Untargeted Metabolomic Identifies Potential Seasonal Biomarkers of Semen Quality in Duroc Boars
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Semen Collection
2.2. Evaluation of Semen Quality
2.3. Isolation of Boar Seminal Plasma
2.4. Metabolite Extraction and Untargeted Metabolomics Analysis
2.4.1. Metabolite Extraction
2.4.2. Untargeted Metabolomics Analysis Using Ultra-Performance Liquid Chromatography–Mass Spectrometry (UPLC-MS)
2.4.3. Multivariate Statistical Analysis
2.4.4. Identification of Potential Biomarkers and Pathway Analysis
2.4.5. Cluster Analysis
2.5. Statistical Analysis
3. Results
3.1. Semen Quality Evaluation
3.2. Metabolomic Profiling and Chemical Classification of Boar Seminal Plasma (SP)
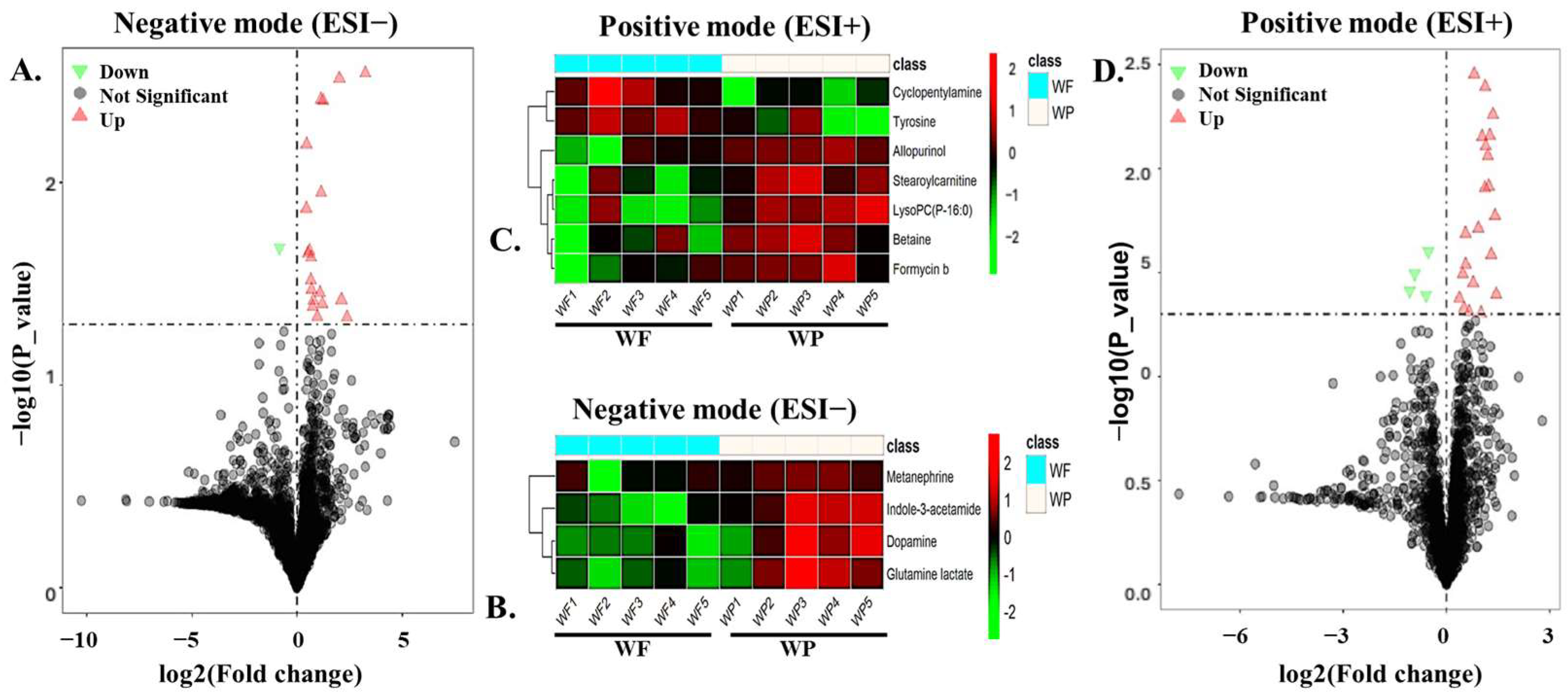
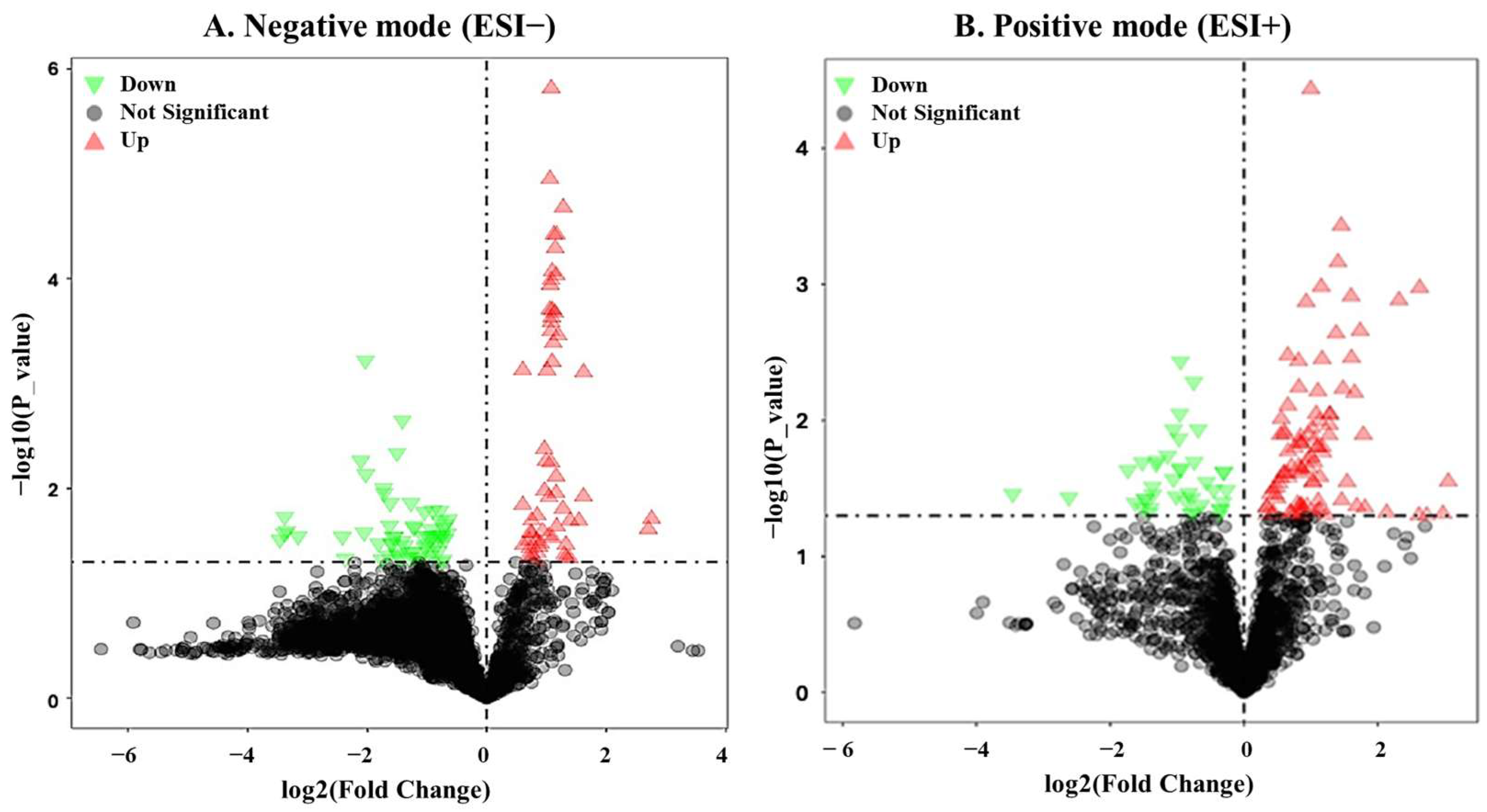
3.3. Identification of DEMs in Boar Seminal Plasma Between Summer and Winter Groups
3.4. Functional Biochemical Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AI | Artificial insemination |
| CASA | Computer-assisted sperm analysis |
| DNA | Deoxyribonucleic acid |
| DEMs | Differentially expressed metabolites |
| DITI | Digital Infrared Thermal Imaging |
| ESI | Electrospray ionization |
| FAO | Fatty acid oxidation |
| FC | Fold change |
| GSH | Glutathione synthesis |
| HCA | Hierarchical clustering analysis |
| HMDB | Human metabolome database |
| KEGG | Kyoto encyclopedia of genes and genomes |
| NEG | Negative |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PLS-DA | Partial least squares discriminant analysis |
| PFAs | Polyhydroxylated fatty alcohols |
| POS | Positive |
| PCA | Principal component analysis |
| QC | Quality control |
| ROS | Reactive oxygen species |
| RNA | Ribonucleic acid |
| SP | Seminal plasma |
| SEM | Standard error of mean |
| SPSS | Statistical package for the social sciences |
| SF | Summer failed |
| SP | Summer passed |
| TCA | Tricarboxylic acid cycle |
| UPLC-MS | Ultra-performance liquid chromatography-mass spectrometry |
| VIP | Variable importance in projection |
| WF | Winter failed |
| WP | Winter passed |
References
- Roca, J.; Parrilla, I.; Bolarin, A.; Martinez, E.; Rodriguez-Martinez, H. Will AI in pigs become more efficient? Theriogenology 2016, 86, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Tsakmakidis, I.; Lymberopoulos, A.; Khalifa, T. Relationship between sperm quality traits and field-fertility of porcine semen. J. Vet. Sci. 2010, 11, 151–154. [Google Scholar] [CrossRef]
- Ciereszko, A.; Ottobre, J.; Glogowski, J. Effects of season and breed on sperm acrosin activity and semen quality of boars. Anim. Reprod. Sci. 2000, 64, 89–96. [Google Scholar] [CrossRef]
- Flowers, W.L. Genetic and phenotypic variation in reproductive traits of AI boars. Theriogenology 2008, 70, 1297–1303. [Google Scholar] [CrossRef]
- Kunavongkrit, A.; Prateep, P. Influence of ambient temperature on reproductive efficiency in pigs: (1) boar semen quality. Pig J. 1995, 35, 43–47. [Google Scholar]
- Kunavongkrit, A.; Suriyasomboon, A.; Lundeheim, N.; Heard, T.W.; Einarsson, S. Management and sperm production of boars under differing environmental conditions. Theriogenology 2005, 63, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Selvam, M.K.P.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Fair, S.; Lonergan, P. Understanding the causes of variation in reproductive wastage among bulls. Animal 2018, 12, s53–s62. [Google Scholar] [CrossRef]
- Drabovich, A.P.; Saraon, P.; Jarvi, K.; Diamandis, E.P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 2014, 11, 278–288. [Google Scholar] [CrossRef]
- Candenas, L.; Chianese, R. Exosome composition and seminal plasma proteome: A promising source of biomarkers of male infertility. Int. J. Mol. Sci. 2020, 21, 7022. [Google Scholar] [CrossRef]
- Du, J.; Shen, J.; Wang, Y.; Pan, C.; Pang, W.; Diao, H.; Dong, W. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget 2016, 7, 58832. [Google Scholar] [CrossRef]
