Nutraceutical Interception of Cachexia: Grape-Derived Compounds as Pathophysiological Network Modulators
Simple Summary
Abstract
1. Introduction
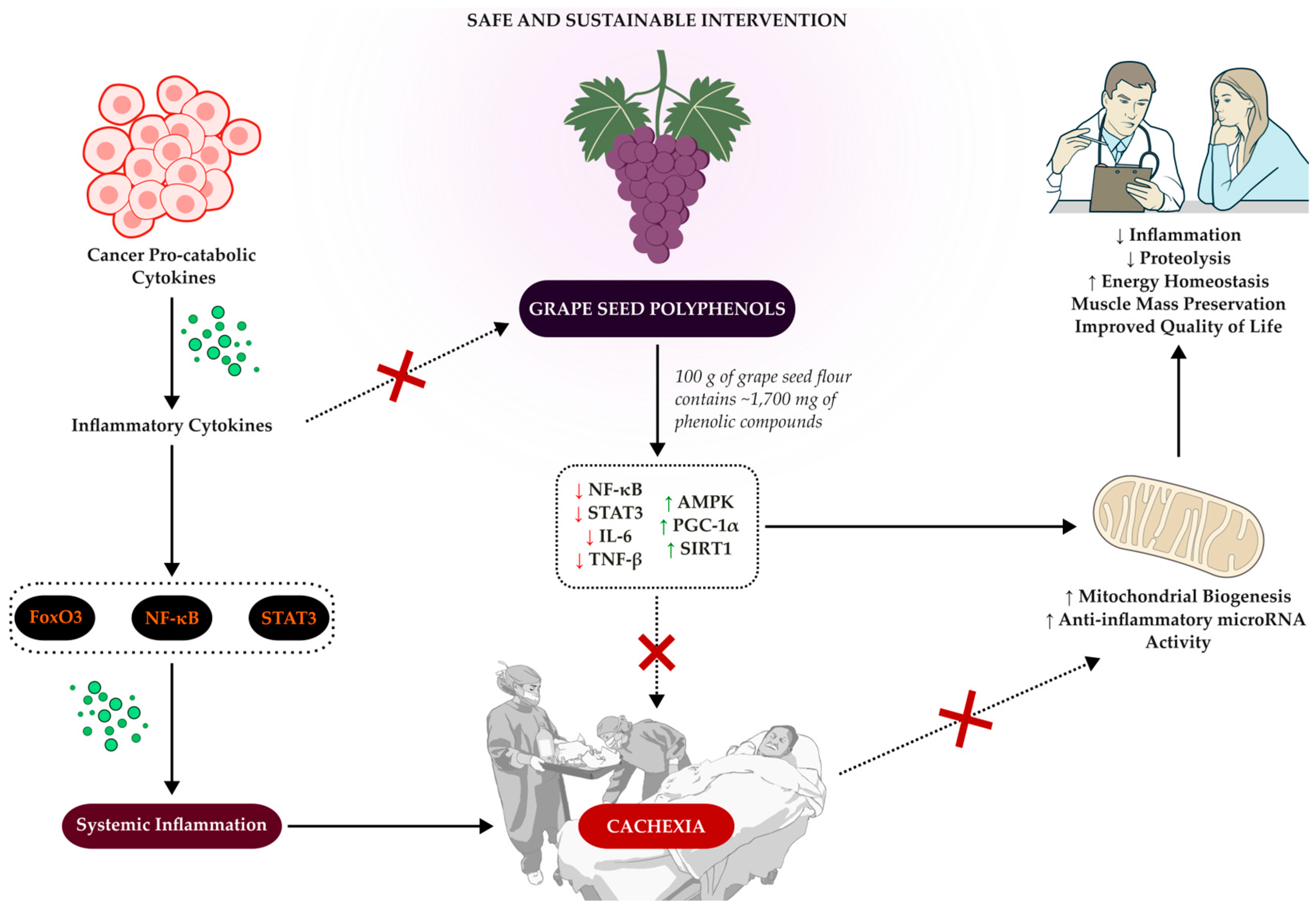
2. Pathophysiology of Cancer-Associated Cachexia
2.1. Molecular Signaling Pathways in Cancer-Associated Cachexia
3. Intracellular and Extracellular Signaling Pathways in Cancer Cachexia
4. Perspectives in the Treatment of Cancer-Related Cachexia
5. Grape Seed Flour: Origin, Processing, and Nutritional Composition
6. Polyphenols and Fiber in Grape Seeds
7. Digestion, Absorption, and Metabolism of Grape Seed Polyphenols
8. Experimental Evidence on Grape Polyphenols and Their Applicability to Cancer-Related Cachexia
9. Clinical Evidence and Safety Profile of Grape Seed Polyphenols
10. Potential Interventions with Grape Seed in the Management of Cachexia
11. Future Perspectives
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 16S rRNA | 16 Svedberg ribosomal RNA |
| ALT | Alanine Aminotransferase |
| AMPK | AMP-activated Protein Kinase |
| AST | Aspartate Aminotransferase |
| CAAE | Certificado de Apresentação para Apreciação Ética |
| CRC | Colorectal Cancer |
| CRP | C-reactive Protein |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DRP1 | Dynamin-related Protein 1 |
| EORTC QLQ-C30 | European Organization for Research and Treatment of Cancer—Quality of Life Questionnaire—Core 30 |
| EPA | Eicosapentaenoic Acid |
| ERR-α | Estrogen-related Receptor Alpha |
| EVs | Extracellular Vesicles |
| FACIT-F | Functional Assessment of Chronic Illness Therapy—Fatigue Scale |
| GLOBOCAN | Global Cancer Observatory |
| GSE | Grape Seed Extract |
| GSF | Grape Seed Flour |
| HDAC | Histone Deacetylase |
| IFN-γ | Interferon gamma |
| IGF-1 | Insulin-like Growth Factor 1 |
