Characterization of Two Novel Heat Shock Protein 70 Transcripts from Sitodiplosis mosellana and Their Response to Larval Diapause and Thermal Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source
2.2. RNA Extraction, cDNA Synthesis, and gDNA Isolation
2.3. Gene Cloning
2.4. Bioinformatic Analysis of SmHsp70s
2.5. 20E Treatment
2.6. Temperature Treatments
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Double-Stranded RNA (dsRNA) Synthesis and Delivery
2.9. Effects of RNAI on Larval Cold Tolerance
2.10. Data Analysis
3. Results
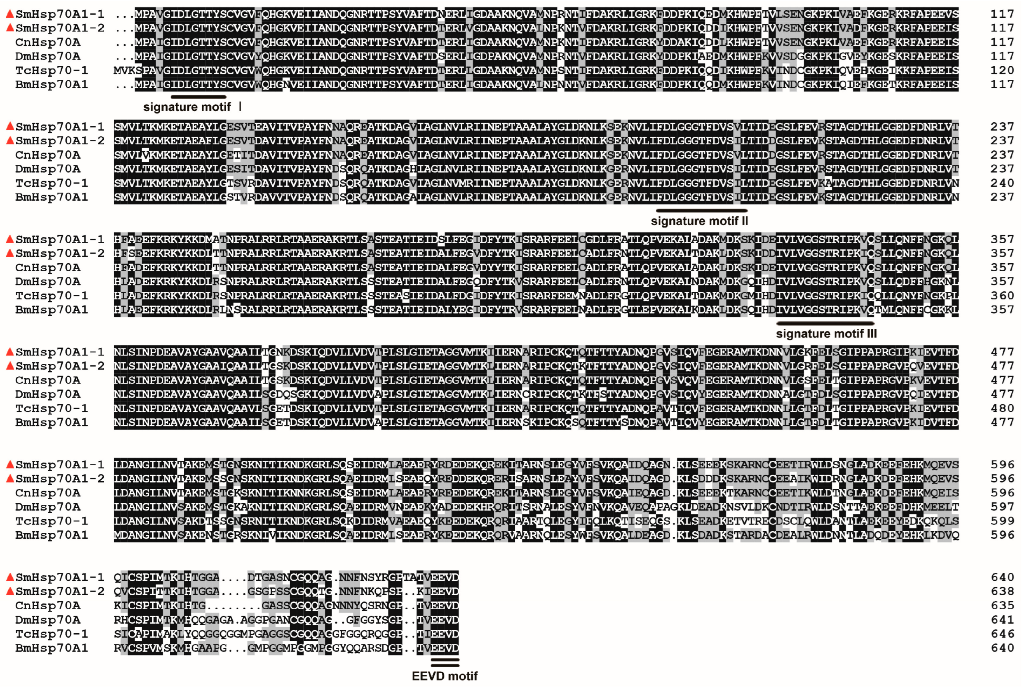
3.1. Characterization of SmHsp70 Genes
3.2. Expression Patterns of SmHsp70 Genes at Different Developmental Stages
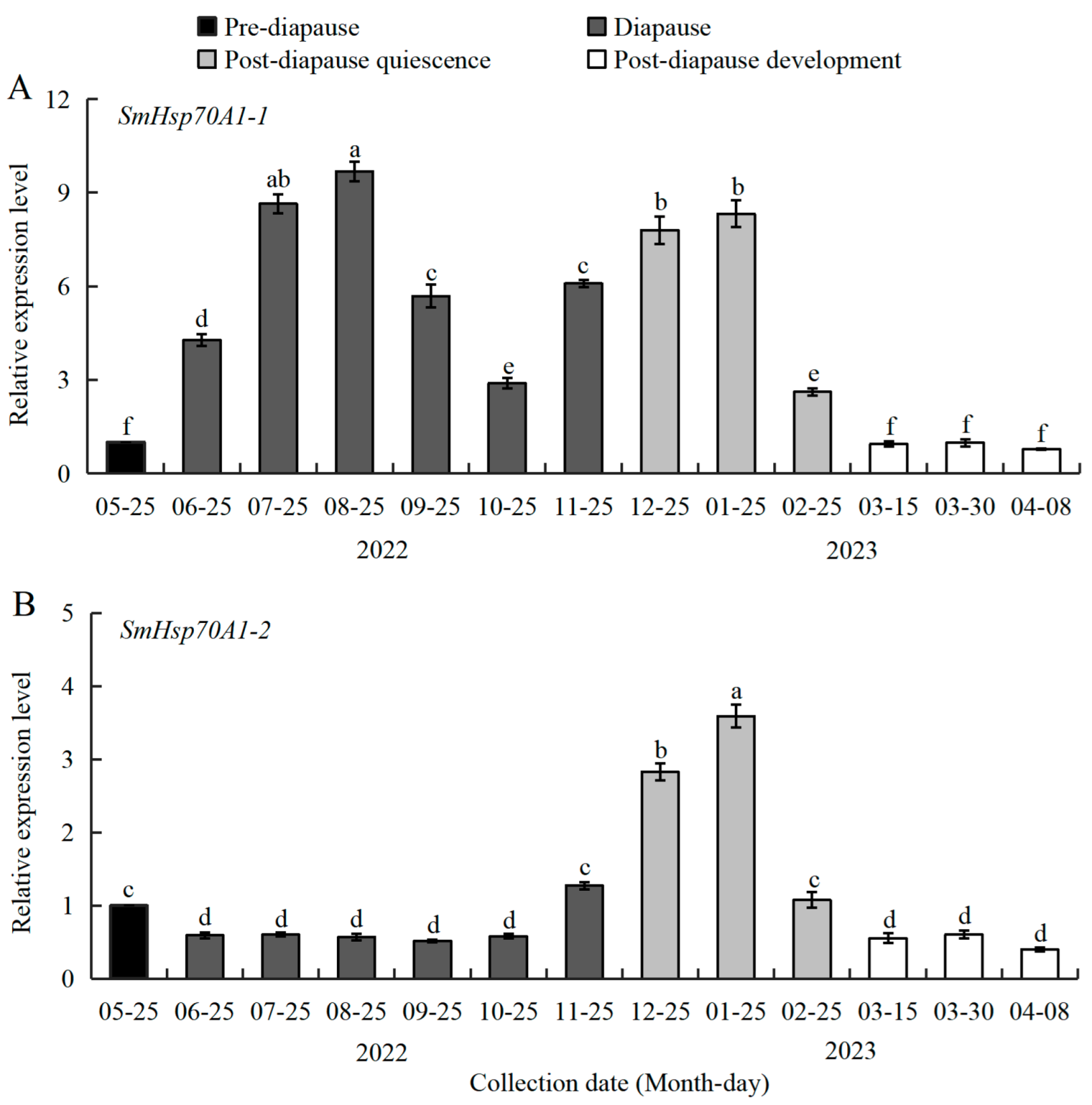
3.3. Expression Patterns of SmHsp70 Genes During Diapause
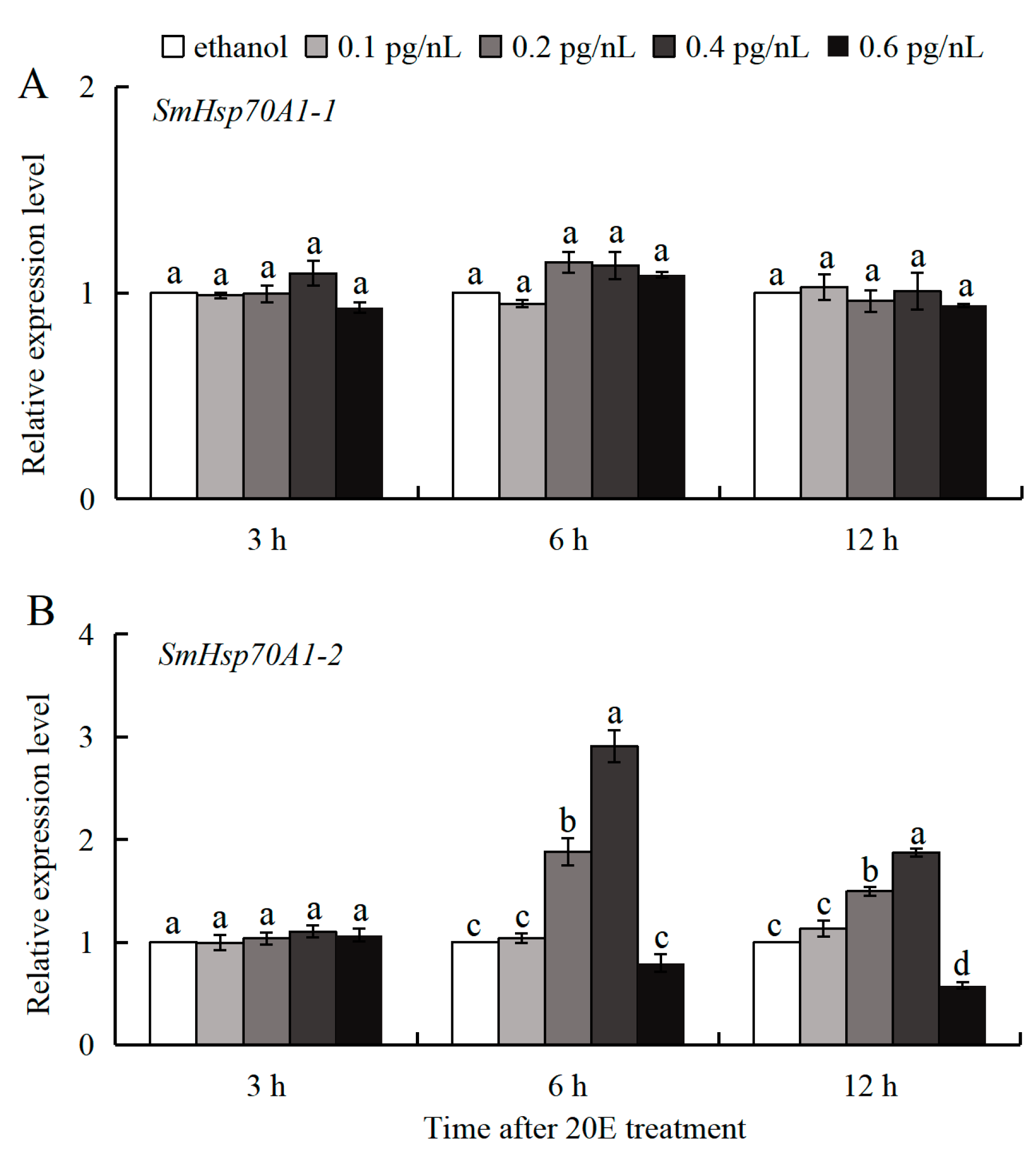
3.4. 20E Regulation of SmHsp70 Genes During Diapause
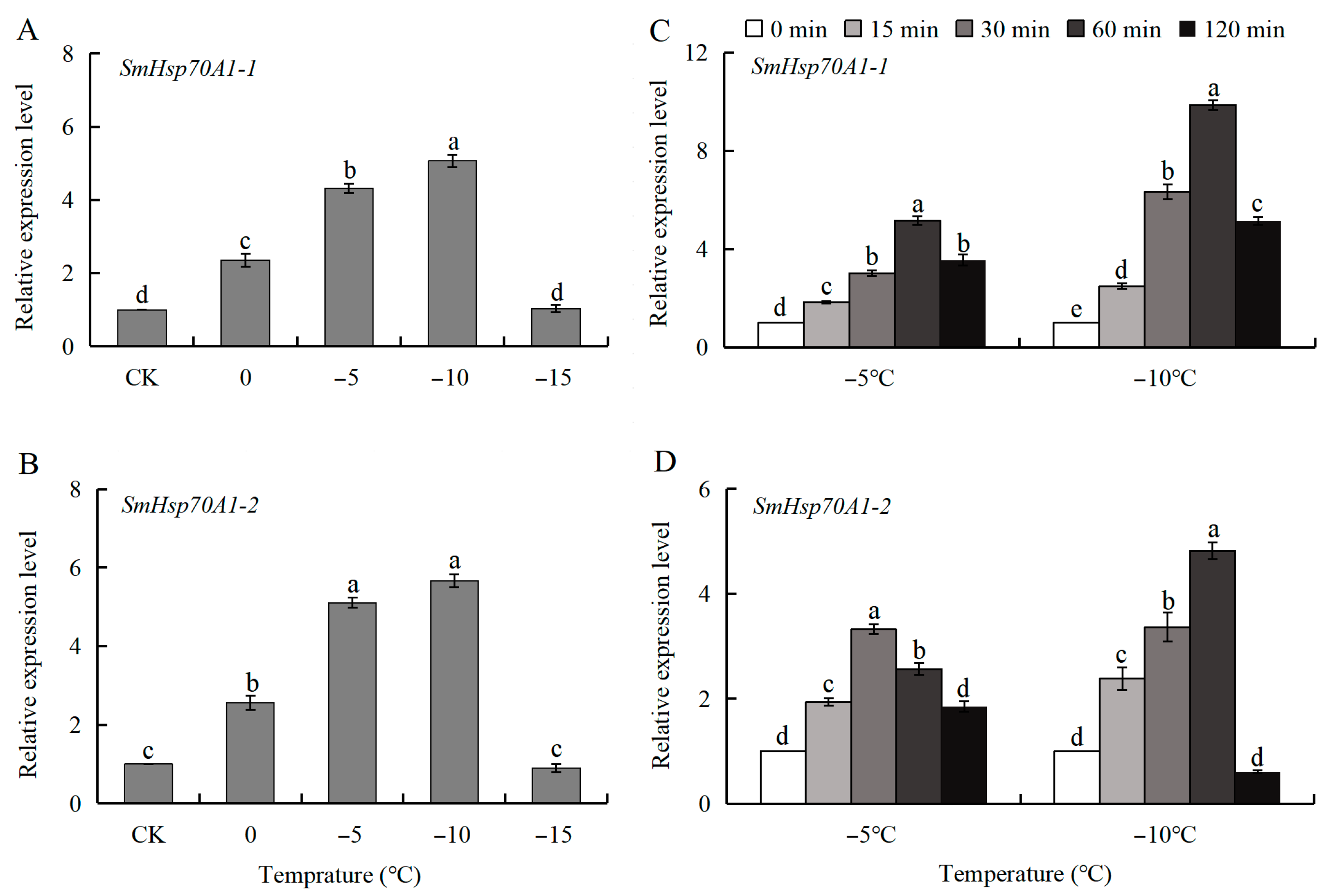
3.5. Patterns of SmHsp70 Gene Expression in Response to Heat and Cold Temperature Stress During Diapause
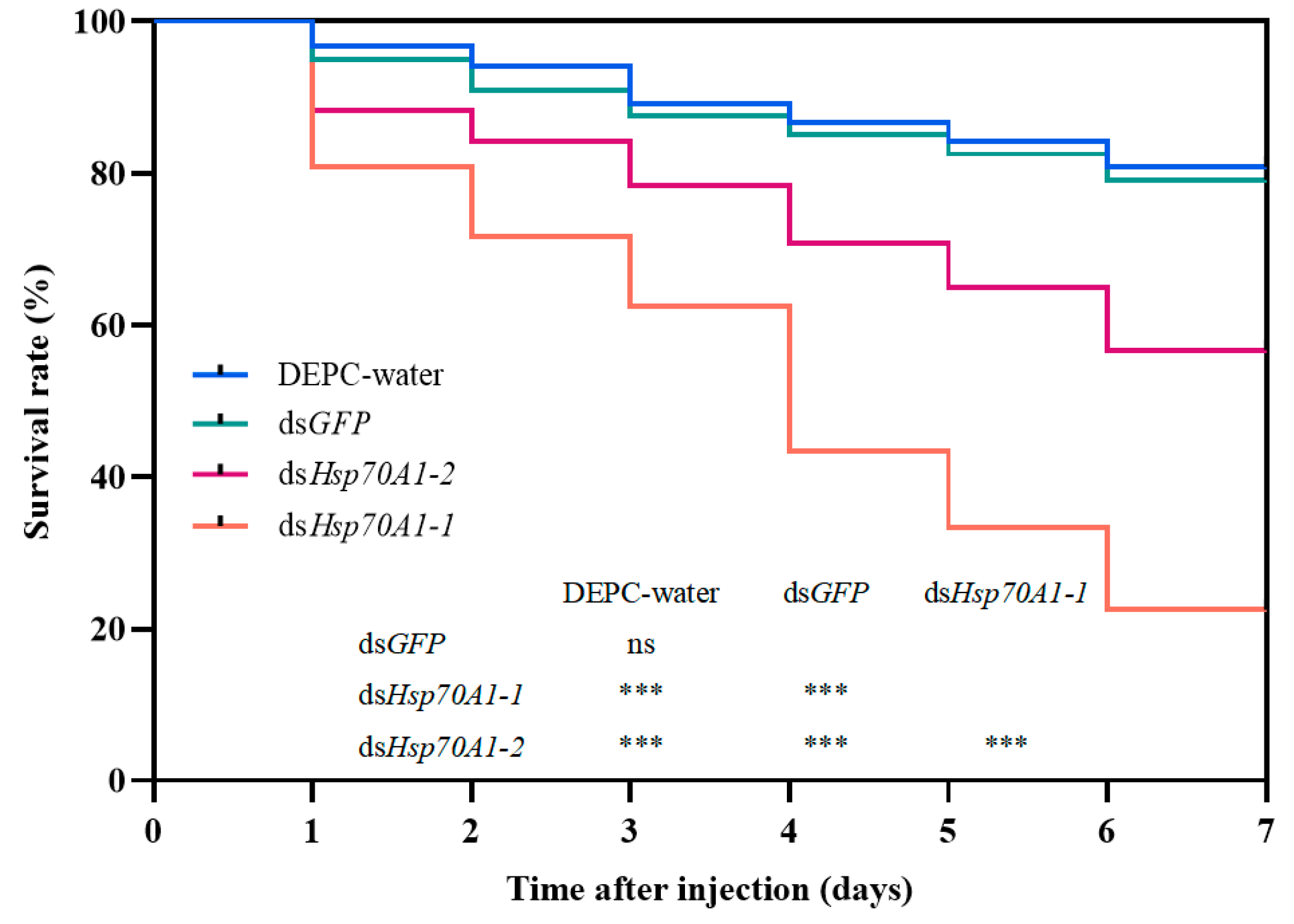
3.6. Silencing of SmHsp70 Genes by RNAI and Assessment of Susceptibility to Cold Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.Q.; Zhang, Y.L.; Wang, X.Q.; Dong, H.; Gao, P.; Jia, L.Y. Characterization of multiple heat-shock protein transcripts from Cydia pomonella: Their response to extreme temperature and insecticide exposure. J. Agric. Food Chem. 2016, 64, 4288–4298. [Google Scholar] [CrossRef]
- Tan, S.Y.; Feng, K.; Zhai, X.D.; Wang, J.J.; Wei, D. Expression and function analysis of two heat shock protein 70 genes (Hsp70s) involved in different high-temperature stresses in Zeugodacus cucurbitae (Diptera: Tephritidae). Entomol. Gen. 2023, 43, 481–490. [Google Scholar] [CrossRef]
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Lubkowska, A.; Pluta, W.; Strońska, A.; Lalko, A. Role of heat shock proteins (HSP70 and HSP90) in viral infection. Int. J. Mol. Sci. 2021, 22, 9366. [Google Scholar] [CrossRef]
- Pei, T.W.; Zhang, M.; Nwanade, C.F.; Meng, H.; Bai, R.W.; Wang, Z.H.; Wang, R.T.; Zhang, T.A.; Liu, J.Z.; Yu, Z.J. Sequential expression of small heat shock proteins contributing to the cold response of Haemaphysalis longicornis (Acari: Ixodidae). Pest Manag. Sci. 2024, 80, 2061–2071. [Google Scholar] [CrossRef]