- Piehl, L.L.; Fischman, M.L.; Hellman, U.; Cisale, H.; Miranda, P.V. Boar seminal plasma exosomes: Effect on sperm function and protein identification by sequencing. Theriogenology 2013, 79, 1071–1082. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Kvist, U.; Saravia, F.; Wallgren, M.; Johannisson, A.; Sanz, L.; Peña, F.; Martínez, E.; Roca, J.; Vázquez, J. The physiological roles of the boar ejaculate. Control Pig Reprod. 2009, 8, 1–21. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal Plasma Proteins: What Role Do They Play? Am. J. Reprod. Immunol. 2011, 66, 11–22. [Google Scholar] [CrossRef]
- Recuero, S.; Fernandez-Fuertes, B.; Bonet, S.; Barranco, I.; Yeste, M. Potential of seminal plasma to improve the fertility of frozen-thawed boar spermatozoa. Theriogenology 2019, 137, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Minai-Tehrani, A.; Jafarzadeh, N.; Gilany, K. Metabolomics: A state-of-the-art technology for better understanding of male infertility. Andrologia 2016, 48, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Aderemi, A.V.; Ayeleso, A.O.; Oyedapo, O.O.; Mukwevho, E. Metabolomics: A scoping review of its role as a tool for disease biomarker discovery in selected non-communicable diseases. Metabolites 2021, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Finelli, R.; Agarwal, A.; Henkel, R. Proteomics and metabolomics—Current and future perspectives in clinical andrology. Andrologia 2021, 53, e13711. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Fernández-López, P.; Ribas-Maynou, J.; Roca, J.; Miró, J.; Yeste, M.; Barranco, I. Metabolite profiling of pig seminal plasma identifies potential biomarkers for sperm resilience to liquid preservation. Front. Cell Dev. Biol. 2021, 9, 669974. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, J.; Li, K.; Qin, J.; Sun, Y.; Zeng, J.; El-Ashram, S.; Zhao, Y. Metabolomic analysis reveals spermatozoa and seminal plasma differences between Duroc and Liang guang Small-spotted pig. Front. Vet. Sci. 2023, 9, 1078928. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Pushparaj, P.N.; Baskaran, S.; Bendou, H. Sperm proteome analysis and identification of fertility-associated biomarkers in unexplained male infertility. Genes 2019, 10, 522. [Google Scholar] [CrossRef]
- Coulter, G.; Senger, P.; Bailey, D. Relationship of scrotal surface temperature measured by infrared thermography to subcutaneous and deep testicular temperature in the ram. Reproduction 1988, 84, 417–423. [Google Scholar] [CrossRef]
- Kastelic, J.; Cook, R.; Pierson, R.; Coulter, G. Relationships among scrotal and testicular characteristics, sperm production, and seminal quality in 129 beef bulls. Can. J. Vet. Res. 2001, 65, 111. [Google Scholar] [PubMed]
- De Ruediger, F.R.; Chacur, M.G.M.; Alves, F.C.P.E.; Oba, E.; de Amorim Ramos, A. Digital infrared thermography of the scrotum, semen quality, serum testosterone levels in Nellore bulls (Bos taurus indicus) and their correlation with climatic factors. Semin. Ciências Agrárias 2016, 37, 221–232. [Google Scholar]
- Knox, R.; Levis, D.; Safranski, T.; Singleton, W. An update on North American boar stud practices. Theriogenology 2008, 70, 1202–1208. [Google Scholar] [CrossRef]
- Flowers, W. Factors affecting the efficient production of boar sperm. Reprod. Domest. Anim. 2015, 50, 25–30. [Google Scholar] [CrossRef]
- Parrish, J.J.; Willenburg, K.L.; Gibbs, K.M.; Yagoda, K.B.; Krautkramer, M.M.; Loether, T.M.; Melo, F.C. Scrotal insulation and sperm production in the boar. Mol. Reprod. Dev. 2017, 84, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Setchell, B. The effects of heat on the testes of mammals. Anim. Reprod. 2018, 3, 81–91. [Google Scholar]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, J.M.; Drabovich, A.P.; Lo, K.C. Seminal biomarkers for the evaluation of male infertility. Asian J. Androl. 2016, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, J.J. A role for seminal plasma in modulating pregnancy outcomes in domestic species. Reproduction 2016, 152, R223–R232. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Funct. Genom. 2002, 48, 155–171. [Google Scholar]