| IGFBP-3 | Insulin-like Growth Factor Binding Protein 3 |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| INCA | Instituto Nacional de Câncer |
| MFSI-SF | Multidimensional Fatigue Symptom Inventory—Short Form |
| mTORC1 | Mechanistic Target of Rapamycin Complex 1 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| ORAC | Oxygen Radical Absorbance Capacity |
| PCNA | Proliferating Cell Nuclear Antigen |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha |
| PTHrP | Parathyroid Hormone-related Protein |
| QoL | Quality of Life |
| RNA | Ribonucleic Acid |
| ReBEC | Registro Brasileiro de Ensaios Clínicos |
| SIRT1 | Sirtuin 1 |
| Smad2/3 | SMAD Family Members 2 and 3 |
| SOCS3 | Suppressor of Cytokine Signaling 3 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TNF-α | Tumor Necrosis Factor alpha |
| UNIFESP | Universidade Federal de São Paulo |
| UPS | Ubiquitin-Proteasome System |
| WAT | White Adipose Tissue |
| WMD | Weighted Mean Difference |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Miller, K.D.; Dale, W.; Mohile, S.G.; Cohen, H.J.; Leach, C.R.; Sauer, A.G.; Jemal, A.; Siegel, R.L. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J. Clin. 2019, 69, 452–467. [Google Scholar] [CrossRef]
- World Health Organization International Agency for Research on Cancer (IARC). World Cancer Report: Cancer Research for Cancer Prevention; World Health Organization International Agency for Research on Cancer (IARC): Lyon, France, 2020; Volume 199.
- Instituto Nacional de Câncer (INCA). Estimativa 2023: Incidência De Câncer No Brasil; Instituto Nacional de Câncer (INCA): Rio de Janeiro-RJ, Brazil, 2022.
- Instituto Nacional de Câncer (INCA). Câncer de cólon e reto 2023. Available online: https://www.gov.br/inca/pt-br/assuntos/cancer/numeros/estimativa/sintese-de-resultados-e-comentarios/cancer-de-colon-e-reto (accessed on 16 December 2024).