- Liu, J.P.; Liu, Y.; Wang, W.; Liang, G.M.; Lu, Y.H. Characterizing three heat shock protein 70 genes of Aphis gossypii and their expression in response to temperature and insecticide stress. J. Agric. Food. Chem. 2025, 73, 2842–2852. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.P.; Wu, H.; Wang, B.L.; Zou, F.M.; Mei, H.S.; Liu, J.; Wang, W.C.; Liu, Q.S. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. Med. Comm. 2022, 3, e161. [Google Scholar] [CrossRef]
- Huang, L.H.; Wang, H.S.; Kang, L. Different evolutionary lineages of large and small heat shock proteins in eukaryotes. Cell Res. 2008, 18, 1074–1076. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Yu, W. Heat shock proteins and viral infection. Front. Immunol. 2022, 13, 947789. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2023, 6, 1025–1037. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef]
- Paim, R.M.M.; Araujo, R.N.; Leis, M.; Sant’anna, M.R.V.; Gontijo, N.F.; Lazzari, C.R.; Pereira, M.H. Functional evaluation of heat shock proteins 70 (HSP70/HSC70) on Rhodnius prolixus (Hemiptera, Reduviidae) physiological responses associated with feeding and starvation. Insect Biochem. Mol. Biol. 2016, 77, 10–20. [Google Scholar] [CrossRef]
- Qin, W.S.; Tyshenko, M.G.; Wu, B.S.; Walker, V.K.; Robertson, R.M. Cloning and characterization of a member of the Hsp70 gene family from Locusta migratoria, a highly thermotolerant insect. Cell Stress Chaperones 2003, 8, 144–152. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Sheng, Y.; Xiao, Y.F.; Zhang, Y.J.; Bai, L.X.; Tan, Y.A.; Xiao, L.B.; Xu, G.C. Identification of heat shock cognate protein 70 gene (Alhsc70) of Apolygus lucorum and its expression in response to different temperature and pesticide stresses. Insect Sci. 2016, 23, 37–49. [Google Scholar] [CrossRef]
- Gehring, W.J.; Wehner, R. Heat shock protein synthesis and thermotolerance in Cataglyphis, an ant from the Sahara desert. Proc. Natl. Acad. Sci. USA 1995, 92, 2994–2998. [Google Scholar] [CrossRef]
- Choi, B.G.; Hepat, R.; Kim, Y. RNA interference of a heat shock protein, Hsp70, loses its protection role in indirect chilling injury to the beet armyworm, Spodoptera exigua. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 168, 90–95. [Google Scholar] [CrossRef]
