Density in EAT
3.6. HIF-1α Expression
3.7. Oxidative Stress
3.8. Mitochondrial Characteristics
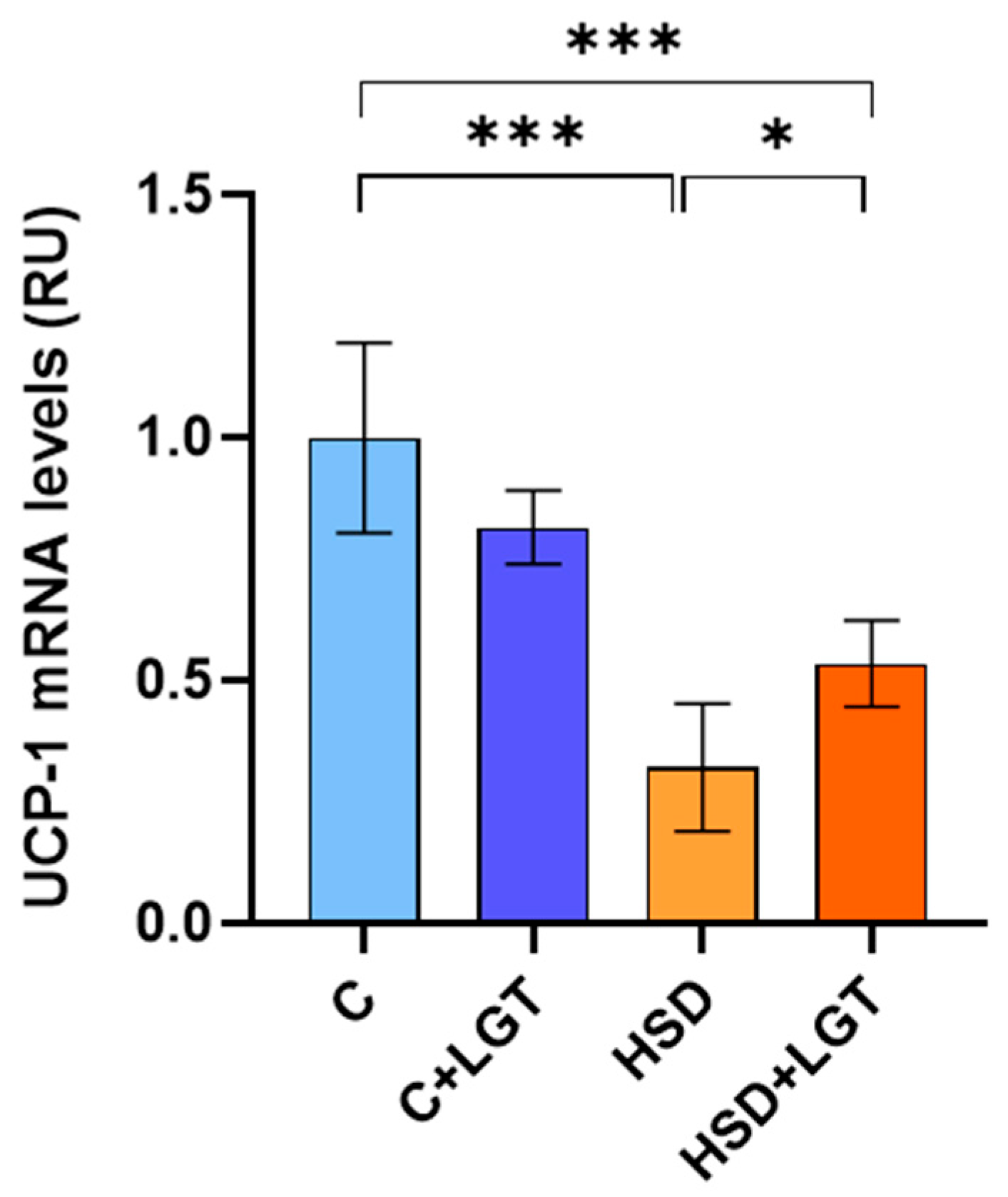
3.9. UCP-1 Expression
3.10. Leptin and Adiponectin Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Vollmer, W.; Sacks, F.M.; Ard, J.; Appel, L.J.; Bray, G.A.; Simons-Morton, D.G.; Conlin, P.R.; Svetkey, L.P.; Erlinger, T.P.; Moore, T.J.; et al. Effects of diet and sodium intake on blood pressure: Subgroup analysis of the DASH-sodium trial. Ann. Intern. Med. 2001, 135, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer-term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomized trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Macgregor, G.A. Salt reduction lowers cardiovascular risk: Metaanalysis of outcome trials. Lancet 2011, 378, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; De Nicola, L.; Conte, G. Dietary salt restriction in chronic kidney disease: A meta-analysis of randomized clinical trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Ministerio de Salud y Desarrollo Social. Encuesta Nacional de Factores de Riesgo; Ministerio de Salud y Desarrollo Social: Buenos Aires, Argentina, 2019. [Google Scholar]
- Kwong, E.J.L.; Whiting, S.; Bunge, A.C.; Leven, Y.; Breda, J.; Rakovac, I.; Cappuccio, F.P.; Wickramasinghe, K. Population-level salt intake in the WHO European Region in 2022: A systematic review. Public Health Nutr. 2023, 26, s6–s19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, A.; Edwards, D.; Farquhar, W. The Influence of Dietary Salt Beyond Blood Pressure. Curr. Hypertens. Rep. 2019, 21, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.C.; Koo, H.; Kim, S.; Chin, H. Estimation of daily salt intake through a 24-hour urine collection in Pohang, Korea. J. Korean Med. Sci. 2014, 29 (Suppl. S2), S87–S90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, Y.; Lee, J.; Chang, Y.; Kim, M.; Sung, E.; Shin, H.; Ryu, S. Dietary sodium and potassium intake in relation to non-alcoholic fatty liver disease. Br. J. Nutr. 2016, 116, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Kitada, K.; Daub, S.; Zhang, Y.; Klein, J.D.; Nakano, D.; Pedchenko, T.; Lantier, L.; LaRocque, L.M.; Marton, A.; Neubert, P.; et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J. Clin. Investig. 