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Dolin, T.G.; Mikkelsen, M.K.; Jakobsen, H.L.; Vinther, A.; Zerahn, B.; Nielsen, D.L.; Johansen, J.S.; Lund, C.M.; Suetta, C. The prevalence of sarcopenia and cachexia in older patients with localized colorectal cancer. J. Geriatr. Oncol. 2023, 14, 101402. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Meyerhardt, J.A. Obesity and Energy Balance in GI Cancer. J. Clin. Oncol. 2016, 34, 4217–4224. [Google Scholar] [CrossRef]
- Bozzetti, F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Ní Bhuachalla, Ē.; Prado, C.M. Cancer-associated malnutrition, cachexia and sarcopenia: The skeleton in the hospital closet 40 years later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef]
- Muscaritoli, M.; the PreMiO Study Group; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Talbert, E.E.; Cuitiño, M.C.; Ladner, K.J.; Rajasekerea, P.V.; Siebert, M.; Shakya, R.; Leone, G.W.; Ostrowski, M.C.; Paleo, B.; Weisleder, N.; et al. Modeling Human Cancer-induced Cachexia. Cell Rep. 2019, 28, 1612–1622.e4. [Google Scholar] [CrossRef]
- Tisdale, M.J. Mechanisms of Cancer Cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2016, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Smuder, A.J.; Criswell, D.S. Mechanistic Links Between Oxidative Stress and Disuse Muscle Atrophy. Antioxid. Redox Signal. 2011, 15, 2519–2528. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Das, S.K.; Eder, S.; Schauer, S.; Diwoky, C.; Temmel, H.; Guertl, B.; Gorkiewicz, G.; Tamilarasan, K.P.; Kumari, P.; Trauner, M.; et al. Adipose Triglyceride Lipase Contributes to Cancer-Associated Cachexia. Science 2011, 333, 233–238. [Google Scholar] [CrossRef]
- Tsoli, M.; Robertson, G. Cancer cachexia: Malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol. Metab. 2013, 24, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.-F.; Peyta, L.; Couet, C.; Servais, S. Implication of liver cardiolipins in mitochondrial energy metabolism disorder in cancer cachexia. Biochimie 2013, 95, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M. Muscle wasting: The gut microbiota as a new therapeutic target? Int. J. Biochem. Cell Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.-P.; et al. Restoring Specific Lactobacilli Levels Decreases Inflammation and Muscle Atrophy Markers in an Acute Leukemia Mouse Model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xi, Q.; Tan, S.; Qu, Y.; Meng, Q.; Zhang, Y.; Cheng, Y.; Wu, G. The metabolite butyrate produced by gut microbiota inhibits cachexia-associated skeletal muscle atrophy by regulating intestinal barrier function and macrophage polarization. Int. Immunopharmacol. 2023, 124, 111001. [Google Scholar] [CrossRef]
- von Haehling, S.; Anker, S.D. Treatment of Cachexia: An Overview of Recent Developments. J. Am. Med. Dir. Assoc. 2014, 15, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.; Zhang, G.; Fattah, E.A.A.; Eissa, N.T.; Li, Y. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2011, 25, 99–110. [Google Scholar] [CrossRef]
- Cosper, P.F.; Leinwand, L.A. Cancer Causes Cardiac Atrophy and Autophagy in a Sexually Dimorphic Manner. Cancer Res. 2011, 71, 1710–1720. [Google Scholar] [CrossRef]
- Garcia, V.R.; López-Briz, E.; Sanchis, R.C.; Perales, J.L.G.; Bort-Martí, S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst. Rev. 2013, 2019, CD004310. [Google Scholar] [CrossRef]
- Ferrer, M.; Anthony, T.G.; Ayres, J.S.; Biffi, G.; Brown, J.C.; Caan, B.J.; Feliciano, E.M.C.; Coll, A.P.; Dunne, R.F.; Goncalves, M.D.; et al. Cachexia: A systemic consequence of progressive, unresolved disease. Cell 2023, 186, 1824–1845. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, A.; Aydogdu, T.; Jin, X.; Zhang, Z.; Zhan, R.; Puzis, L.; Koniaris, L.G.; Zimmers, T.A. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E410–E421. [Google Scholar] [CrossRef]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef]
- Davinelli, S.; De Stefani, D.; De Vivo, I.; Scapagnini, G. Polyphenols as Caloric Restriction Mimetics Regulating Mitochondrial Biogenesis and Mitophagy. Trends Endocrinol. Metab. 2020, 31, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Dumont, S.; Le Pennec, S.; Donnart, A.; Teusan, R.; Steenman, M.; Chevalier, C.; Houlgatte, R.; Savagner, F. Transcriptional orchestration of mitochondrial homeostasis in a cellular model of PGC-1-related coactivator-dependent thyroid tumor. Oncotarget 2018, 9, 15883–15894. [Google Scholar] [CrossRef] [PubMed]