- Gu, J.; Ye, Y.; Zheng, Z.W.; Luo, W.; Gong, Y.J.; Feng, Q.L.; Li, S.; Huang, L.H. Cytoplasmic Hsp70s promote EcR transport into the nucleus by responding to various stimuli. Insect Biochem. Mol. Biol. 2023, 157, 103964. [Google Scholar] [CrossRef]
- King, A.M.; MacRae, T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Schebeck, M.; Lehmann, P.; Laparie, M.; Bentz, B.J.; Ragland, G.J.; Battisti, A.; Hahn, D.A. Seasonality of forest insects: Why diapause matters. Trends Ecol. Evol. 2024, 39, 757–770. [Google Scholar] [CrossRef]
- Zhang, J.; Miano, F.N.; Jiang, T.; Peng, Y.C.; Zhang, W.N.; Xiao, H.J. Characterization of three heat shock protein genes in Pieris melete and their expression patterns in response to temperature stress and pupal diapause. Insects 2022, 13, 430. [Google Scholar] [CrossRef]
- Rinehart, J.P.; Li, A.Q.; Yocum, G.D.; Robich, R.M.; Hayward, S.A.L.; Denlinger, D.L. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA 2007, 104, 11130–11137. [Google Scholar] [CrossRef]
- Yocum, G.D. Differential expression of two HSP70 transcripts in response to cold shock, thermoperiod, and adult diapause in the Colorado potato beetle. J. Insect Physiol. 2001, 47, 1139–1145. [Google Scholar] [CrossRef]
- Moribe, Y.; Oka, K.; Niimi, T.; Yamashita, O.; Yaginuma, T. Expression of heat shock protein 70a mRNA in Bombyx mori diapause eggs. J. Insect Physiol. 2010, 56, 1246–1252. [Google Scholar] [CrossRef]
- Uzelac, I.; Avramov, M.; Knežić, T.; Tatić, V.; Gošić-Dondo, S.; Popović, Ž.D. Prolonged heat stress during winter diapause alters the expression of stress-response genes in Ostrinia nubilalis (Hbn.). Int. J. Mol. Sci. 2024, 25, 3100. [Google Scholar] [CrossRef] [PubMed]
- Tungjitwitayakul, J.; Tatun, N.; Singtripop, T.; Sakurai, S. Characteristic expression of three heat shock-responsive genes during larval diapause in the bamboo borer Omphisa fuscidentalis. Zool. Sci. 2008, 25, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Gkouvitsas, T.; Kontogiannatos, D.; Kourti, A. Cognate Hsp70 gene is induced during deep larval diapause in the moth Sesamia nonagrioides. Insect Mol. Biol. 2009, 18, 253–264. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Denlinger, D.L. Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J. Insect Physiol. 2010, 56, 138–150. [Google Scholar] [CrossRef]
