Hydrogen-Rich Water Attenuates Diarrhea in Weaned Piglets via Oxidative Stress Alleviation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Grouping
2.2. Animal Management
2.3. Growth Performance Measurement
2.4. Sample Collection and Processing
2.5. Assessment of Antioxidant Parameters
2.6. Intestinal Morphometric Analysis
2.7. Gut Microbiota Profiling
2.8. Hepatic Metabolomics Profiling
2.9. Statistical Analysis
3. Results
3.1. Effects of Hydrogen-Rich Water on Growth Performance and Diarrhea Incidence in Weaned Piglets
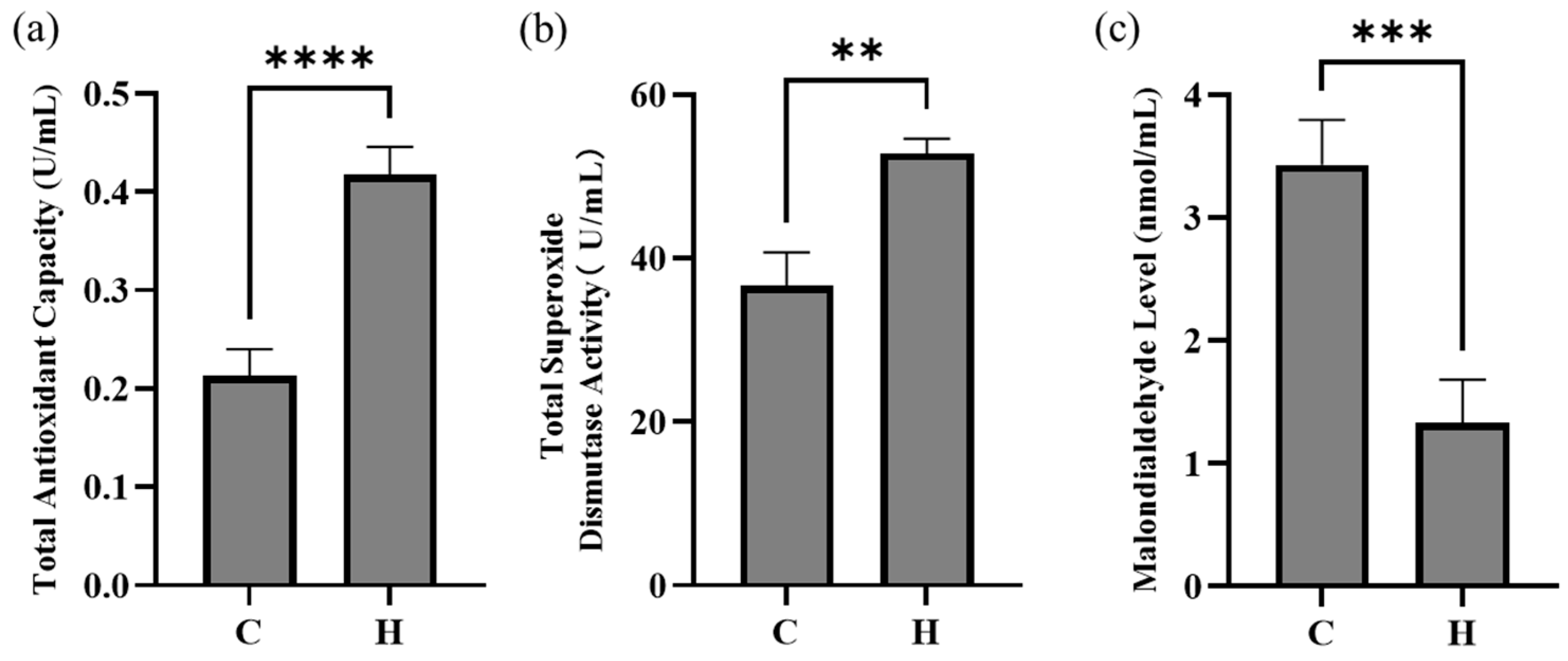
3.2. Effects of Hydrogen-Rich Water on Serum Antioxidant Parameters
3.3. Impacts of Hydrogen-Rich Water on Intestinal Morphology
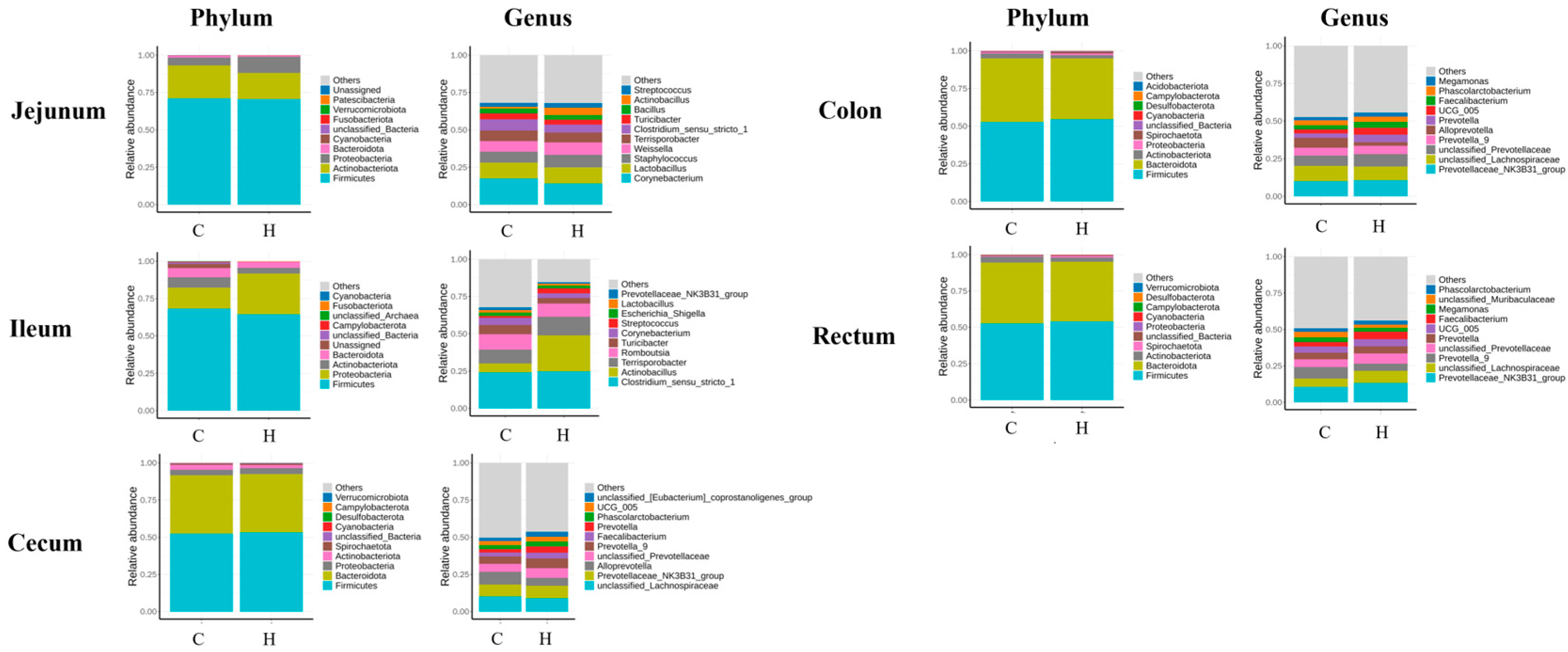
3.4. Modulation of Gut Microbiota by Hydrogen-Rich Water
3.5. Gut Microbiota Composition Analysis
3.6. LEfSe (Linear Discriminant Analysis Effect Size) Analysis
3.7. Prediction Analysis of Microbial Functional Genes
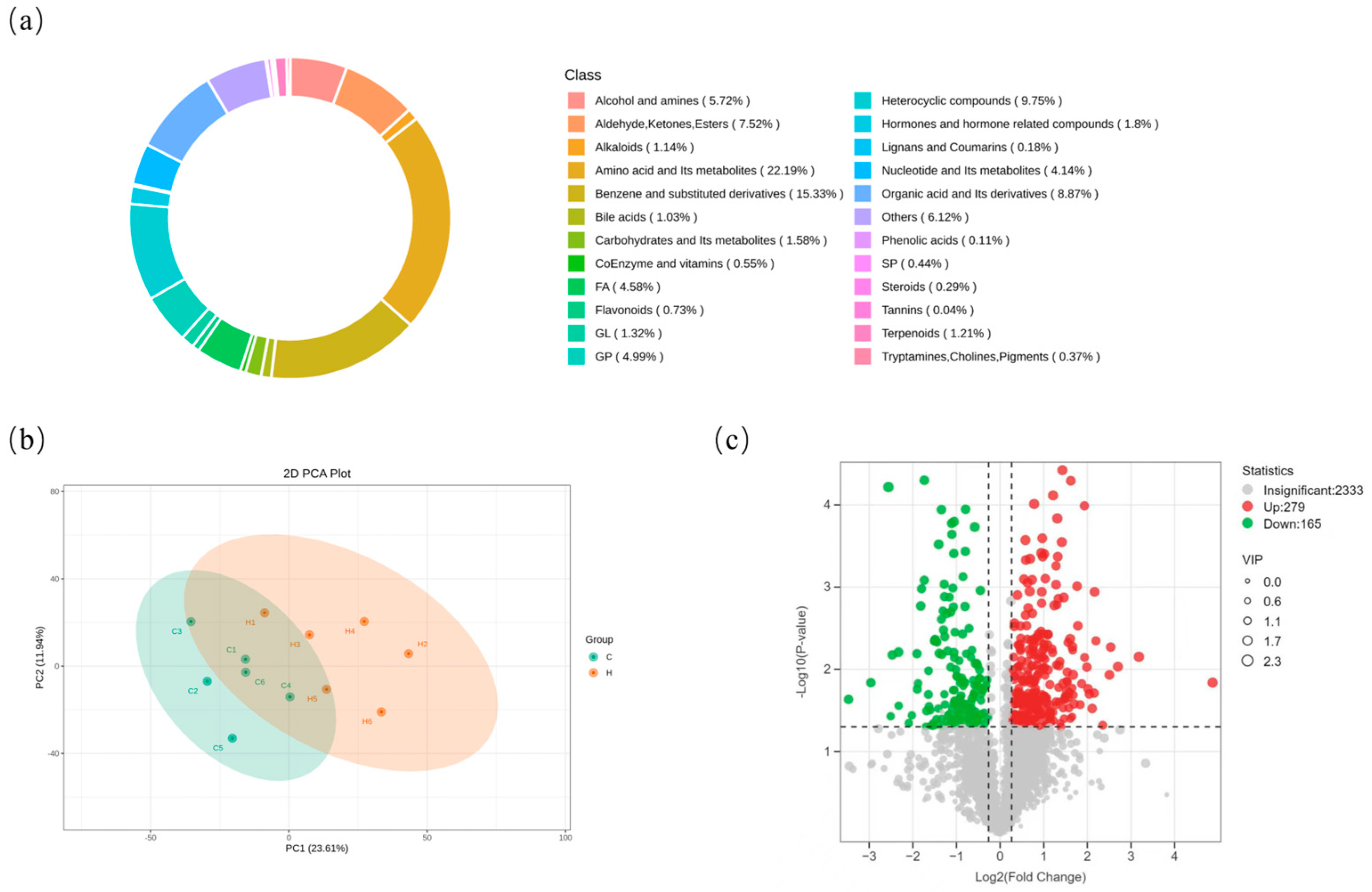
3.8. Effects of Hydrogen-Rich Water on Liver Metabolites in Weaned Piglets
3.9. Analysis of Differentially Abundant Metabolites and KEGG Pathway Enrichment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HRW | Hydrogen-rich water |
| ADG | Average daily gain |
| ADFI | Average daily feed intake |
| FCR | Feed conversion ratio |
| VH/CD | Villus height-to-crypt depth |
References
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Cao, S.; Hou, L.; Sun, L.; Gao, J.; Gao, K.; Yang, X.; Jiang, Z.; Wang, L. Intestinal morphology and immune profiles are altered in piglets by early-weaning. Int. Immunopharmacol. 2022, 105, 108520. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, P.; Yao, K.; Lin, Q.; Wang, M.; Hou, Z.; Tang, W.; Diao, H. Diarrhea induced by insufficient fat absorption in weaned piglets: Causes and nutrition regulation. Anim. Nutr. 2024, 16, 299–305. [Google Scholar] [CrossRef]