- Deepinder, F.; Chowdary, H.T.; Agarwal, A. Role of metabolomic analysis of biomarkers in the management of male infertility. Expert Rev. Mol. Diagn. 2007, 7, 351–358. [Google Scholar] [CrossRef]
- Velho, A.L.C.; Menezes, E.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Metabolomic markers of fertility in bull seminal plasma. PLoS ONE 2018, 13, e0195279. [Google Scholar] [CrossRef]
- Saraf, K.K.; Kumaresan, A.; Dasgupta, M.; Karthikkeyan, G.; Prasad, T.S.K.; Modi, P.K.; Ramesha, K.; Jeyakumar, S.; Manimaran, A. Metabolomic fingerprinting of bull spermatozoa for identification of fertility signature metabolites. Mol. Reprod. Dev. 2020, 87, 692–703. [Google Scholar] [CrossRef]
- Song, C.; Chang, L.; Wang, B.; Zhang, Z.; Wei, Y.; Dou, Y.; Qi, K.; Yang, F.; Li, X.; Li, X. Seminal plasma metabolomics analysis of differences in liquid preservation ability of boar sperm. J. Anim. Sci. 2023, 101, skad392. [Google Scholar] [CrossRef]
- Sui, H.; Sheng, M.; Luo, H.; Liu, G.; Meng, F.; Cao, Z.; Zhang, Y. Characterization of freezability-associated metabolites in boar semen. Theriogenology 2023, 196, 88–96. [Google Scholar] [CrossRef]
- Xu, B.; Wang, R.; Wang, Z.; Liu, H.; Wang, Z.; Zhang, W.; Zhang, Y.; Su, R.; Liu, Z.; Liu, Y. Evaluation of lipidomic change in goat sperm after cryopreservation. Front. Vet. Sci. 2022, 9, 1004683. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, W.; Liu, Y.; Liu, Y.; Liang, H.; Xu, Q.; Liu, Z.; Weng, X. Plasma membrane lipid composition and metabolomics analysis of Yorkshire boar sperms with high and low resistance to cryopreservation. Theriogenology 2023, 206, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.W.; Okun, J.G.; Hoffmann, G.F.; Koelker, S.; Morath, M.A. Impact of short-and medium-chain organic acids, acylcarnitines, and acyl-CoAs onmitochondrial energy metabolism. Biochim. Biophys. Acta (BBA)-Bioenerg. 2008, 1777, 1276–1282. [Google Scholar] [CrossRef]
- Kim, B.H.; Ju, W.S.; Kim, J.-S.; Kim, S.-U.; Park, S.J.; Ward, S.M.; Lyu, J.H.; Choo, Y.-K. Effects of gangliosides on spermatozoa, oocytes, and preimplantation embryos. Int. J. Mol. Sci. 2019, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Tcheng, M.; Minden, M.D.; Spagnuolo, P.A. Avocado-derived avocadyne is a potent inhibitor of fatty acid oxidation. J. Food Biochem. 2022, 46, e13895. [Google Scholar] [CrossRef] [PubMed]
- Juyena, N.S.; Stelletta, C. Seminal plasma: An essential attribute to spermatozoa. J. Androl. 2012, 33, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Castillo, J.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol. Cell. Proteom. 2013, 12, 330–342. [Google Scholar] [CrossRef]
- Ey, J.; Schömig, E.; Taubert, D. Dietary sources and antioxidant effects of ergothioneine. J. Agric. Food Chem. 2007, 55, 6466–6474. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, X.; Li, Y.; Cheng, Y.; Li, J. Ergothioneine Improves the Quality of Boar Sperm During In Vitro Liquid Preservation by Regulating Mitochondrial Respiratory Chain. Animals 2025, 15, 1450. [Google Scholar] [CrossRef]
- Franzoni, F.; Colognato, R.; Galetta, F.; Laurenza, I.; Barsotti, M.; Di Stefano, R.; Bocchetti, R.; Regoli, F.; Carpi, A.; Balbarini, A. An in vitro study on the free radical scavenging capacity of ergothioneine: Comparison with reduced glutathione, uric acid and trolox. Biomed. Pharmacother. 2006, 60, 453–457. [Google Scholar] [CrossRef]
- Ari, U.C.; Kulaksiz, R.; Yildiz, S.; Lehimcioğlu, N.; Öztürkler, Y. Effect of N-acetylcysteine (NAC) on post-thaw semen quality of Tushin rams: Higher doses of NAC may be toxic: P6. Reprod. Domest. Anim. 2015, 50, 43. [Google Scholar]
- Salih, S.A.; Daghigh-Kia, H.; Mehdipour, M.; Najafi, A. Does ergothioneine and thawing temperatures improve rooster semen post-thawed quality? Poult. Sci. 2021, 100, 101405. [Google Scholar] [CrossRef]
- Nikodemus, D.; Lazic, D.; Bach, M.; Bauer, T.; Pfeiffer, C.; Wiltzer, L.; Lain, E.; Schömig, E.; Gründemann, D. Paramount levels of ergothioneine transporter SLC22A4 mRNA in boar seminal vesicles and cross-species analysis of ergothioneine and glutathione in seminal plasma. J. Physiol. Pharmacol. 2011, 62, 411. [Google Scholar]