- Miao, J.; Huang, J.R.; Wu, Y.Q.; Gong, Z.J.; Li, H.L.; Zhang, G.Y.; Duan, Y.; Li, T.; Jiang, Y.L. Climate factors associated with the population dynamics of Sitodiplosis mosellana (Diptera: Cecidomyiidae) in central China. Sci. Rep. 2019, 9, 12361. [Google Scholar] [CrossRef]
- Shrestha, G.; Reddy, G.V.P. Field efficacy of insect pathogen, botanical, and jasmonic acid for the management of wheat midge Sitodiplosis mosellana and the impact on adult parasitoid Macroglenes penetrans populations in spring wheat. Insect Sci. 2019, 26, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, X.B.; Liu, H.; Fu, Y.; Cheng, Y.M.; Zhang, L.J.; Shi, W.P.; Zhang, Y.; Chen, J.L. Transcriptomic and metabolomic analysis of wheat kernels in response to the feeding of orange wheat blossom midges (Sitodiplosis mosellana) in the field. J. Agric. Food Chem. 2022, 70, 1477–1493. [Google Scholar] [CrossRef]
- Wang, Y.; Long, Z.R.; Feng, A.R.; Cheng, W.N. Effects of initial population number, wheat varieties and precipitation on infestation of Sitodiplosis mosellana (Diptera: Cecidomyiidae). Acta Agric. Boreali-Occident. Sin. 2015, 24, 165–171. [Google Scholar]
- Cheng, W.N.; Long, Z.R.; Zhang, Y.D.; Liang, T.T.; Zhu-Salzman, K.Y. Effects of temperature, soil moisture and photoperiod on diapause termination and post-diapause development of the wheat blossom midge, Sitodiplosis mosellana (Géhin) (Diptera: Cecidomyiidae). J. Insect Physiol. 2017, 103, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Li, D.; Wang, Y.; Liu, Y.; Zhu-Salzman, K.Y. Cloning of heat shock protein genes (hsp70, hsc70 and hsp90) and their expression in response to larval diapause and thermal stress in the wheat blossom midge, Sitodiplosis mosellana. J. Insect Physiol. 2016, 95, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Li, X.J.; Zhao, J.J.; Zhu-Salzman, K.Y. Cloning and characterization of methoprene-tolerant (Met) and krüppel homolog 1 (Kr-h1) genes in the wheat blossom midge, Sitodiplosis mosellana. Insect Sci. 2020, 27, 292–303. [Google Scholar] [CrossRef]