- Ying, J.; Zhang, K.; Huang, Y.; Zhu, X.; Ruan, Y.; Lin, H.; Wu, G. Molecular hydrogen: Mechanism against oxidative stress and application in periodontitis: A review. Medicine 2025, 104, e41800. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.M.; Hou, Y.C.; Liao, M.T.; Tsai, K.W.; Hu, W.C.; Yeh, C.C.; Lu, K.C. Potential role of molecular hydrogen therapy on oxidative stress and redox signaling in chronic kidney disease. Biomed. Pharmacother. 2024, 176, 116802. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Sun, T.; Wang, Y.Z.; Wan, Q.; Li, W.Z.; Yang, W.C. Molecular hydrogen alleviates lung injury after traumatic brain injury: Pyroptosis and apoptosis. Eur. J. Pharmacol. 2022, 914, 174664. [Google Scholar] [CrossRef]
- Dhillon, G.; Buddhavarapu, V.; Grewal, H.; Sharma, P.; Verma, R.K.; Munjal, R.; Devadoss, R.; Kashyap, R. Hydrogen Water: Extra Healthy or a Hoax?—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 973. [Google Scholar] [CrossRef]
- Yang, C.M.; Han, Q.J.; Wang, K.L.; Xu, Y.L.; Lan, J.H.; Cao, G.T. Astragalus and Ginseng Polysaccharides Improve Developmental, Intestinal Morphological, and Immune Functional Characters of Weaned Piglets. Front. Physiol. 2019, 10, 418. [Google Scholar] [CrossRef]
- Zheng, W.; Ji, X.; Zhang, Q.; Du, W.; Wei, Q.; Yao, W. Hydrogen-Rich Water and Lactulose Protect Against Growth Suppression and Oxidative Stress in Female Piglets Fed Fusarium Toxins Contaminated Diets. Toxins 2018, 10, 228. [Google Scholar] [CrossRef]
- Gómez, G.; Laviano, H.D.; García-Casco, J.M.; Escudero, R.; Muñoz, M.; Heras-Molina, A.; González-Bulnes, A.; Óvilo, C.; López-Bote, C.; Rey, A.I. Different Effect of Vitamin E or Hydroxytyrosol Supplementation to Sow’s Diet on Oxidative Status and Performances of Weaned Piglets. Antioxidants 2023, 12, 1504. [Google Scholar] [CrossRef]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef]
- Jermsutjarit, P.; Mebumroong, S.; Watcharavongtip, P.; Lin, H.; Tantituvanont, A.; Kaeoket, K.; Piñeyro, P.; Nilubol, D. Evolution and virulence of porcine epidemic diarrhea virus following in vitro and in vivo propagation. Sci. Rep. 2024, 14, 12279. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Z.; Ni, H.; Meng, Y.; Xu, Y.; Xu, H.; Zheng, Y.; Zhang, Y.; Xue, G.; Shang, Y. Hydrogen-rich water alleviates asthma airway inflammation by modulating tryptophan metabolism and activating aryl hydrocarbon receptor via gut microbiota regulation. Free Radic. Biol. Med. 2024, 224, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, B.; Zhang, G.; Hu, J.; Shu, D.; Han, J.; Hu, M.; Tu, M.; Qiao, W.; Liu, R.; et al. Effects of combined treatment with hydrogen-rich electrolyzed water and tea polyphenols on oxidative stress, intestinal injury and intestinal flora disruption in heat-stressed mice. J. Therm. Biol. 2024, 123, 103921. [Google Scholar] [CrossRef]
- Song, L.; Zhang, Y.; Zhu, C.; Ding, X.; Yang, L.; Yan, H. Hydrogen-rich water partially alleviate inflammation, oxidative stress and intestinal flora dysbiosis in DSS-induced chronic ulcerative colitis mice. Adv. Med. Sci. 2022, 67, 29–38. [Google Scholar] [CrossRef]
- Yang, K.; Li, G.; Li, Q.; Wang, W.; Zhao, X.; Shao, N.; Qiu, H.; Liu, J.; Xu, L.; Zhao, J. Distribution of gut microbiota across intestinal segments and their impact on human physiological and pathological processes. Cell Biosci. 2025, 15, 47. [Google Scholar] [CrossRef]
- Lan, C.; Li, H.; Shen, Y.; Liu, Y.; Wu, A.; He, J.; Cai, J.; Tian, G.; Mao, X.; Huang, Z.; et al. Next-generation probiotic candidates targeting intestinal health in weaned piglets: Both live and heat-killed Akkermansia muciniphila prevent pathological changes induced by enterotoxigenic Escherichia coli in the gut. Anim. Nutr. 2024, 17, 110–122. [Google Scholar] [CrossRef]
- Tian, R.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Zhai, Q. Effect of inulin, galacto-oligosaccharides, and polyphenols on the gut microbiota, with a focus on Akkermansia muciniphila. Food Funct. 2024, 15, 4763–4772. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Liu, X.; Wu, J.; Sun, X.; Li, Z.; Geng, Y.J.; Liu, F.; Zhou, Y. Attenuation of Cardiac Ischaemia-reperfusion Injury by Treatment with Hydrogen-rich Water. Curr. Mol. Med. 2019, 19, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, U.; Louis, P.; Goesmann, A.; Henrissat, B.; Duncan, S.H.; Flint, H.J. Complete genome of a new Firmicutes species belonging to the dominant human colonic microbiota (‘Ruminococcus bicirculans’) reveals two chromosomes and a selective capacity to utilize plant glucans. Environ. Microbiol. 2014, 16, 2879–2890. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.Y.; Xie, Y.Y.; Zang, X.W.; Zhong, Y.F.; Ma, X.J.; Sun, H.Z.; Liu, J.X. Deciphering functional groups of rumen microbiome and their underlying potentially causal relationships in shaping host traits. Imeta 2024, 3, e225. [Google Scholar] [CrossRef]
- Abdugheni, R.; Wang, W.Z.; Wang, Y.J.; Du, M.X.; Liu, F.L.; Zhou, N.; Jiang, C.Y.; Wang, C.Y.; Wu, L.; Ma, J.; et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. Imeta 2022, 1, e58. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.K.; Finley, L.W.S. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Deng, Q.; Zhang, Z.; Zhao, H.; Tang, J.; Chen, X.; Liu, G.; Tian, G.; Cai, J.; Jia, G. Glucagon-like peptide 2 attenuates intestinal mucosal barrier injury through the MLCK/pMLC signaling pathway in a piglet model. J. Cell. Physiol. 2021, 236, 3015–3032. [Google Scholar] [CrossRef]
- Domenichini, A.; Adamska, A.; Falasca, M. ABC transporters as cancer drivers: Potential functions in cancer development. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 52–60. [Google Scholar] [CrossRef]
- Lana, J.V.; Rios, A.; Takeyama, R.; Santos, N.; Pires, L.; Santos, G.S.; Rodrigues, I.J.; Jeyaraman, M.; Purita, J.; Lana, J.F. Nebulized Glutathione as a Key Antioxidant for the Treatment of Oxidative Stress in Neurodegenerative Conditions. Nutrients 2024, 16, 2476. [Google Scholar] [CrossRef]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A.E.; Zemskov, E.A.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef]