- Alvarenga, M.A.; Papa, F.O.; Landim-Alvarenga, F.; Medeiros, A. Amides as cryoprotectants for freezing stallion semen: A review. Anim. Reprod. Sci. 2005, 89, 105–113. [Google Scholar] [CrossRef]
- Stoll, M.L.; Kumar, R.; Lefkowitz, E.; Cron, R.Q.; Morrow, C.D.; Barnes, S. Fecal metabolomics in pediatric spondyloarthritis implicate decreased metabolic diversity and altered tryptophan metabolism as pathogenic factors. Genes Immun. 2016, 17, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Olds-Clarke, P. Mice carrying two t haplotypes: Sperm populations with reduced zona pellucida binding are deficient in capacitation. Biol. Reprod. 1999, 61, 305–311. [Google Scholar] [CrossRef]
- Zmijewski, J.; Landar, A.; Watanabe, N.; Dickinson, D.; Noguchi, N.; Darley-Usmar, V. Cell signalling by oxidized lipids and the role of reactive oxygen species in the endothelium. Biochem. Soc. Trans. 2005, 33, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Stival, C.; Puga Molina, L.d.C.; Paudel, B.; Buffone, M.G.; Visconti, P.E.; Krapf, D. Sperm capacitation and acrosome reaction in mammalian sperm. Adv. Anat. Embryol. Cell Biol. 2016, 220, 93–106. [Google Scholar]
- Pyttel, S.; Nimptsch, A.; Böttger, J.; Zschörnig, K.; Jakop, U.; Wegener, J.; Müller, K.; Paasch, U.; Schiller, J. Changes of murine sperm phospholipid composition during epididymal maturation determined by MALDI-TOF mass spectrometry. Theriogenology 2014, 82, 396–402. [Google Scholar] [CrossRef]
- Longobardi, V.; Kosior, M.A.; Pagano, N.; Fatone, G.; Staropoli, A.; Vassetti, A.; Vinale, F.; Campanile, G.; Gasparrini, B. Changes in bull semen metabolome in relation to cryopreservation and fertility. Animals 2020, 10, 1065. [Google Scholar] [CrossRef]
- Urra, J.A.; Villaroel-Espíndola, F.; Covarrubias, A.A.; Rodríguez-Gil, J.E.; Ramírez-Reveco, A.; Concha, I.I. Presence and function of dopamine transporter (DAT) in stallion sperm: Dopamine modulates sperm motility and acrosomal integrity. PLoS ONE 2014, 9, e112834. [Google Scholar] [CrossRef]
- Kanno, H.; Kurata, S.; Hiradate, Y.; Hara, K.; Yoshida, H.; Tanemura, K. High concentration of dopamine treatment may induce acceleration of human sperm motility. Reprod. Med. Biol. 2022, 21, e12482. [Google Scholar] [CrossRef]
- Ramírez, A.R.; Castro, M.A.; Angulo, C.; Ramió, L.; Rivera, M.M.; Torres, M.; Rigau, T.; Rodríguez-Gil, J.E.; Concha, I.I. The presence and function of dopamine type 2 receptors in boar sperm: A possible role for dopamine in viability, capacitation, and modulation of sperm motility. Biol. Reprod. 2009, 80, 753–761. [Google Scholar] [CrossRef]
- Pérez-Escuredo, J.; Dadhich, R.K.; Dhup, S.; Cacace, A.; Van Hée, V.F.; De Saedeleer, C.J.; Sboarina, M.; Rodriguez, F.; Fontenille, M.-J.; Brisson, L. Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle 2016, 15, 72–83. [Google Scholar] [CrossRef]
- Zhu, Z.; Fan, X.; Lv, Y.; Lin, Y.; Wu, D.; Zeng, W. Glutamine protects rabbit spermatozoa against oxidative stress via glutathione synthesis during cryopreservation. Reprod. Fertil. Dev. 2017, 29, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, P.B.; Sarıözkan, S.; Bucak, M.N.; Ulutaş, P.A.; Akalın, P.P.; Büyükleblebici, S.; Canturk, F. Effect of glutamine and sugars after bull spermatozoa cryopreservation. Theriogenology 2011, 75, 1459–1465. [Google Scholar] [CrossRef]
- De Mercado, E.; Hernandez, M.; Sanz, E.; Rodriguez, A.; Gomez, E.; Vazquez, J.; Martinez, E.; Roca, J. Evaluation of l-glutamine for cryopreservation of boar spermatozoa. Anim. Reprod. Sci. 2009, 115, 149–157. [Google Scholar] [CrossRef]
- Tang, W.; Wu, J.; Jin, S.; He, L.; Lin, Q.; Luo, F.; He, X.; Feng, Y.; He, B.; Bing, P. Glutamate and aspartate alleviate testicular/epididymal oxidative stress by supporting antioxidant enzymes and immune defense systems in boars. Sci. China Life Sci. 2020, 63, 116–124. [Google Scholar] [CrossRef]
- Xu, B.; Bai, X.; Zhang, J.; Li, B.; Zhang, Y.; Su, R.; Wang, R.; Wang, Z.; Lv, Q.; Zhang, J. Metabolomic analysis of seminal plasma to identify goat semen freezability markers. Front. Vet. Sci. 2023, 10, 1132373. [Google Scholar] [CrossRef]