- Cheng, W.N.; Li, J.J.; Li, Y.P.; Li, X.L.; Wu, J.X.; Wang, H.L. Quantitative analysis of ecdysteroid in adults and the pre-diapause, diapause and post-diapause larvae of wheat blossom midge, Sitodiplosis mosellana Gehin. Acta Phytophy. Sin. 2009, 36, 163–167. [Google Scholar]
- Doane, J.F.; Olfert, O. Seasonal development of wheat midge, Sitodiplosis mosellana (Géhin) (Diptera: Cecidomyiidae), in Saskatchewan, Canada. Crop Prot. 2008, 27, 951–958. [Google Scholar] [CrossRef]
- Ni, H.X.; Ding, H.J.; Sun, J.R. Occurrence dynamic and integrated control strategies of Sitodiplosis mosellana. Chin. Agric. Sci. Bull. 1994, 10, 20–23. [Google Scholar]
- Qiu, X.B.; Shao, Y.M.; Miao, S.; Wang, L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci. 2006, 63, 2560–2570. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Gotelli, N.J.; Cahan, S.H. The evolution of heat shock protein sequences, cis-regulatory elements, and expression profiles in the eusocial Hymenoptera. BMC Evol. Biol. 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Goyal, B.; Tushir, S.; Sharma, A.; Singh, S.; Tatu, U.; Pandey, K.; Chakraborti, S. Unveiling role of HSP70 genes for development and survival of Indian malaria vector Anopheles culicifacies. Int. J. Biol. Macromol. 2025, 308, 142173. [Google Scholar] [CrossRef]
- Silver, J.T.; Noble, E.G. Regulation of survival gene hsp70. Cell Stress Chaperones 2012, 17, 1–9. [Google Scholar] [CrossRef]
- Wang, X.R.; Wang, C.; Ban, F.X.; Zhu, D.T.; Liu, S.S.; Wang, X.W. Genome-wide identification and characterization of HSP gene superfamily in whitefly (Bemisia tabaci) and expression profiling analysis under temperature stress. Insect Sci. 2019, 26, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.D.; Li, D.T.; Jiang, M.X.; Zhang, C.X. HSP70/DNAJ family of genes in the brown planthopper, Nilaparvata lugens: Diversity and function. Genes 2021, 12, 394. [Google Scholar] [CrossRef]
- Bukau, B.; Weissman, J.; Horwich, A. Molecular chaperones and protein quality control. Cell 2006, 125, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef]
- Fuertes, M.A.; Pérez, J.M.; Soto, M.; Menéndez, M.; Alonso, C. Thermodynamic stability of the C-terminal domain of the human inducible heat shock protein 70. Biochim. Biophys. Acta 2004, 1699, 45–56. [Google Scholar] [CrossRef]