2017, 127, 1944–1959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- White, U. Adipose tissue expansion in obesity, health, and disease. Front. Cell Dev. Biol. 2023, 11, 1188844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, Q.; An, Y.A.; Scherer, P.E. Mitochondrial regulation and white adipose tissue homeostasis. Trends Cell Biol. 2022, 32, 351–364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miksztowicz, V.; Morales, C.; Zago, V.; Friedman, S.; Schreier, L.; Berg, G. Effect of insulin-resistance on circulating and adipose tissue MMP-2 and MMP-9 activity in rats fed a sucrose-rich diet. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marcelin, G.; Gautier, E.L.; Clément, K. Adipose Tissue Fibrosis in Obesity: Etiology and Challenges. Annu. Rev. Physiol. 2022, 84, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Touceda, V.; Fontana Estevez, F.; Cacciagiú, L.; Finocchietto, P.; Bustos, R.; Vidal, A.; Berg, G.; Morales, C.; González, G.; Miksztowicz, V. Liraglutide improves adipose tissue remodeling and mitochondrial dynamics in a visceral obesity model induced by a high-fat diet. Curr. Res. Pharmacol. Drug Discov. 2024, 6, 100185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fonseca-Alaniz, M.H.; Takada, J.; Andreotti, S.; de Campos, T.B.; Campaña, A.B.; Borges-Silva, C.N.; Lima, F.B. High sodium intake enhances insulin-stimulated glucose uptake in rat epididymal adipose tissue. Obesity 2008, 16, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, P.; Sun, F.; Li, Q.; Chen, J.; Yu, H.; Li, L.; Wei, X.; He, H.; Lu, Z.; et al. Sodium Intake Regulates Glucose Homeostasis through the PPARδ/Adiponectin-Mediated SGLT2 Pathway. Cell Metab. 2016, 23, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Milano, W.; De Biasio, V.; Di Munzio, W.; Foggia, G.; Capasso, A. Obesity: The New Global Epidemic Pharmacological Treatment, Opportunities and Limits for Personalized Therapy. Endocr. Metab. Immune Disord.-Drug Targets 2020, 20, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Iepsen, E.W.; Torekov, S.S.; Holst, J.J. Liraglutide for Type 2 diabetes and obesity: A 2015 update. Expert Rev. Cardiovasc. Ther. 2015, 13, 753–767. [Google Scholar] [CrossRef] [PubMed]
- FDA. US Food & Drug Administration. 2010. Available online: https://web.archive.org/web/20130307084113/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm198638.htm (accessed on 12 December 2024).