- Day, C.R.; Kempson, S.A. Betaine chemistry, roles, and potential use in liver disease. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 1098–1106. [Google Scholar] [CrossRef]
- Lugar, D.; Krom, W.; Mings, P.; Stewart, K. Effects of supplemental betaine to semen extenders on semen quality in boars. Transl. Anim. Sci. 2018, 2, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Vigueras-Villaseñor, R.M.; Molina-Ortiz, D.; Reyes-Torres, G.; del Ángel, D.S.; Moreno-Mendoza, N.A.; Cruz, M.E.G.; Cuevas-Alpuche, O.; Rojas-Castañeda, J.C. Effect of allopurinol on damage caused by free radicals to cryptorchid testes. Acta Histochem. 2009, 111, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, A.; Kodama, H.; Kumagai, J.; Fukuda, J.; Kawamura, K.; Tanikawa, H.; Sato, N.; Tanaka, T. Xanthine oxidase inhibitors suppress testicular germ cell apoptosis induced by experimental cryptorchidism. Mol. Hum. Reprod. 2002, 8, 118–123. [Google Scholar] [CrossRef]
- Buchanan, J.G.; Jumaah, A.O.; Kerr, G.; Talekar, R.R.; Wightman, R.H. C-nucleoside studies. Part 22. cine-Substitution in 1, 4-dinitropyrazoles: Further model studies, an improved synthesis of formycin and pyrazofurin and the synthesis of some 3 (5)-alkylsulphonyl-4-amino-5 (3)-β-D-ribofuranosylpyrazoles. J. Chem. Soc. Perkin Trans. 1991, 1, 1077–1083. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, K.; Gupta, G.K.; Sharma, A.K. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. [Google Scholar] [CrossRef]
- Liu, R.; Li, P.; Fan, C.; Wang, S.; Liu, Z.; Zhao, G.; Yang, K. Effect of supplementation with L-Citrulline on rumen microbiota structure, plasma metabolites, reproductive hormones, and antioxidant capacity of Hu ewes. Front. Microbiol. 2025, 16, 1606437. [Google Scholar] [CrossRef]
- Zasiadczyk, L.; Fraser, L.; Kordan, W.; Wasilewska, K. Individual and seasonal variations in the quality of fractionated boar ejaculates. Theriogenology 2015, 83, 1287–1303. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Ortega, M.D.; Martinez-Alborcia, M.J.; Vazquez, J.M.; Martinez, E.A.; Roca, J. Season of ejaculate collection influences the freezability of boar spermatozoa. Cryobiology 2013, 67, 299–304. [Google Scholar] [CrossRef]
- Wysokińska, A.; Kondracki, S.; Kowalewski, D.; Adamiak, A.; Muczyńska, E. Effect of seasonal factors on the ejaculate properties of crossbred Duroc x Pietrain and Pietrain x Duroc boars as well as purebred Duroc and Pietrain boars. Bull. Vet. Inst. Pulawy 2009, 53, 677–685. [Google Scholar]
- Adamiak, A.; Kondracki, S.; Wysokińska, A. Influence of season of the year on physical properties of ejaculates from Polish Large White and Polish Landrace boars. Rocz. Nauk. Zootech. 2010, 37, 159–167. [Google Scholar]
- Shackman, H.M.; Ding, W.; Bolgar, M.S. A novel route to recognizing quaternary ammonium cations using electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2014, 26, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Mohanarao, G.J.; Atreja, S. Identification of capacitation associated tyrosine phosphoproteins in buffalo (Bubalus bubalis) and cattle spermatozoa. Anim. Reprod. Sci. 2011, 123, 40–47. [Google Scholar] [CrossRef]
- Barbonetti, A.; Vassallo, M.R.C.; Cordeschi, G.; Venetis, D.; Carboni, A.; Sperandio, A.; Felzani, G.; Francavilla, S.; Francavilla, F. Protein tyrosine phosphorylation of the human sperm head during capacitation: Immunolocalization and relationship with acquisition of sperm-fertilizing ability. Asian J. Androl. 2010, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Humphries, P.; Pretorius, E.; Naude, H. Direct and indirect cellular effects of aspartame on the brain. Eur. J. Clin. Nutr. 2008, 62, 451–462. [Google Scholar] [CrossRef]
- Jabbari, S.; Sadeghi, M.R.; Akhondi, M.M.; Habibi, A.E.; Amirjanati, N.; Lakpour, N.; Asgharpour, L.; Ardekani, A.M. Tyrosine phosphorylation pattern in sperm proteins isolated from normospermic and teratospermic men. J. Reprod. Infertil. 2009, 10, 185. [Google Scholar]