- Park, H.; Ahn, I.Y.; Lee, H.E. Expression of heat shock protein 70 in the thermally stressed antarctic clam Laternula elliptica. Cell Stress Chaperones 2007, 12, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kanakala, S.; Kontsedalov, S.; Lebedev, G.; Ghanim, M. Plant-mediated Silencing of the whitefly Bemisia tabaci cyclophilin B and heat shock protein 70 impairs insect development and virus transmission. Front. Physiol. 2019, 10, 557. [Google Scholar] [CrossRef]
- Mahroof, R.; Zhu, K.Y.; Neven, L.; Subramanyam, B.; Bai, J.F. Expression patterns of three heat shock protein 70 genes among developmental stages of the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 141, 247–256. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Y.; Huo, Z.J.; Zhou, X.R.; Shan, Y.M.; Pang, B.P. Molecular cloning and expression profiling of the heat shock protein gene GdHsp70 in Galeruca daurica (Coleoptera: Chrysomelidae). Acta Entomol. Sin. 2017, 60, 865–875. [Google Scholar]
- Lu, K.; Chen, X.; Liu, W.T.; Zhang, Z.C.; Wang, Y.; You, K.K.; Li, Y.; Zhang, R.B.; Zhou, Q. Characterization of heat shock protein 70 transcript from Nilaparvata lugens (Stål): Its response to temperature and insecticide stresses. Pestic. Biochem. Physiol. 2017, 142, 102–110. [Google Scholar] [CrossRef]
- Dong, B.; Liu, X.Y.; Li, B.; Li, M.Y.; Li, S.G.; Liu, S. A heat shock protein protects against oxidative stress induced by lambda-cyhalothrin in the green peach aphid Myzus persicae. Pestic. Biochem. Physiol. 2022, 181, 104995. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, Y.; Zheng, J.C.; Liang, L.N.; Hoffmann, A.A.; Ma, C.S. Response of heat shock protein genes of the oriental fruit moth under diapause and thermal stress reveals multiple patterns dependent on the nature of stress exposure. Cell Stress Chaperones 2016, 21, 653–663. [Google Scholar] [CrossRef]
- Ding, J.H.; Zheng, L.X.; Chu, J.; Liang, X.H.; Wang, J.; Gao, X.W.; Wu, F.A.; Sheng, S. Characterization, and functional analysis of Hsp70 and Hsp90 gene families in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Front. Physiol. 