- Komen, J.C.; Thorburn, D.R. Turn up the power—Pharmacological activation of mitochondrial biogenesis in mouse models. Br. J. Pharmacol. 2014, 171, 1818–1836. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Diaz, F.; Iommarini, L.; Aure, K.; Lombes, A.; Moraes, C.T. PGC-1α/β induced expression partially compensates for respiratory chain defects in cells from patients with mitochondrial disorders. Hum. Mol. Genet. 2009, 18, 1805–1812. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo Transcription Factors Induce the Atrophy-Related Ubiquitin Ligase Atrogin-1 and Cause Skeletal Muscle Atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 Coordinately Activates Protein Degradation by the Autophagic/Lysosomal and Proteasomal Pathways in Atrophying Muscle Cells. Cell Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef]
- Romanello, V.; Guadagnin, E.; Gomes, L.; Roder, I.; Sandri, C.; Petersen, Y.; Milan, G.; Masiero, E.; Del Piccolo, P.; Foretz, M.; et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010, 29, 1774–1785. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- de Castro, G.S.; Simoes, E.; Lima, J.D.; Ortiz-Silva, M.; Festuccia, W.T.; Tokeshi, F.; Alcântara, P.S.; Otoch, J.P.; Coletti, D.; Seelaender, M. Human Cachexia Induces Changes in Mitochondria, Autophagy and Apoptosis in the Skeletal Muscle. Cancers 2019, 11, 1264. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S.; et al. Reversal of Cancer Cachexia and Muscle Wasting by ActRIIB Antagonism Leads to Prolonged Survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Macovei, Ș.O.; Bocșan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape Pomace Polyphenols as a Source of Compounds for Management of Oxidative Stress and Inflammation—A Possible Alternative for Non-Steroidal Anti-Inflammatory Drugs? Molecules 2022, 27, 6826. [Google Scholar] [CrossRef]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Bowers, M.; Cucchiaro, B.; Reid, J.; Slee, A. Non-steroidal anti-inflammatory drugs for treatment of cancer cachexia: A systematic review. J. Cachexia Sarcopenia Muscle 2023, 14, 2473–2497. [Google Scholar] [CrossRef]
- Mehrzad, V.; Afshar, R.; Akbari, M. Pentoxifylline treatment in patients with cancer cachexia: A double-blind, randomized, placebo-controlled clinical trial. Adv. Biomed. Res. 2016, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Scott, A.M.; Hoogenraad, N.J.; Osellame, L.D. Mediators and clinical treatment for cancer cachexia: A systematic review. JCSM Rapid Commun. 2021, 4, 166–186. [Google Scholar] [CrossRef]
- Nishie, K.; Nishie, T.; Sato, S.; Hanaoka, M. Update on the treatment of cancer cachexia. Drug Discov. Today 2023, 28, 103689. [Google Scholar] [CrossRef] [PubMed]
- Avancini, A.; Trestini, I.; Tregnago, D.; Lanza, M.; Menis, J.; Belluomini, L.; Milella, M.; Pilotto, S. A multimodal approach to cancer-related cachexia: From theory to practice. Expert Rev. Anticancer Ther. 2021, 21, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Braha, A.; Albai, A.; Timar, B.; Negru, Ș.; Sorin, S.; Roman, D.; Popovici, D. Nutritional Interventions to Improve Cachexia Outcomes in Cancer—A Systematic Review. Medicina 2022, 58, 966. [Google Scholar] [CrossRef] [PubMed]
- Orsso, C.E.; Montes-Ibarra, M.; Findlay, M.; van der Meij, B.S.; de van der Schueren, M.A.E.; Landi, F.; Laviano, A.; Prado, C.M. Mapping ongoing nutrition intervention trials in muscle, sarcopenia, and cachexia: A scoping review of future research. J. Cachexia Sarcopenia Muscle 2022, 13, 1442–1459. [Google Scholar] [CrossRef]
- López, A.P.; i Figuls, M.R.; Cuchi, G.U.; Berenstein, E.G.; Pasies, B.A.; Alegre, M.B.; Herdman, M. Systematic review of megestrol acetate in the treatment of anorexia-cachexia syndrome. J. Pain Symptom Manag. 2004, 27, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Zeraattalab-Motlagh, S.; Barkhordar, M.; Vaezi, M.; Ghoreishy, S.M.; Ghavami, A.; Hosseini, Y.; Travica, N.; Mohammadi, H. Dose-dependent effect of megestrol acetate supplementation in cancer patients with anorexia–cachexia syndrome: A meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 1254–1263. [Google Scholar] [CrossRef]