- Sui, H.; Wang, S.; Liu, G.; Meng, F.; Cao, Z.; Zhang, Y. Effects of heat stress on motion characteristics and metabolomic profiles of boar spermatozoa. Genes 2022, 13, 1647. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.J. Carbohydrate metabolism and its diseases. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 45–81. [Google Scholar]
- Marin, S.; Chiang, K.; Bassilian, S.; Lee, W.-N.P.; Boros, L.G.; Fernández-Novell, J.M.; Centelles, J.J.; Medrano, A.; Rodriguez-Gil, J.E.; Cascante, M. Metabolic strategy of boar spermatozoa revealed by a metabolomic characterization. FEBS Lett. 2003, 554, 342–346. [Google Scholar] [CrossRef]
- Parrilla, I.; Martinez, E.A.; Gil, M.A.; Cuello, C.; Roca, J.; Rodriguez-Martinez, H.; Martinez, C.A. Boar seminal plasma: Current insights on its potential role for assisted reproductive technologies in swine. Anim. Reprod. 2020, 17, e20200022. [Google Scholar] [CrossRef]
- Wang, C.; Ye, T.; Bao, J.; Dong, J.; Wang, W.; Li, C.; Ding, H.; Chen, H.; Wang, X.; Shi, J. 5-methylcytidine effectively improves spermatogenesis recovery in busulfan-induced oligoasthenospermia mice. Eur. J. Pharmacol. 2024, 967, 176405. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, G.; Stegmayr, B.; Niklasson, F. Sperm motility and interactions among seminal uridine, xanthine, urate, and ATPase in fertile and infertile men. Arch. Androl. 1985, 15, 21–27. [Google Scholar] [CrossRef]
- Niemeyer, T.; Dietz, C.; Fairbanks, L.; Schroeder-Printzen, I.; Henkel, R.; Löeffler, M. Evaluation of uridine metabolism in human and animal spermatozoa. Nucleosides Nucleotides Nucleic Acids 2006, 25, 1215–1219. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, Y.; Wang, J.; Liu, J.; Zhang, J.; Zhang, C.; Zhou, L.; Cao, J.; Jiang, L. Lipidomic and transcriptomic characteristics of boar seminal plasma extracellular vesicles associated with sperm motility. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2024, 1870, 159561. [Google Scholar] [CrossRef]
- Medica, A.J.; Aitken, R.J.; Nicolson, G.L.; Sheridan, A.R.; Swegen, A.; De Iuliis, G.N.; Gibb, Z. Glycerophospholipids protect stallion spermatozoa from oxidative damage in vitro. Reprod. Fertil. 2021, 2, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Costa, C.; Bassaizteguy, V.; Santos, M.; Cardozo, R.; Montes, J.; Settineri, R.; Nicolson, G.L. Incubation of human sperm with micelles made from glycerophospholipid mixtures increases sperm motility and resistance to oxidative stress. PLoS ONE 2018, 13, e0197897. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Li, J.; Xiong, X.; Yin, S.; Fu, W.; Wang, P.; Liu, J.; Xiong, Y. Differential metabolites screening in yak (Bos grunniens) seminal plasma after cryopreservation and the evaluation of the effect of galactose on post-thaw sperm motility. Theriogenology 2024, 215, 249–258. [Google Scholar] [CrossRef]
- Beskow, A.P.; Fernandes, C.G.; Leipnitz, G.; da Silva, L.d.B.; Seminotti, B.; Amaral, A.U.; Wyse, A.T.; Wannmacher, C.M.; Vargas, C.R.; Dutra-Filho, C.S. Influence of ketone bodies on oxidative stress parameters in brain of developing rats in vitro. Metab. Brain Dis. 2008, 23, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Asquith, K.L.; Baleato, R.M.; McLaughlin, E.A.; Nixon, B.; Aitken, R.J. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J. Cell Sci. 2004, 117, 3645–3657. [Google Scholar] [CrossRef]
- Naz, R.K.; Rajesh, P.B. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod. Biol. Endocrinol. 2004, 2, 75. [Google Scholar] [CrossRef][Green Version]
- Fait, G.; Vered, Y.; Yogev, L.; Gamzu, R.; Lessing, J.; Paz, G.; Yavetz, H. High levels of catecholamines in human semen: A preliminary study. Andrologia 2001, 33, 347–350. [Google Scholar] [CrossRef]
- Arcelay, E.; Salicioni, A.M.; Wertheimer, E.; Visconti, P.E. Identification of proteins undergoing tyrosine phosphorylation during mouse sperm capacitation. Int. J. Dev. Biol. 2008, 52, 463–472. [Google Scholar] [CrossRef]