2021, 12, 753914. [Google Scholar] [CrossRef]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Feder, J.H.; Rossi, J.M.; Solomon, J.; Solomon, N.; Lindquist, S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev. 1992, 6, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Krebs, R.A.; Feder, M.E. Deleterious consequences of Hsp70 overexpression in Drosophila melanogaster larvae. Cell Stress Chaperones 1997, 2, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Lezzi, M. Chromosome puffing: Supramolecular aspects of ecdysone action. In Metamorphosis; Academic Pres: San Diego, CA, USA, 1996; pp. 145–173. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, F.X.; Cai, M.J.; Zhao, W.L.; Li, X.R.; Wang, J.X.; Zhao, X.F. The hormone-dependent function of Hsp90 in the crosstalk between 20-hydroxyecdysone and juvenile hormone signaling pathways in insects is determined by differential phosphorylation and protein interactions. Biochim. Biophys. Acta 2013, 1830, 5184–5192. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Liu, Y.; Li, Q.; Lu, Y.H. Heat shock protein 70 and Cathepsin B genes are involved in the thermal tolerance of Aphis gossypii. Pest Manag. Sci. 2023, 79, 2075–2086. [Google Scholar] [CrossRef]
- Fang, S.M.; Zhang, Q.; Zhang, Y.L.; Zhang, G.Z.; Zhang, Z.; Yu, Q.Y. Heat shock protein 70 family in response to multiple abiotic stresses in the silkworm. Insects 2021, 12, 928. [Google Scholar] [CrossRef]
- Sokhansanj, S.; Venkatesam, V.S.; Wood, H.C.; Doane, J.F.; Spurr, D.T. Thermal kill of wheat midge and Hessian fly. Postharvest Biol. Technol. 1992, 2, 65–71. [Google Scholar] [CrossRef]
- Barnes, H.F. Studies of fluctuations in insect populations. VIII. The wheat blossom midge on broadbalk, 1932–1940, with a discussion of the results obtained 1927–40. J. Anim. Ecol. 1941, 10, 94–120. [Google Scholar] [CrossRef]
- Yuan, F. The Wheat Blossom Midges Sitodiplosis mosellana (Gehin) and Contarinia tritici (Kirby): Their Plague Principle and Control; Science Press: Beijing, China, 2004. [Google Scholar]
- Jiang, F.; Chang, G.F.; Li, Z.Z.; Abouzaid, M.; Du, X.Y.; Hull, J.J.; Ma, W.H.; Lin, Y.J. The HSP/co-chaperone network in environmental cold adaptation of Chilo suppressalis. Int. J. Biol. Macromol. 2021, 187, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.P.; Yocum, G.D.; Denlinger, D.L. Developmental upregulation of inducible hsp70 transcripts, but not the cognate form, during pupal diapause in the flesh fly, Sarcophaga crassipalpis. Insect Biochem. Mol. Biol. 2000, 30, 515–521. [Google Scholar] [CrossRef]