- Rezaei, S.; de Oliveira, L.C.; Ghanavati, M.; Shadnoush, M.; Akbari, M.E.; Akbari, A.; Hadizadeh, M.; Ardehali, S.H.; Wakabayashi, H.; Elhelali, A.; et al. The effect of anamorelin (ONO-7643) on cachexia in cancer patients: Systematic review and meta-analysis of randomized controlled trials. J. Oncol. Pharm. Pract. 2023, 29, 1725–1735. [Google Scholar] [CrossRef]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Smith, T.J.; Loprinzi, C.L.; Panel, F.T.C.C.E.; Bruera, E.; del Fabbro, E.; Dixon, S.; Fallon, M.; et al. Cancer Cachexia: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 2023, 41, 4178–4179. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D.; Forbes, S.C.; Fairman, C.M.; Gualano, B.; Roschel, H. Creatine supplementation for older adults: Focus on sarcopenia, osteoporosis, frailty and Cachexia. Bone 2022, 162, 116467. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Stene, G.B.; Baracos, V.; Bye, A.; Dajani, O.; Hendifar, A.E.; Strasser, F.; Chasen, M.R.; et al. Results from a randomised, open-label trial of a multimodal intervention (exercise, nutrition and anti-inflammatory medication) plus standard care versus standard care alone to attenuate cachexia in patients with advanced cancer undergoing chemotherapy. J. Clin. Oncol. 2024, 42, LBA12007. [Google Scholar] [CrossRef]
- Kim, B.-H.; Maeng, C.H.; Chon, J.; Kang, W.S.; Kang, K.; Woo, M.; Hong, I.K.; Lee, J.; Lee, K.Y. Effect of multimodal intervention care on cachexia in patients with advanced cancer compared to conventional management (MIRACLE): An open-label, parallel, randomized, phase 2 trial. J. Clin. Oncol. 2023, 41, e24135. [Google Scholar] [CrossRef]
- Mantovani, G.; Macciò, A.; Madeddu, C.; Serpe, R.; Massa, E.; Dessì, M.; Panzone, F.; Contu, P. Randomized Phase III Clinical Trial of Five Different Arms of Treatment in 332 Patients with Cancer Cachexia. Oncologist 2010, 15, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Cancer-associated cachexia—Understanding the tumour macroenvironment and microenvironment to improve management. Nat. Rev. Clin. Oncol. 2023, 20, 250–264. [Google Scholar] [CrossRef] [PubMed]
- (OIV) IO of V and W. State of the World Vine and Wine Sector in 2022; OIV: Paris, France, 2023. [Google Scholar]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, K.; Hosseinian, F.; Rod, M. The Market Potential of Grape Waste Alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B.; Laureano, O.; da Silva, J.M.R. Monomeric, Oligomeric, and Polymeric Flavan-3-ol Composition of Wines and Grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef]
- Beveridge, T.H.J.; Girard, B.; Kopp, T.; Drover, J.C.G. Yield and Composition of Grape Seed Oils Extracted by Supercritical Carbon Dioxide and Petroleum Ether: Varietal Effects. J. Agric. Food Chem. 2005, 53, 1799–1804. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.C.; Gascueña, J.M.; Romero, E.G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT-Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Lavelli, V.; Sri Harsha, P.S.C.; Ferranti, P.; Scarafoni, A.; Iametti, S. Grape skin phenolics as inhibitors of mammalian α-glucosidase and α-amylase—Effect of food matrix and processing on efficacy. Food Funct. 2016, 7, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.; Moreira, R.; Chenlo, F.; Morel, M.H. Effect of water and guar gum content on thermal properties of chestnut flour and its starch. Food Hydrocoll. 2013, 33, 192–198. [Google Scholar] [CrossRef]
- Vinson, J.A.; Proch, J.; Bose, P. MegaNatural® Gold Grapeseed Extract: In Vitro Antioxidant and In Vivo Human Supplementation Studies. J. Med. Food 2001, 4, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Laight, D.; Rooprai, H.K.; Shaw, K.M.; Cummings, M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet. Med. 2009, 26, 526–531. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Krzeminski, R.; Gralak, M.; Martin-Belloso, O.; Delgado-Licon, E.; Haruenkit, R.; Katrich, E.; Park, Y.-S.; et al. Fresh Israeli Jaffa Blond (Shamouti) Orange and Israeli Jaffa Red Star Ruby (Sunrise) Grapefruit Juices Affect Plasma Lipid Metabolism and Antioxidant Capacity in Rats Fed Added Cholesterol. J. Agric. Food Chem. 2004, 52, 4853–4859. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.C.; Kahmann, A.; Anzanello, M.J.; Rodrigues, R.C.; Rodrigues, E.; Mercali, G.D. Acid hydrolysis conditions do affect the non-extractable phenolic compounds composition from grape peel and seed. Food Res. Int. 2023, 174, 113636. [Google Scholar] [CrossRef]