- van Overveld, F.W.; Haenen, G.R.; Rhemrev, J.; Vermeiden, J.P.; Bast, A. Tyrosine as important contributor to the antioxidant capacity of seminal plasma. Chem. Biol. Interact. 2000, 127, 151–161. [Google Scholar] [CrossRef]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [PubMed]
- Sato, Y.; Son, J.H.; Meizel, S. The mouse sperm glycine receptor/chloride channel: Cellular localization and involvement in the acrosome reaction initiated by glycine. J. Androl. 2000, 21, 99–106. [Google Scholar] [CrossRef]
- Li, Y.; Si, W.; Zhang, X.; Dinnyes, A.; Ji, W. Effect of amino acids on cryopreservation of cynomolgus monkey (Macaca fascicularis) sperm. Am. J. Primatol. Off. J. Am. Soc. Primatol. 2003, 59, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.R.; Zeng, J.; Wang, X.; Roelofs, M.C.; Huang, W.; Chiozzi, R.Z.; Hevler, J.F.; Heck, A.J.; Dutcher, S.K.; Brown, A. Structural specializations of the sperm tail. Cell 2023, 186, 2880–2896.e2817. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Peng, L.; Xu, J.; Guo, D.; Cao, W.; Xu, Y.; Li, S. Betaine attenuate chronic restraint stress-induced changes in testicular damage and oxidative stress in male mice. Reprod. Biol. Endocrinol. 2022, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, C.; Chen, Y.; Zhao, Y.; Tan, M.; He, B. Protective Effects of Betaine on Boar Sperm Quality during Liquid Storage and Transport. Animals 2024, 14, 2711. [Google Scholar] [CrossRef]


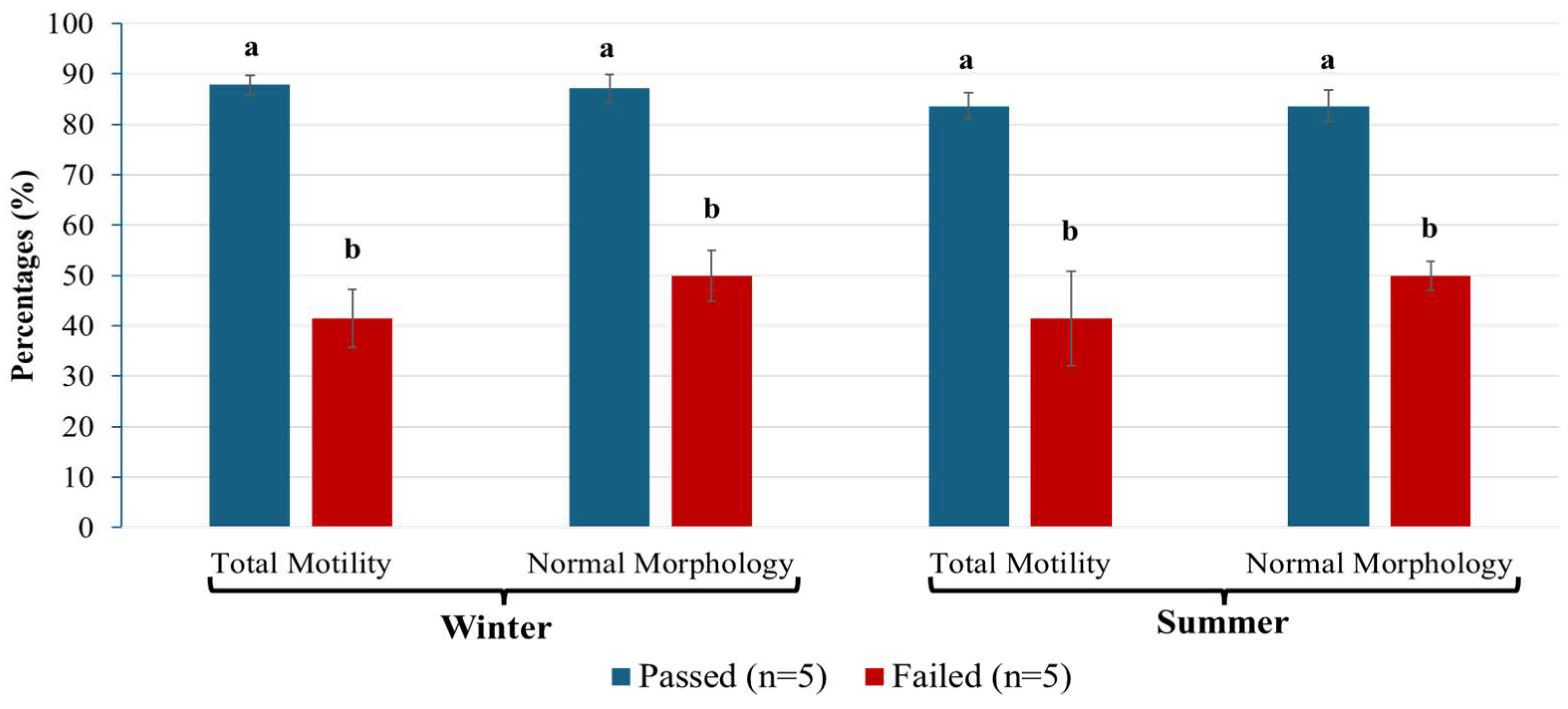
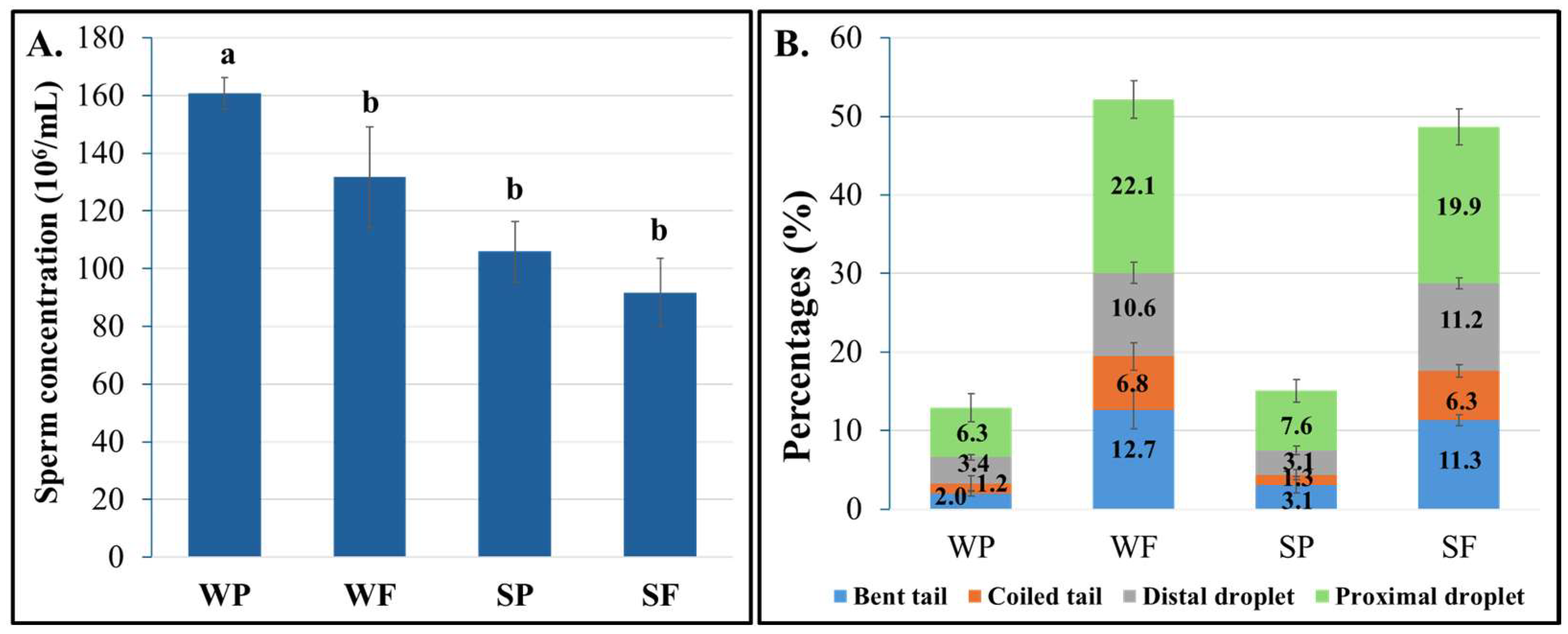






| Replicates (Weeks) | Semen Samples | Failed (Mean ± Sem; %) | Passed (Mean ± Sem; %) | ||
|---|---|---|---|---|---|
| N | Motility | Morphology | Motility | Morphology | |
| I | 6 | 86 ± 1 | 56 ±1 | 90 ± 2 | 82 ± 3 |
| II | 16 | 47 ± 3 | 57 ± 2 | 90 ± 1 | 83 ± 2 |
| III | 7 | 64 ± 6 | 49 ± 1 | 88 ± 1 | 87 ± 1 |
| IV | 6 | 45 ± 7 | 61 ± 6 | 87 ± 5 | 87 ± 3 |
| V | 8 | 63 ± 5 | 41 ± 5 | 88 ± 1 | 85 ± 1 |
| VI | 11 | 43 ± 4 | 52 ± 2 | 90 ± 0.2 | 71 ± 4 |
| VII | 7 | 75 ± 2 | 46 ± 1 | 80 ± 3 | 81 ± 1 |
| VIII | 6 | n.a. | n.a. | 89 ± 1 | 73 ± 6 |
| IX | 8 | 63 ± 4 | 58 ± 3 | 86 ± 1 | 83 ± 2 |
| Global averages | 55 ± 0.7 | 53 ± 0.4 | 88 ± 0.2 | 84 ± 0.2 | |
| N = 38 | N = 37 | ||||
| HMDB ID | Compound Name | Chemical Formula | FC (WF/WP) | p-Value | VIP | ESI |
|---|---|---|---|---|---|---|
| HMDB0029739 | Indole-3-acetamide | C10H10N2O | 2.352 | 0.004 | 2.639 | Negative |
| HMDB0010407 | LysoPC(P-16:0) | C24H50NO6P | 2.203 | 0.008 | 2.378 | Positive |
| HMDB0014581 | Allopurinol | C5H4N4O | 2.301 | 0.009 | 2.350 | Positive |
| HMDB0252811 | Glutamine lactate | C8H14N2O6 | 1.573 | 0.023 | 1.899 | Negative |
| HMDB0000848 | Stearoylcarnitine | C25H49NO4 | 2.469 | 0.026 | 2.048 | Positive |
| HMDB0000073 | Dopamine | C8H11NO2 | 1.556 | 0.030 | 1.939 | Negative |
| HMDB0250671 | Cyclopentylamine | C5H11N | 0.528 | 0.032 | 2.511 | Positive |
| HMDB0000043 | Betaine | C5H12NO2 | 1.391 | 0.032 | 1.845 | Positive |
| HMDB0252449 | Formycin b | C10H12N4O5 | 1.715 | 0.036 | 2.029 | Positive |
| HMDB0004063 | Metanephrine | C10H15NO3 | 1.694 | 0.038 | 1.805 | Negative |
| HMDB0000158 | Tyrosine | C9H11NO3 | 0.477 | 0.039 | 2.284 | Positive |
| HMDB ID | Compound Name | Chemical Formula | FC (WF/WP) | p-Value | VIP | ESI |
|---|---|---|---|---|---|---|
| HMDB0034126 | Gyrocyanin | C17H12O5 | 2.1 | 1.52 × 10−6 | 2.0 | Negative |
| HMDB0251405 | Dimethylpropiothetin | C5H10O2S | 2.2 | 3.81 × 10−5 | 1.9 | Negative |
| HMDB0000788 | Orotidine | C10H12N2O8 | 2.1 | 1.97 × 10−4 | 1.8 | Negative |
| HMDB0040150 | Dihydro-5-methyl-2(3H)-thiophenone | C5H8OS | 2.1 | 1.98 × 10−4 | 1.8 | Negative |
| HMDB0015599 | Enoximone | C12H12N2O2S | 2.1 | 2.60 × 10−4 | 1.8 | Negative |
| HMDB0000425 | 3-Deoxyglycerogalacto-2-nonulosonic acid | C9H16O9 | 2.1 | 3.16 × 10−4 | 1.8 | Negative |
| HMDB0241782 | Nona-2,4,7-trienedioylcarnitine | C16H23NO6 | 2.7 | 3.70 × 10−4 | 2.3 | Positive |
| HMDB0035473 | Avocadyne | C17H32O3 | 2.6 | 6.88 × 10−4 | 2.4 | Positive |
| HMDB0000296 | Uridine | C9H12N2O6 | 2.0 | 7.50 × 10−4 | 1.7 | Negative |
| HMDB0029738 | Indole-3-methyl acetate | C11H11NO2 | 6.2 | 1.06 × 10−3 | 2.3 | Positive |