| Primer Name | Sequence (5′ to 3′) | Purpose |
|---|---|---|
| Hsp70A1-1 sense | GCGAAAGAAAGGAGGAGAAG | ORF and gDNA cloning |
| Hsp70A1-1 antisense | CAGGCTCTCTTATTGTACGAG | |
| Hsp70A1-2 sense | ATGCCTGCAGTTGGAATTG | |
| Hsp70A1-2 antisense | GCAAAGAGTGATTTCTTCCTCG | |
| dsHsp70A1-1 sense | taatacgactcactatagggAAAACCAAGTCGCCATGAAC | dsRNA synthesis |
| dsHsp70A1-1 antisense | taatacgactcactatagggTATCCAATCCGTAGGCCAAG | |
| dsHsp70A1-2 sense | taatacgactcactatagggGAAGGCGAACGAGCTATGAC | |
| dsHsp70A1-2 antisense | taatacgactcactatagggCGATCGATTTCTGCTTGTGA | |
| dsGFP sense | taatacgactcactatagggGTGTTCAATGCTTTTCCCGT | |
| dsGFP antisense | taatacgactcactatagggCAATGTTGTGGCGAATTTTG | |
| Hsp70A1-1 sense | AATCAACCCAGACGAGGCAG | qPCR |
| Hsp70A1-1 antisense | GCCCAATGAGAGTGGTGTCA | |
| Hsp70A1-2 sense | CGTAATTGCTGCGAGGAAGC | |
| Hsp70A1-2 antisense | GTGATTGGCGAGCAAACCTG | |
| GAPDH sense | CCATCAAAGCAAGCAAGA | |
| GAPDH antisense | CAGCACGGAGCACAAGAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Tang, W.; Liu, X.; Ma, Q.; Zhu-Salzman, K.; Cheng, W. Characterization of Two Novel Heat Shock Protein 70 Transcripts from Sitodiplosis mosellana and Their Response to Larval Diapause and Thermal Stress. Biology 2025, 14, 1147. https://doi.org/10.3390/biology14091147
Huang Q, Tang W, Liu X, Ma Q, Zhu-Salzman K, Cheng W. Characterization of Two Novel Heat Shock Protein 70 Transcripts from Sitodiplosis mosellana and Their Response to Larval Diapause and Thermal Stress. Biology. 2025; 14(9):1147. https://doi.org/10.3390/biology14091147
Chicago/Turabian StyleHuang, Qitong, Wenqian Tang, Xiaobin Liu, Qian Ma, Keyan Zhu-Salzman, and Weining Cheng. 2025. "Characterization of Two Novel Heat Shock Protein 70 Transcripts from Sitodiplosis mosellana and Their Response to Larval Diapause and Thermal Stress" Biology 14, no. 9: 1147. https://doi.org/10.3390/biology14091147
APA StyleHuang, Q., Tang, W., Liu, X., Ma, Q., Zhu-Salzman, K., & Cheng, W. (2025). Characterization of Two Novel Heat Shock Protein 70 Transcripts from Sitodiplosis mosellana and Their Response to Larval Diapause and Thermal Stress. Biology, 14(9), 1147. https://doi.org/10.3390/biology14091147