- Das, T.; Chatterjee, N.; Capanoglu, E.; Lorenzo, J.M.; Das, A.K.; Dhar, P. The synergistic ramification of insoluble dietary fiber and associated non-extractable polyphenols on gut microbial population escorting alleviation of lifestyle diseases. Food Chem. X 2023, 18, 100697. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.; Singh, R.P.; Kaul, N.; Agarwal, R.; Agarwal, C. Dietary Feeding of Grape Seed Extract Prevents Intestinal Tumorigenesis in APCmin/+ Mice. Neoplasia 2010, 12, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Downing, L.E.; Ferguson, B.S.; Rodriguez, K.; Ricketts, M. A grape seed procyanidin extract inhibits HDAC activity leading to increased Pparα phosphorylation and target-gene expression. Mol. Nutr. Food Res. 2017, 61, 1600347. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. Rev. Anim Feed. Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Hirota, S. Interactions of flavonoids with α-amylase and starch slowing down its digestion. Food Funct. 2018, 9, 677–687. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; el, S.N.; Karakaya, S.; et al. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef]
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein–phenolic compound interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Konishi, Y.; Kobayashi, S. Transepithelial Transport of Chlorogenic Acid, Caffeic Acid, and Their Colonic Metabolites in Intestinal Caco-2 Cell Monolayers. J. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef]
- Herremans, K.M.; Riner, A.N.; Cameron, M.E.; Trevino, J.G. The microbiota and cancer cachexia. Int. J. Mol. Sci. 2019, 20, 6267–6279. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, G.; Liang, X.; Zhu, M.; Du, M. Grape seed extract prevents skeletal muscle wasting in interleukin 10 knockout mice. BMC Complement. Altern. Med. 2014, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Lambert, K.; Coisy-Quivy, M.; Bisbal, C.; Sirvent, P.; Hugon, G.; Mercier, J.; Avignon, A.; Sultan, A. Grape polyphenols supplementation reduces muscle atrophy in a mouse model of chronic inflammation. Nutrition 2015, 31, 1275–1283. [Google Scholar] [CrossRef]
- Myburgh, K.H.; Kruger, M.J.; Smith, C. Accelerated skeletal muscle recovery after in vivo polyphenol administration. J. Nutr. Biochem. 2012, 23, 1072–1079. [Google Scholar] [CrossRef]
- Qin, X.; Niu, W.; Zhao, K.; Luo, Y.; Wang, W.; He, Y.; Yang, F.; Cao, B.; Du, M.; Su, H. Resveratrol enhances post-injury muscle regeneration by regulating antioxidant and mitochondrial biogenesis. Curr. Res. Food Sci. 2025, 10, 100972. [Google Scholar] [CrossRef] [PubMed]
- Derry, M.M.; Raina, K.; Balaiya, V.; Jain, A.K.; Shrotriya, S.; Huber, K.M.; Serkova, N.J.; Agarwal, R.; Agarwal, C. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: Interlinking miRNA with cytokine signaling and inflammation. Cancer Prev. Res. 2013, 6, 625–633. [Google Scholar] [CrossRef]
- Foshati, S.; Rouhani, M.H.; Amani, R. The effect of grape seed extract supplementation on oxidative stress and inflammation: A systematic review and meta-analysis of controlled trials. Int. J. Clin. Pract. 2021, 75, e14469. [Google Scholar] [CrossRef] [PubMed]
- Malta, F.A.P.S.; Gonçalves, D.C. A triple-masked, two-center, randomized parallel clinical trial to assess the superiority of eight weeks of grape seed flour supplementation against placebo for weight loss attenuation during perioperative period in patients with cachexia associated with colorectal cancer: A study protocol. Front. Endocrinol. 2024, 14, 1146479. [Google Scholar] [CrossRef]