| HMDB0036186 | Pyrazinemethanethiol | C5H6N2S | 3.3 | 2.20 × 10−3 | 2.3 | Positive |
| HMDB0003045 | Ergothioneine | C9H15N3O2S | 3.0 | 3.48 × 10−3 | 2.2 | Positive |
| HMDB0245936 | 3-Methyltetrahydrophthalic anhydride | C9H10O3 | 0.2 | 5.42 × 10−3 | 2.0 | Negative |
| HMDB0247230 | 7-Amino-4-methylcoumarin | C10H9NO2 | 2.2 | 1.11 × 10−2 | 1.5 | Negative |
| HMDB0259391 | 5-Cholesten-3beta-25-diol-3-sulfate | C27H46O5S | 0.3 | 1.11 × 10−2 | 1.8 | Negative |
| HMDB0041404 | 2-Methylcyclododecanone | C13H24O | 1.5 | 1.27 × 10−2 | 2.2 | Positive |
| HMDB0256086 | Palmitoleamide | C16H31NO | 1.5 | 1.27 × 10−2 | 2.3 | Positive |
| HMDB0252449 | Formycin b | C10H12N4O5 | 2.1 | 1.38 × 10−2 | 2.0 | Positive |
| HMDB0001889 | Theophylline | C7H8N4O2 | 1.5 | 1.44 × 10−2 | 1.8 | Negative |
| HMDB0014581 | Allopurinol | C5H4N4O | 1.8 | 1.48 × 10−2 | 2.1 | Positive |
| HMDB ID | Compound Name | Chemical Formula | FC (WF/WP) | p-Value | VIP | ESI | Season |
|---|---|---|---|---|---|---|---|
| HMDB0029739 | Indole-3-acetamide | C10H10N2O | 2.4 | 0.004 | 2.6 | Negative | Winter |
| HMDB0010407 | LysoPC(P-16:0) | C24H50NO6P | 2.2 | 0.008 | 2.4 | Positive | Winter |
| HMDB0014581 | Allopurinol | C5H4N4O | 2.3 | 0.009 | 2.4 | Positive | Winter |
| HMDB0000848 | Stearoylcarnitine | C25H49NO4 | 2.5 | 0.026 | 2.0 | Positive | Winter |
| HMDB0252449 | Formycin b | C10H12N4O5 | 1.7 | 0.036 | 2.0 | Positive | Winter |
| HMDB0000158 | Tyrosine | C9H11NO3 | 0.5 | 0.039 | 2.3 | Positive | Winter |
| HMDB0241782 | Nona-2,4,7-trienedioylcarnitine | C16H23NO6 | 2.74 | 0.0004 | 2.3 | Positive | Summer |
| HMDB0035473 | Avocadyne | C17H32O3 | 2.65 | 0.0007 | 2.4 | Positive | Summer |
| HMDB0029738 | Indole-3-methyl acetate | C11H11NO2 | 6.18 | 0.0011 | 2.3 | Positive | Summer |
| HMDB0003045 | Ergothioneine | C9H15N3O2S | 3.05 | 0.0035 | 2.2 | Positive | Summer |
| HMDB0256086 | Palmitoleamide | C16H31NO | 1.46 | 0.0127 | 2.3 | Positive | Summer |
| HMDB0252449 | Formycin b | C10H12N4O5 | 2.08 | 0.0138 | 2.0 | Positive | Summer |
| HMDB0014581 | Allopurinol | C5H4N4O | 1.81 | 0.0148 | 2.1 | Positive | Summer |
| HMDB0243861 | 1-Deoxymanno-heptulose | C7H14O6 | 2.15 | 0.0158 | 2.1 | Positive | Summer |
| HMDB0060512 | Sterol | C17H28O | 1.51 | 0.0245 | 2.0 | Positive | Summer |
| HMDB0000378 | 2-Methylbutyroylcarnitine | C12H23NO4 | 0.32 | 0.0404 | 2.2 | Positive | Summer |
| HMDB0041862 | Glucaro-1,4-lactone | C6H8O7 | 1.74 | 0.0409 | 2.0 | Positive | Summer |
| Season | Pathway Name | Selected Metabolites in Pathway | p-Value | ESI |
|---|---|---|---|---|
| Summer | Starch and sucrose metabolism | Glucaro-1,4-lactone, 3-Deoxyglycerogalacto-2-nonulosonic acid | 0.0011 | Both |
| Summer | Pyrimidine metabolism | Uridine, Cytidine | 0.0021 | Negative |
| Summer | Glycerophospholipid metabolism | Glycerophosphocholine | 0.0158 | Negative |
| Summer | Ether lipid metabolism | Glycerophosphocholine | 0.0158 | Negative |
| Summer | Butanoate metabolism | 3-Hydroxybutyric acid | 0.0216 | Negative |
| Winter | Tyrosine metabolism | Tyrosine, Dopamine, Metanephrine | 0.0311 | Both |
| Winter | Glycine, serine, and threonine metabolism | Betaine | 0.0468 | Positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlamini, N.H.; Kameni, S.L.; Feugang, J.M. Untargeted Metabolomic Identifies Potential Seasonal Biomarkers of Semen Quality in Duroc Boars. Biology 2025, 14, 995. https://doi.org/10.3390/biology14080995
Dlamini NH, Kameni SL, Feugang JM. Untargeted Metabolomic Identifies Potential Seasonal Biomarkers of Semen Quality in Duroc Boars. Biology. 2025; 14(8):995. https://doi.org/10.3390/biology14080995
Chicago/Turabian StyleDlamini, Notsile H., Serge L. Kameni, and Jean M. Feugang. 2025. "Untargeted Metabolomic Identifies Potential Seasonal Biomarkers of Semen Quality in Duroc Boars" Biology 14, no. 8: 995. https://doi.org/10.3390/biology14080995
APA StyleDlamini, N. H., Kameni, S. L., & Feugang, J. M. (2025). Untargeted Metabolomic Identifies Potential Seasonal Biomarkers of Semen Quality in Duroc Boars. Biology, 14(8), 995. https://doi.org/10.3390/biology14080995