- Song, J.; Li, Z.; Zhou, L.; Chen, X.; Sew, W.Q.G.; Herranz, H.; Ye, Z.; Olsen, J.V.; Li, Y.; Nygaard, M.; et al. FOXO-regulated OSER1 reduces oxidative stress and extends lifespan in multiple species. Nat. Commun. 2024, 15, 7144. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, R.; Sun, K.; Yan, C.; Jiang, J.; Kong, F.; Shi, J. The deubiquitinase USP11 ameliorates intervertebral disc degeneration by regulating oxidative stress-induced ferroptosis via deubiquitinating and stabilizing Sirt3. Redox Biol. 2023, 62, 102707. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Teng, C.; Wang, J.; Yu, J.; Jin, C.; Wang, L.; Wu, L.; Lin, Z.; Yu, Z.; et al. Orientin downregulating oxidative stress-mediated endoplasmic reticulum stress and mitochondrial dysfunction through AMPK/SIRT1 pathway in rat nucleus pulposus cells in vitro and attenuated intervertebral disc degeneration in vivo. Apoptosis 2022, 27, 1031–1048. [Google Scholar] [CrossRef]





| Growth Performance | C | H | p-Value |
|---|---|---|---|
| ADFI (g/d) | 377.05 ± 3.04 a | 388.23 ± 1.53 b | 0.005 |
| ADG (g/d) | 239.65 ± 11.50 | 265.39 ± 14.61 | 0.094 |
| FGR | 1.62 ± 0.10 | 1.55 ± 0.14 | 0.632 |
| Diarrhea rate (%) | 27.38 a | 9.22 b | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Yang, J.; Lu, Z.; Liang, Q.; Yang, X.; Wang, J.; Guo, J.; Zhao, Y. Hydrogen-Rich Water Attenuates Diarrhea in Weaned Piglets via Oxidative Stress Alleviation. Biology 2025, 14, 997. https://doi.org/10.3390/biology14080997
Zhang P, Yang J, Lu Z, Liang Q, Yang X, Wang J, Guo J, Zhao Y. Hydrogen-Rich Water Attenuates Diarrhea in Weaned Piglets via Oxidative Stress Alleviation. Biology. 2025; 14(8):997. https://doi.org/10.3390/biology14080997
Chicago/Turabian StyleZhang, Pengfei, Jingyu Yang, Zhuoda Lu, Qianxi Liang, Xing Yang, Junchao Wang, Jinbiao Guo, and Yunxiang Zhao. 2025. "Hydrogen-Rich Water Attenuates Diarrhea in Weaned Piglets via Oxidative Stress Alleviation" Biology 14, no. 8: 997. https://doi.org/10.3390/biology14080997
APA StyleZhang, P., Yang, J., Lu, Z., Liang, Q., Yang, X., Wang, J., Guo, J., & Zhao, Y. (2025). Hydrogen-Rich Water Attenuates Diarrhea in Weaned Piglets via Oxidative Stress Alleviation. Biology, 14(8), 997. https://doi.org/10.3390/biology14080997