- Wu, C.; Suzuki, K. The Effects of Flavonoids on Skeletal Muscle Mass, Muscle Function, and Physical Performance in Individuals with Sarcopenia: A Systematic Review of Randomized Controlled Trials. Nutrients 2023, 15, 3897. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Tan, R.; Liu, Y. Effect of flavonoids on skeletal muscle mass, strength and physical performance in middle-aged and older adults with or without Sarcopenia: A meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 1013449. [Google Scholar] [CrossRef]
- Medoro, A.; Scapagnini, G.; Davinelli, S. Polyphenol Supplementation and Sarcopenia: A Systematic Review and Meta-Analysis of Clinical Trials. J. Frailty Aging 2024, 13, 432–440. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Valls, J.; Vitrac, X.; Mérrillon, J.-M.; Arola, L.; Ardévól, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; et al. Grape-Seed Procyanidins Act as Antiinflammatory Agents in Endotoxin-Stimulated RAW 264.7 Macrophages by Inhibiting NFkB Signaling Pathway. J. Agric. Food Chem. 2007, 55, 4357–4365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Raffoul, J.J. Potential Anticancer Properties of Grape Antioxidants. J. Oncol. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Ngum, J.A.; Tatang, F.J.; Toumeni, M.H.; Nguengo, S.N.; Simo, U.S.F.; Mezajou, C.F.; Kameni, C.; Ngongang, N.N.; Tchinda, M.F.; Dongmo, F.F.D.; et al. An overview of natural products that modulate the expression of non-coding RNAs involved in oxidative stress and inflammation-associated disorders. Front. Pharmacol. 2023, 14, 1144836. [Google Scholar] [CrossRef]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Busch, C.; Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 2008, 19, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Chodari, L.; Aytemir, M.D.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A.; de Oliveira, F.L. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxid. Med. Cell. Longev. 2021, 2021, 4946711. [Google Scholar] [CrossRef]
- Traynard, V. Resveratrol, Multiple Bioactivities for a Wide Range of Health Benefits—New Innovative Extracts for Nutraceutical, Pharmaceutical, and Cosmetics Applications. In Resveratrol—Recent Advances, Application, and Therapeutic Potential; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Jiang, S.; Li, H.; Chen, L.; Wu, Y.; Essien, A.E.; Opoku, M.; Naranmandakh, S.; Liu, S.; et al. SIRT1 signaling pathways in sarcopenia: Novel mechanisms and potential therapeutic targets. Biomed. Pharmacother. 2024, 177, 116917. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.C. Grape Seed Flour Supplementation as a strategy to Reverse Muscle Mass Loss in Perioperative Colorectal Cancer Cachexia Patients: A Translational Study. 2023. Available online: https://ensaiosclinicos.gov.br/rg/RBR-5p6nv8b (accessed on 28 August 2025).
- Blauwhoff-Buskermolen, S.; Ruijgrok, C.; Ostelo, R.W.; de Vet, H.C.W.; Verheul, H.M.W.; de van der Schueren, M.A.E.; Langius, J.A.E. The assessment of anorexia in patients with cancer: Cut-off values for the FAACT-A/CS and the VAS for appetite. Support Care Cancer 2016, 24, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Edirisinghe, I.; Choy, Y.Y.; Waterhouse, A.; Burton-Freeman, B. Effects of grape seed extract beverage on blood pressure and metabolic indices in individuals with pre-hypertension: A randomised, double-blinded, two-arm, parallel, placebo-controlled trial. Br. J. Nutr. 2016, 115, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Sano, A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem. Toxicol. 2017, 108, 519–523. [Google Scholar] [CrossRef]
- Laviano, A.; Giraldi, G.D.L.; Koverech, A. Does nutrition support have a role in managing cancer cachexia? Curr. Opin. Support. Palliat. Care 2016, 10, 288–292. [Google Scholar] [CrossRef]
- NIAID Visual & Medical Arts. (10/7/2024). Cytokines. NIAID NIH BIOART Source. Available online: https://bioart.niaid.nih.gov/bioart/98 (accessed on 1 June 2025).
- NIAID Visual & Medical Arts. (10/7/2024). Doctor Patient. NIAID NIH BIOART Source. Available online: https://bioart.niaid.nih.gov/bioart/130 (accessed on 1 June 2025).
- NIAID Visual & Medical Arts. (10/7/2024). Hospital Setting. NIAID NIH BIOART Source. Available online: https://bioart.niaid.nih.gov/bioart/206 (accessed on 1 June 2025).
- NIAID Visual & Medical Arts. (10/7/2024). Mitochondria. NIAID NIH BIOART Source. Available online: https://bioart.niaid.nih.gov/bioart/352 (accessed on 1 June 2025).