- FDA. US Food & Drug Administration. 2020. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-weight-management-drug-patients-aged-12-and-older (accessed on 12 December 2024).
- Müller, T.; Finan, B.; Bloom, S.; D’ALessio, D.; Drucker, D.; Flatt, P.; Fritsche, A.; Gribble, F.; Grill, H.; Habener, J.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Challa, T.D.; Beaton, N.; Arnold, M.; Rudofsky, G.; Langhans, W.; Wolfrum, C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J. Biol. Chem. 2012, 287, 6421–6430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Du, J.; Zhu, E.; Zhang, J.; Han, J.; Zhao, W.; Sun, B.; Tian, D. Liraglutide suppresses proliferation and induces adipogenic differentiation of 3T3-L1 cells via the Hippo-YAP signaling pathway. Mol. Med. Rep. 2018, 17, 4499–4507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamura, K.; Minami, K.; Kudo, M.; Iemoto, K.; Takahashi, H.; Seino, S. Liraglutide improves pancreatic Beta cell mass and function in alloxan-induced diabetic mice. PLoS ONE 2015, 10, e0126003. [Google Scholar] [CrossRef]
- Liu, Y.T.; He, T.; Li, H.Q.; Jiang, P. Liraglutide improves pancreatic islet β cell apoptosis in rats with type 2 diabetes mellitus by inhibiting the IKKε/NF-κB pathway. Eur. Rev. Med. Pharmacol Sci. 2021, 25, 4818–4828. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- González, G.E.; Rhaleb, N.-E.; D’AMbrosio, M.A.; Nakagawa, P.; Liao, T.-D.; Peterson, E.L.; Leung, P.; Dai, X.; Janic, B.; Liu, Y.-H.; et al. Cardiac-deleterious role of galectin-3 in chronic angiotensin II-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1287–H1296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldrick, R.B. Morphological changes in the adipocyte during fat deposition and mobilization. Am. J. Physiol. 1967, 212, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Stoilov, I.; Starcher, B.C.; Mecham, R.P.; Broekelmann, T.J. Measurement of elastin, collagen, and total protein levels in tissues. Methods Cell Biol. 2018, 143, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Estevez, F.S.F.; Betazza, M.C.; Miksztowicz, V.; Seropian, I.M.; Silva, M.G.; Penas, F.; Touceda, V.; Selser, C.; Villaverde, A.; Goren, N.; et al. Genetic Deletion of Galectin-3 Exacerbates Age-Related Myocardial Hypertrophy and Fibrosis in Mice. Cell Physiol. Biochem. 2022, 56, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Chanmuang, S.; Kim, B.M.; Gu, S.Y.; Son, Y.J.; Le, H.G.; Nam, Y.D.; Song, E.J.; Ham, K.S.; Kim, H.J. Effects of sea salt intake on metabolites, steroid hormones, and gut microbiota in rats. PLoS ONE 2022, 17, e0269014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, H.; Gao, X.; Du, H.; Lin, Z.; Huang, X. Changes in microbial and metabolic profiles of mice fed with long-term high salt diet. BMC Gastroenterol. 2025, 25, 375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysiological cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muñoz-Durango, N.; Fuentes, C.A.; Castillo, A.E.; González-Gómez, L.M.; Vecchiola, A.; Fardella, C.E.; Kalergis, A.M. Role of the Renin-Angiotensin-Aldosterone System beyond Blood Pressure Regulation: Molecular and Cellular Mechanisms Involved in End-Organ Damage during Arterial Hypertension. Int. J. Mol. Sci. 2016, 17, 797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanaka, H.; Sun, T.; Kinashi, H.; Kamiya, K.; Yamaguchi, M.; Nobata, H.; Sakata, F.; Kim, H.; Mizuno, M.; Kunoki, S.; et al. Interleukin-6 blockade reduces salt-induced cardiac inflammation and fibrosis in subtotal nephrectomized mice. Am. J. Physiol. Renal Physiol. 2022, 323, F654–F665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yuan, F.; Ji, W.J.; Guo, Z.Z.; Zhang, L.; Lu, R.Y.; Liu, X.; Liu, H.M.; Zhang, W.C.; Jiang, T.M.; et al. High-salt intake induced visceral adipose tissue hypoxia and its association with circulating monocyte subsets in humans. Obesity 2014, 22, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Jusman, S.W.; Halim, A.; Wanandi, S.I.; Sadikin, M. Expression of hypoxia-inducible factor-1alpha (HIF-1alpha) related to oxidative stress in liver of rat-induced by systemic chronic normobaric hypoxia. Acta Med. Indones. 2010, 42, 17–23. [Google Scholar] [PubMed]
- Kobayashi, Y.; Oguro, A.; Imaoka, S. Feedback of hypoxia-inducible factor-1alpha (HIF-1alpha) transcriptional activity via redox factor-1 (Ref-1) induction by reactive oxygen species (ROS). Free. Radic. Res. 2021, 55, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Touceda, V.; Finocchietto, P.; Morales, C.; Cevey, A.; López, G.; Bermejo, M.; Quintanilla, M.; Barchuk, M.; Friedman, S.; Goren, N.; et al. GLP-1 agonists and insulin resistance: Effects of Liraglutide on adipose tissue remodeling. LXIV Reunión Anual de la Sociedad Argentina de Investigación Clínica (SAIC). Fund. Rev. Med. 2019, 79 (Suppl. S4), 201. [Google Scholar]
- Yanes, B.; Rainero, E. The Interplay between Cell-Extracellular Matrix Interaction and Mitochondria Dynamics in Cancer. Cancers 2022, 14, 1433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yapa, N.M.B.; Lisnyak, V.; Reljic, B.; Ryan, M.T. Mitochondrial dynamics in health and disease. FEBS Lett. 2021, 595, 1184–1204. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Lanza, I.R.; Swain, J.M.; Sarr, M.G.; Nair, K.S.; Jensen, M.D. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J. Clin. Endocrinol. Metab. 2014, 99, E209–E216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Dobrian, A.D.; Schriver, S.D.; Lynch, T.; Prewitt, R.L. Effect of salt on hypertension and oxidative stress in a rat model of diet-induced obesity. Am. J. Physiol. Renal Physiol. 2003, 285, F619–F628. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Harper, M.E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 2011, 51, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Q.; Burley, G.; Li, L.C.; Lin, S.; Shi, Y.C. The role of dietary salt in metabolism and energy balance: Insights beyond cardiovascular disease. Diabetes Obes. Metab. 2023, 25, 1147–1161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baldelli, S.; Aiello, G.; Mansilla Di Martino, E.; Campaci, D.; Muthanna, F.M.S.; Lombardo, M. The Role of Adipose Tissue and Nutrition in the Regulation of Adiponectin. Nutrients 2024, 16, 2436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| C | HSD | |
|---|---|---|
| Initial body weight (g) | 24.5 ± 1.0 | 24.3 ± 1.3 |
| Pre-treatment weight (g) | 31.3 ± 1.6 | 25.8 ± 1.6 *** |
| Food consumption (g/day/100 g b.w.) | 19.5 ± 1.7 | 23.2 ± 3.2 ** |
| Water intake (mL/day/100 g b.w.) | 27 ± 5 | 122 ± 16 *** |
| Caloric intake (kcal/day/100 g b.w.) | 37 ± 4 | 40 ± 6 |
| Initial SBP (mmHg) | 110 ± 9 | 108 ± 5 |
| Pre-treatment SBP(mmHg) | 107 ± 6 | 125 ± 2 *** |
| C | C + LGT | HSD | HSD + LGT | |