- NIAID Visual & Medical Arts. (10/7/2024). Generic Cells. NIAID NIH BIOART Source. Available online: https://bioart.niaid.nih.gov/bioart/172 (accessed on 1 June 2025).
| 100 g | |
|---|---|
| Energy value (kcal) | 159 |
| Total carbohydrates (g) | 28 |
| Total sugars (g) | 3.4 |
| Proteins (g) | 8.6 |
| Total fats (g) | 1.3 |
| Dietary fiber (g) | 46 |
| Phenolic compounds (mg) | 1703 |
| Hydrolysable tannins (mg) | 307.7 |
| Hydroxybenzoic acids and derivatives (mg) | 153.8 |
| Non-extractable proanthocyanidins (mg) | 1287.1 |
| Hydroxycinnamic acids (mg) | 24.9 |
| Anthocyanins (mg) | ND |
| Study (Year) | Model Used | Intervention | Main Outcomes | Relevance to Cancer Cachexia |
|---|---|---|---|---|
| Wang et al. [98] | IL10-KO mice (chronic inflammation and muscle atrophy model) | GSE (0.2 mg/g/day in water) | 15% preservation of muscle mass; ↓ TNF-α by 40% | Directly mimics colorectal cancer-associated cachexia |
| Lambert et al. [100] | LPS-induced muscle atrophy (caspase-3 mediated) | 50 mg/kg of mixed polyphenols | ↓ caspase-3 by 30%; ↓ muscle loss by 22% | Mechanistically relevant (PTHrP pathway activation in CRC |
| Myburgh et al. [101] | Acute muscle injury (gastrocnemius) | Grape seed procyanidins (20 mg/kg/day) | ↑ satellite cell activation; ↓ local TNF-α by 50% | Limited due to acute, non-cachexia model |
| Qin et al. [102] | Post-injury muscle regeneration | Resveratrol (450 mg/kg) | ↑ PGC-1α; ↑ mitochondrial function (35%) | Impractical dosage; not representative of cachexia |
| Derry et al. [103] | Colorectal cancer induced by AOM | GSE (0.25–0.5%) | ↓ tumor formation (55%); ↓ PCNA (65%) | Focus on tumorigenesis, not cachexia parameters |
| Study (Year) | Population | Intervention | Main Outcomes | Relevance to Cachexia |
|---|---|---|---|---|
| Kar et al. [80] | Elderly (mean age 61.8) | GSE (600 mg/day, 4 weeks) | ↓ hs-CRP by 34%; ↑ glutathione by 28% | Biomarkers relevant to cachexia (inflammation, oxidative stress) |
| Foshati et al. [104] | Meta-analysis (15 trials) | GSE (various doses) | ↓ CRP (by 0.8 mg/L); ↓ glucose, LDL, TG | No muscle outcomes; baseline CRP < cachexia levels |
| Malta & Gonçalves [105] | Colorectal cancer patients (perioperative) | GSF (8 g/day, 8 weeks) | Primary: ↓ weight loss; Secondary: muscle strength, inflammation, QoL | Ongoing RCT; first study focused on cancer cachexia |
| Item | Details |
| Study Design |
|
| Population |
|
| Intervention |
|
| Duration |
|
| Primary Endpoint |
|
| Secondary Endpoints |
|
| Safety Monitoring |
|
| Ethics |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdi, A.M.O.H.; Rizzardi, M.L.; Soares, J.M.; Schiessel, D.L.; Coletti, D.; Seelaender, M.C.L.; Gonçalves, D.C. Nutraceutical Interception of Cachexia: Grape-Derived Compounds as Pathophysiological Network Modulators. Biology 2025, 14, 1159. https://doi.org/10.3390/biology14091159
Verdi AMOH, Rizzardi ML, Soares JM, Schiessel DL, Coletti D, Seelaender MCL, Gonçalves DC. Nutraceutical Interception of Cachexia: Grape-Derived Compounds as Pathophysiological Network Modulators. Biology. 2025; 14(9):1159. https://doi.org/10.3390/biology14091159
Chicago/Turabian StyleVerdi, Anderson Matheus Oliveira Haas, Mariana Lemos Rizzardi, Jaqueline Machado Soares, Dalton Luiz Schiessel, Dario Coletti, Marilia Cerqueira Leite Seelaender, and Daniela Caetano Gonçalves. 2025. "Nutraceutical Interception of Cachexia: Grape-Derived Compounds as Pathophysiological Network Modulators" Biology 14, no. 9: 1159. https://doi.org/10.3390/biology14091159
APA StyleVerdi, A. M. O. H., Rizzardi, M. L., Soares, J. M., Schiessel, D. L., Coletti, D., Seelaender, M. C. L., & Gonçalves, D. C. (2025). Nutraceutical Interception of Cachexia: Grape-Derived Compounds as Pathophysiological Network Modulators. Biology, 14(9), 1159. https://doi.org/10.3390/biology14091159