|---|---|---|---|---|
| Final body weight (g) | 32.5 ± 1.8 | 31.6 ± 2.1 | 27.0 ± 2.7 ***a,**b | 26.8 ± 1.7 ***a,b |
| Food consumption (g/day/100 g b.w.) | 17.7 (12–29.8) | 17.2 (9.6–24.4) | 21.8 (16.5–28.2) | 19.5 (17.1–23.0) |
| Water intake (mL/day/100 g b.w.) | 24 (19–36) | 18 (12–35) | 129 (91–158) ***a,b | 81 (73–108) *a,b |
| Caloric intake (kcal/day/100 g b.w.) | 36 ± 8 | 33 ± 6 | 38 ± 6 | 35 ± 3 |
| FER (%) | 0.85 (0.59–1.23) | 1.05 (0.93–1.05) | 0.24 (0.01–0.72) *a,b | 0.25 (0.20–0.41) *a,b |
| SBP(mmHg) | 114 ± 8 | 103 ± 9 | 133 ± 10 **a,***b | 117 ± 8 *b,*c |
| EAT mass (mg) | 614 ± 171 | 445 ± 158 *a | 305 ± 100 ***a,*b | 281 ± 67 ***a,*b |
| Tibia length (mm) | 16.6 ± 0.6 | 16.4 ± 0.6 | 16.5 ± 0.4 | 16.7 ± 0.8 |
| Final body weight/tibia length (g/mm) | 2.0 ± 0.1 | 1.9 ± 0.1 | 1.6 ± 0.2 ***a,b | 1.6 ± 0.1 ***a,b |
| EAT mass/tibia length (mg/mm) | 36.9 ± 10.0 | 27.1 ± 8.8 | 19.0 ± 5.6 ***a | 17.8 ± 5.1 ***a,*b |
| C | C + LGT | HSD | HSD + LGT | |
|---|---|---|---|---|
| Sodium (mmol/L) | 151 ± 1 | 152 ± 2 | 153 ± 6 | 153 ± 6 |
| Creatinine (mg/dL) | 0.17 ± 0.04 | 0.13 ± 0.03 | 0.14 ± 0.03 | 0.16 ± 0.08 |
| Glucose (mg/dL) | 222 ± 34 | 203 ± 44 | 229 ± 75 | 257 ± 75 |
| Triglycerides (mg/dL) | 63 (38–91) | 67 (44–103) | 48 (33–70) | 59 (28–85) |
| Total cholesterol (mg/dL) | 92 ± 10 | 80 ± 11 | 85 ± 11 | 89 ± 10 |
| HDL cholesterol (mg/dL) | 77 ± 11 | 66 ± 11 | 69 ± 13 | 74 ± 5 |
| Non-HDL cholesterol (mg/dL) | 18 ± 6 | 17 ± 5 | 17 ± 3 | 19 ± 3 |
| C | C + LGT | HSD | HSD + LGT | |
|---|---|---|---|---|
| Urinary volume (mL/24 h) | 0.4 ± 0.1 | 0.6 ± 0.3 | 14.5 ± 0.9 ***a,b | 10.7 ± 2.2 *a |
| uNa (μg/24 h) | 47 ± 23 | 54 ± 24 | 3186 ± 1046 ***a,b | 2152 ± 344 *a,b |
| FENa (%) | 0.32 ± 0.18 | 0.27 ± 0.12 | 8.39 ± 2.56 ***a,b | 10.70 ± 2.89 ***a,b |
| Creatinine (μg/24 h) | 138 ± 38 | 190 ± 108 | 287 ± 70 | 411 ± 233 *a |
| Standardized CrCl (µL/min) | 70 ± 30 | 81 ± 36 | 150 ± 33 *a,b | 86 ± 25 *c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touceda, V.; Cacciagiú, L.; Moglie, I.B.; Wiszniewski, M.; Sanchez, V.; De Lucca, R.C.; Vidal, A.; Finocchietto, P.; Friedman, S.; González, G.E.; et al. High Salt Intake Affects Visceral Adipose Tissue Homeostasis: Beneficial Effects of GLP-1 Agonists. Biology 2025, 14, 1171. https://doi.org/10.3390/biology14091171
Touceda V, Cacciagiú L, Moglie IB, Wiszniewski M, Sanchez V, De Lucca RC, Vidal A, Finocchietto P, Friedman S, González GE, et al. High Salt Intake Affects Visceral Adipose Tissue Homeostasis: Beneficial Effects of GLP-1 Agonists. Biology. 2025; 14(9):1171. https://doi.org/10.3390/biology14091171
Chicago/Turabian StyleTouceda, Vanessa, Leonardo Cacciagiú, Ignacio Barbani Moglie, Morena Wiszniewski, Valeria Sanchez, Romina C. De Lucca, Agustina Vidal, Paola Finocchietto, Silvia Friedman, Germán E. González, and et al. 2025. "High Salt Intake Affects Visceral Adipose Tissue Homeostasis: Beneficial Effects of GLP-1 Agonists" Biology 14, no. 9: 1171. https://doi.org/10.3390/biology14091171
APA StyleTouceda, V., Cacciagiú, L., Moglie, I. B., Wiszniewski, M., Sanchez, V., De Lucca, R. C., Vidal, A., Finocchietto, P., Friedman, S., González, G. E., & Miksztowicz, V. (2025). High Salt Intake Affects Visceral Adipose Tissue Homeostasis: Beneficial Effects of GLP-1 Agonists. Biology, 14(9), 1171. https://doi.org/10.3390/biology14091171









